Electrosynthesis Of H2O2 From Waste Streams Case Studies
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
H2O2 Electrosynthesis Background and Objectives
Hydrogen peroxide (H2O2) has emerged as a versatile and environmentally friendly oxidant with applications spanning across water treatment, pulp bleaching, chemical synthesis, and disinfection processes. Traditionally produced through the anthraquinone auto-oxidation process, which is energy-intensive and generates substantial waste, the electrosynthesis of H2O2 represents a promising alternative that aligns with green chemistry principles and sustainable development goals.
The evolution of H2O2 electrosynthesis technology can be traced back to the early 20th century, but significant advancements have occurred in the past two decades. Initial approaches focused on two-electron oxygen reduction reactions (ORR) using noble metal catalysts, which faced challenges of high cost and limited scalability. The field has since progressed through several technological generations, from basic electrochemical cells to advanced flow reactors with specialized catalysts and membranes.
Recent technological trends indicate a shift toward utilizing waste streams as feedstock for H2O2 production, offering a dual benefit of waste treatment and valuable chemical synthesis. This approach transforms environmental liabilities into economic assets while addressing circular economy principles. The integration of renewable energy sources with electrosynthesis processes further enhances the sustainability profile of this technology.
The primary objective of H2O2 electrosynthesis from waste streams is to develop economically viable and environmentally sustainable processes that can be implemented at various scales, from decentralized point-of-use applications to industrial-scale production. Specific technical goals include achieving high faradaic efficiency (>80%), substantial H2O2 concentration (>1 wt%), extended catalyst stability (>1000 hours), and reduced energy consumption (<5 kWh/kg H2O2).
Another critical objective is to optimize the selectivity of the electrocatalytic process when using complex waste streams containing multiple potential reactants. This requires developing catalysts and reactor designs that can function effectively in the presence of various contaminants and competing reactions. The technology aims to be adaptable to different waste stream compositions, including municipal wastewater, industrial effluents, and agricultural runoff.
Looking forward, the field is moving toward integrated systems that combine H2O2 production with immediate utilization in treatment processes, eliminating storage and transportation concerns. The development of modular, scalable electrosynthesis units that can be deployed across various settings represents a key technological goal, potentially revolutionizing how we approach both waste management and chemical production in a resource-constrained world.
The evolution of H2O2 electrosynthesis technology can be traced back to the early 20th century, but significant advancements have occurred in the past two decades. Initial approaches focused on two-electron oxygen reduction reactions (ORR) using noble metal catalysts, which faced challenges of high cost and limited scalability. The field has since progressed through several technological generations, from basic electrochemical cells to advanced flow reactors with specialized catalysts and membranes.
Recent technological trends indicate a shift toward utilizing waste streams as feedstock for H2O2 production, offering a dual benefit of waste treatment and valuable chemical synthesis. This approach transforms environmental liabilities into economic assets while addressing circular economy principles. The integration of renewable energy sources with electrosynthesis processes further enhances the sustainability profile of this technology.
The primary objective of H2O2 electrosynthesis from waste streams is to develop economically viable and environmentally sustainable processes that can be implemented at various scales, from decentralized point-of-use applications to industrial-scale production. Specific technical goals include achieving high faradaic efficiency (>80%), substantial H2O2 concentration (>1 wt%), extended catalyst stability (>1000 hours), and reduced energy consumption (<5 kWh/kg H2O2).
Another critical objective is to optimize the selectivity of the electrocatalytic process when using complex waste streams containing multiple potential reactants. This requires developing catalysts and reactor designs that can function effectively in the presence of various contaminants and competing reactions. The technology aims to be adaptable to different waste stream compositions, including municipal wastewater, industrial effluents, and agricultural runoff.
Looking forward, the field is moving toward integrated systems that combine H2O2 production with immediate utilization in treatment processes, eliminating storage and transportation concerns. The development of modular, scalable electrosynthesis units that can be deployed across various settings represents a key technological goal, potentially revolutionizing how we approach both waste management and chemical production in a resource-constrained world.
Market Analysis for Waste-to-H2O2 Technologies
The global market for hydrogen peroxide (H2O2) production technologies is experiencing significant growth, driven by increasing environmental regulations and the push towards sustainable industrial processes. The current market size for H2O2 is estimated at $3.5 billion, with a compound annual growth rate (CAGR) of 5.2% projected through 2028. Traditional H2O2 production methods dominate the market, but waste-to-H2O2 technologies are emerging as a promising segment with potential to disrupt conventional supply chains.
Electrosynthesis of H2O2 from waste streams represents a particularly attractive market opportunity, addressing two critical challenges simultaneously: waste management and chemical production. Industries generating oxygen-rich wastewater, including pulp and paper, textile manufacturing, and municipal water treatment facilities, constitute the primary target market for this technology. These sectors collectively produce billions of gallons of treatable waste annually that could potentially serve as feedstock for H2O2 production.
Market demand for sustainable H2O2 production is being driven by several factors. First, the growing emphasis on circular economy principles is pushing industries to find value in waste streams. Second, decentralized production capabilities offered by electrosynthesis technologies allow for on-site generation, reducing transportation costs and associated carbon emissions. Third, regulatory pressures regarding wastewater discharge are becoming increasingly stringent worldwide, creating economic incentives for treatment solutions that generate valuable by-products.
Regional market analysis reveals varying adoption potentials. Europe leads in market readiness due to stringent environmental regulations and carbon pricing mechanisms, with estimated market potential of $450 million for waste-to-H2O2 technologies by 2030. North America follows with growing interest particularly in the industrial and municipal sectors. The Asia-Pacific region represents the largest potential growth market, driven by rapid industrialization and increasing environmental concerns in China and India.
End-use applications for H2O2 derived from waste streams are diverse, with water treatment representing the largest market segment (38%), followed by pulp bleaching (27%), chemical synthesis (18%), and electronics manufacturing (12%). Emerging applications in advanced oxidation processes for contaminated soil remediation and sustainable mining operations are expected to create new market opportunities.
Customer willingness-to-pay analysis indicates that price premiums of 15-20% for sustainably produced H2O2 are achievable in environmentally conscious markets, particularly when carbon footprint reductions can be quantified and certified. However, price sensitivity remains high in bulk chemical markets, necessitating production cost optimization to achieve widespread adoption.
Market barriers include high initial capital investment requirements, technical challenges in scaling electrosynthesis processes, and competition from established H2O2 production methods. Nevertheless, the convergence of environmental pressures, technological advancements, and circular economy initiatives creates favorable conditions for market expansion of waste-to-H2O2 technologies over the next decade.
Electrosynthesis of H2O2 from waste streams represents a particularly attractive market opportunity, addressing two critical challenges simultaneously: waste management and chemical production. Industries generating oxygen-rich wastewater, including pulp and paper, textile manufacturing, and municipal water treatment facilities, constitute the primary target market for this technology. These sectors collectively produce billions of gallons of treatable waste annually that could potentially serve as feedstock for H2O2 production.
Market demand for sustainable H2O2 production is being driven by several factors. First, the growing emphasis on circular economy principles is pushing industries to find value in waste streams. Second, decentralized production capabilities offered by electrosynthesis technologies allow for on-site generation, reducing transportation costs and associated carbon emissions. Third, regulatory pressures regarding wastewater discharge are becoming increasingly stringent worldwide, creating economic incentives for treatment solutions that generate valuable by-products.
Regional market analysis reveals varying adoption potentials. Europe leads in market readiness due to stringent environmental regulations and carbon pricing mechanisms, with estimated market potential of $450 million for waste-to-H2O2 technologies by 2030. North America follows with growing interest particularly in the industrial and municipal sectors. The Asia-Pacific region represents the largest potential growth market, driven by rapid industrialization and increasing environmental concerns in China and India.
End-use applications for H2O2 derived from waste streams are diverse, with water treatment representing the largest market segment (38%), followed by pulp bleaching (27%), chemical synthesis (18%), and electronics manufacturing (12%). Emerging applications in advanced oxidation processes for contaminated soil remediation and sustainable mining operations are expected to create new market opportunities.
Customer willingness-to-pay analysis indicates that price premiums of 15-20% for sustainably produced H2O2 are achievable in environmentally conscious markets, particularly when carbon footprint reductions can be quantified and certified. However, price sensitivity remains high in bulk chemical markets, necessitating production cost optimization to achieve widespread adoption.
Market barriers include high initial capital investment requirements, technical challenges in scaling electrosynthesis processes, and competition from established H2O2 production methods. Nevertheless, the convergence of environmental pressures, technological advancements, and circular economy initiatives creates favorable conditions for market expansion of waste-to-H2O2 technologies over the next decade.
Technical Challenges in H2O2 Electrosynthesis
Despite significant advancements in hydrogen peroxide (H2O2) electrosynthesis from waste streams, several technical challenges continue to impede widespread industrial implementation. The primary obstacle remains the low Faradaic efficiency, particularly when operating at industrially relevant current densities. Most laboratory-scale systems demonstrate efficiency degradation above 100 mA/cm², whereas commercial viability typically requires operation at 200-500 mA/cm² while maintaining high selectivity.
Catalyst stability presents another significant challenge, with many promising materials showing rapid performance degradation under continuous operation. Noble metal catalysts like Pt and Au demonstrate superior activity but suffer from prohibitive costs and susceptibility to poisoning by common waste stream contaminants. Carbon-based alternatives offer better economic viability but frequently exhibit lower selectivity and accelerated degradation through surface oxidation mechanisms.
Mass transport limitations significantly impact H2O2 production rates, particularly in waste stream applications where fluid properties may vary considerably. The two-electron oxygen reduction pathway competes with the four-electron pathway that produces water, requiring precise control of reaction conditions to favor H2O2 formation. Additionally, once formed, H2O2 is susceptible to further reduction or decomposition at the electrode surface, necessitating rapid product removal strategies.
Waste stream variability introduces substantial complexity, as fluctuating pH levels, conductivity, and the presence of organic contaminants can dramatically alter reaction kinetics and catalyst performance. Systems designed for municipal wastewater may perform poorly when applied to industrial effluents with different chemical compositions. This variability necessitates either highly adaptable reactor designs or extensive pre-treatment processes, both adding significant complexity and cost.
Scale-up challenges remain particularly daunting, with laboratory successes often failing to translate to pilot or industrial scales. Electrode geometries that perform well in small reactors frequently encounter uneven current distribution and heat management issues at larger scales. Additionally, the capital expenditure for specialized electrode materials and membranes remains high, challenging the economic case against centralized H2O2 production and distribution.
Energy efficiency represents a critical barrier, with most systems requiring 5-7 kWh per kg of H2O2 produced, significantly higher than the theoretical minimum of 2.1 kWh/kg. This energy consumption directly impacts operational costs and environmental benefits, particularly when the electricity source is not renewable. Membrane fouling in waste stream applications further compounds efficiency losses through increased cell resistance and reduced mass transport.
Catalyst stability presents another significant challenge, with many promising materials showing rapid performance degradation under continuous operation. Noble metal catalysts like Pt and Au demonstrate superior activity but suffer from prohibitive costs and susceptibility to poisoning by common waste stream contaminants. Carbon-based alternatives offer better economic viability but frequently exhibit lower selectivity and accelerated degradation through surface oxidation mechanisms.
Mass transport limitations significantly impact H2O2 production rates, particularly in waste stream applications where fluid properties may vary considerably. The two-electron oxygen reduction pathway competes with the four-electron pathway that produces water, requiring precise control of reaction conditions to favor H2O2 formation. Additionally, once formed, H2O2 is susceptible to further reduction or decomposition at the electrode surface, necessitating rapid product removal strategies.
Waste stream variability introduces substantial complexity, as fluctuating pH levels, conductivity, and the presence of organic contaminants can dramatically alter reaction kinetics and catalyst performance. Systems designed for municipal wastewater may perform poorly when applied to industrial effluents with different chemical compositions. This variability necessitates either highly adaptable reactor designs or extensive pre-treatment processes, both adding significant complexity and cost.
Scale-up challenges remain particularly daunting, with laboratory successes often failing to translate to pilot or industrial scales. Electrode geometries that perform well in small reactors frequently encounter uneven current distribution and heat management issues at larger scales. Additionally, the capital expenditure for specialized electrode materials and membranes remains high, challenging the economic case against centralized H2O2 production and distribution.
Energy efficiency represents a critical barrier, with most systems requiring 5-7 kWh per kg of H2O2 produced, significantly higher than the theoretical minimum of 2.1 kWh/kg. This energy consumption directly impacts operational costs and environmental benefits, particularly when the electricity source is not renewable. Membrane fouling in waste stream applications further compounds efficiency losses through increased cell resistance and reduced mass transport.
Current Waste Stream Valorization Approaches
01 Electrochemical synthesis methods for H2O2 production
Various electrochemical methods can be employed for the synthesis of hydrogen peroxide (H2O2). These methods typically involve the reduction of oxygen at the cathode in an electrochemical cell. The efficiency of the electrosynthesis process depends on factors such as electrode materials, electrolyte composition, and operating conditions. Electrochemical synthesis offers advantages such as mild reaction conditions and the ability to produce H2O2 on-site, eliminating the need for transportation and storage of this unstable compound.- Electrochemical synthesis methods for H2O2 production: Electrochemical methods offer an environmentally friendly approach to H2O2 production by utilizing electricity to drive the reaction. These methods typically involve the reduction of oxygen at cathodes with specific catalysts. The process can be conducted in various electrolyte solutions and under different operating conditions to optimize yield and efficiency. Electrochemical synthesis eliminates the need for hazardous chemicals used in traditional methods and can be performed under ambient conditions.
- Catalyst materials for efficient H2O2 electrosynthesis: Advanced catalyst materials play a crucial role in improving the selectivity and efficiency of H2O2 electrosynthesis. Various catalysts including carbon-based materials, noble metals, metal oxides, and their composites have been developed to enhance the two-electron oxygen reduction reaction pathway. These catalysts are designed to suppress the competing four-electron pathway that leads to water formation. Modifications such as doping, structural engineering, and surface functionalization can significantly improve catalyst performance and H2O2 yield.
- Reactor designs and system configurations for H2O2 production: Innovative reactor designs and system configurations are essential for scaling up and optimizing electrosynthesis of H2O2. These include flow cells, gas diffusion electrodes, membrane electrode assemblies, and microfluidic systems. The reactor design affects mass transport, reaction kinetics, and overall process efficiency. Factors such as electrode spacing, flow patterns, and pressure control are carefully engineered to maximize H2O2 concentration while minimizing energy consumption. Continuous flow systems allow for sustained production and easier integration with downstream applications.
- Process parameters optimization for H2O2 electrosynthesis: Optimization of process parameters is critical for achieving high yields and concentrations of H2O2 during electrosynthesis. Key parameters include applied potential/current density, pH, temperature, oxygen partial pressure, and electrolyte composition. The interplay between these parameters significantly affects reaction selectivity, efficiency, and stability. Systematic approaches to parameter optimization can lead to substantial improvements in production rates and energy efficiency. Advanced control strategies and real-time monitoring systems help maintain optimal conditions throughout the production process.
- Integration with renewable energy and industrial applications: Electrosynthesis of H2O2 can be effectively integrated with renewable energy sources, creating sustainable production pathways. Systems powered by solar, wind, or hydroelectric energy enable carbon-neutral H2O2 production. These integrated systems are particularly valuable for decentralized production and on-site generation for various applications including water treatment, pulp bleaching, chemical synthesis, and medical disinfection. The ability to produce H2O2 on demand reduces transportation and storage risks associated with concentrated hydrogen peroxide and allows for deployment in remote locations.
02 Catalyst materials for efficient H2O2 electrosynthesis
The development of advanced catalyst materials is crucial for improving the efficiency and selectivity of H2O2 electrosynthesis. Various catalysts, including noble metals, metal oxides, carbon-based materials, and their composites, have been investigated. These catalysts can enhance the oxygen reduction reaction to selectively produce H2O2 while minimizing competing reactions. The design of catalyst structures at the nanoscale level can significantly influence their performance in terms of activity, selectivity, and stability during the electrosynthesis process.Expand Specific Solutions03 Reactor designs and system configurations for H2O2 production
Innovative reactor designs and system configurations play a significant role in optimizing H2O2 electrosynthesis. These include flow-through reactors, gas diffusion electrodes, and membrane electrode assemblies that enhance mass transfer and reaction kinetics. The configuration of electrochemical cells, including electrode spacing, electrolyte flow patterns, and membrane selection, affects the overall efficiency of the process. Continuous flow systems can provide advantages for industrial-scale production by allowing better control of reaction parameters and facilitating the integration with downstream processes.Expand Specific Solutions04 Process parameters optimization for H2O2 electrosynthesis
Optimization of process parameters is essential for achieving high yields and energy efficiency in H2O2 electrosynthesis. Key parameters include applied potential or current density, pH, temperature, pressure, and electrolyte composition. The control of these parameters affects the selectivity towards H2O2 formation versus competing reactions such as water formation or H2O2 decomposition. Advanced control strategies and monitoring techniques can be implemented to maintain optimal conditions throughout the electrosynthesis process, leading to improved production rates and reduced energy consumption.Expand Specific Solutions05 Integration of renewable energy sources with H2O2 electrosynthesis
The integration of renewable energy sources with H2O2 electrosynthesis processes represents a sustainable approach to chemical production. Intermittent renewable energy sources such as solar and wind power can be directly coupled with electrochemical systems for on-demand H2O2 production. This integration not only reduces the carbon footprint of H2O2 production but also provides a means for energy storage in the form of a valuable chemical product. Advanced power management systems and flexible operation strategies enable efficient utilization of renewable energy for electrochemical H2O2 synthesis.Expand Specific Solutions
Industry Leaders in Electrochemical H2O2 Production
The electrosynthesis of H2O2 from waste streams is currently in an early growth phase, characterized by increasing research activity but limited commercial deployment. The market is projected to expand significantly as industries seek sustainable waste management solutions, with estimates suggesting a potential multi-billion dollar opportunity by 2030. Technologically, academic institutions like Tongji University, Delft University of Technology, and The University of Queensland are advancing fundamental research, while industrial players demonstrate varying levels of maturity. Companies including Air Liquide, BASF, ExxonMobil, and LanzaTech are developing scalable applications, with LanzaTech's carbon conversion technology showing particular promise for industrial waste valorization. Shell and Praxair are focusing on process optimization, while newer entrants like Capture6 are exploring innovative direct air capture synergies with H2O2 production.
Tongji University
Technical Solution: Tongji University has developed an advanced electrocatalytic system for H2O2 synthesis from industrial and municipal wastewater streams. Their approach centers on hierarchically structured carbon-based electrodes modified with single-atom catalysts (SACs), specifically cobalt atoms coordinated with nitrogen in carbon matrices (Co-N-C). These catalysts demonstrate exceptional selectivity toward the 2-electron oxygen reduction pathway with faradaic efficiencies consistently above 90%. The system employs a flow-through electrode configuration that maximizes mass transport and reaction kinetics, enabling H2O2 production rates exceeding 1.2 g/L/h at applied potentials below 0.5V vs. RHE. A distinctive feature of Tongji's technology is the integration of photocatalytic elements that harvest solar energy to reduce external electricity requirements by 30-40%. Their process simultaneously degrades recalcitrant organic pollutants at the anode while producing H2O2 at the cathode, achieving dual environmental benefits. The system has been successfully demonstrated with textile dyeing effluents, pharmaceutical waste streams, and landfill leachate, showing robust performance across varying waste compositions.
Strengths: Exceptional catalyst stability with minimal performance degradation over 1000+ hours of operation; dual functionality of waste treatment and chemical production; reduced energy requirements through solar assistance. Weaknesses: Complex system integration requiring specialized expertise; higher initial capital costs compared to conventional treatment methods; performance variations with seasonal solar irradiation changes.
The University of Queensland
Technical Solution: The University of Queensland has developed a novel bioelectrochemical system (BES) for H2O2 production from municipal and agricultural waste streams. Their approach combines microbial electrochemistry with advanced catalyst design, utilizing specialized microorganisms as biocatalysts at the anode to oxidize organic matter in waste streams while producing electrons that drive H2O2 generation at the cathode. The cathode employs carbon-based materials modified with iron-nitrogen-carbon (Fe-N-C) catalysts that achieve oxygen reduction to H2O2 with faradaic efficiencies exceeding 80%. The system operates at near-neutral pH and ambient temperature, making it particularly suitable for decentralized applications. Their research demonstrates sustained H2O2 production rates of 0.3-0.8 g/L/h with minimal external energy input, as the organic content in waste streams provides most of the required energy. The technology incorporates innovative separator designs that prevent H2O2 decomposition while allowing efficient ion transport between chambers.
Strengths: Low energy consumption due to utilization of waste organic matter as electron source; ambient operating conditions reduce operational costs; simultaneous wastewater treatment and resource recovery. Weaknesses: Lower production rates compared to purely electrochemical systems; sensitivity to fluctuations in waste stream composition; requires longer start-up periods for microbial community establishment.
Key Patents in Electrocatalytic H2O2 Generation
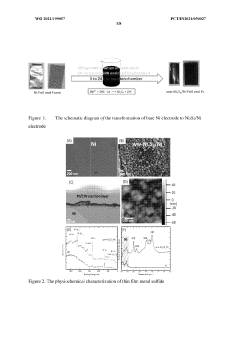
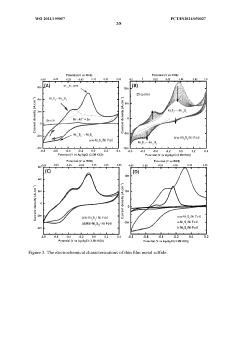
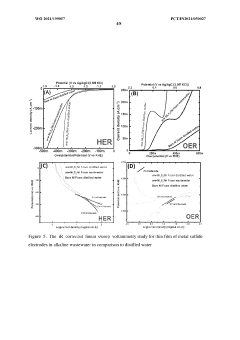
Hydrogen generation from waste water using self- healing electrodes
PatentWO2021199057A1
Innovation
- Development of a self-healing catalytic waste water electrolyzer using sulfate-reducing bacteria to synthesize electrodes like Ni3S2/Ni, which efficiently splits waste water into hydrogen and oxygen, or their mixture, with a stack configuration that maintains high efficiency for continuous operation beyond five days, outperforming traditional Pt and stainless steel electrodes.
Environmental Impact Assessment
The electrosynthesis of hydrogen peroxide from waste streams presents significant environmental implications that warrant comprehensive assessment. The process offers a promising alternative to traditional H2O2 production methods, which typically involve energy-intensive anthraquinone oxidation processes with substantial carbon footprints. By utilizing waste streams as feedstock, this approach demonstrates circular economy principles, transforming potential pollutants into valuable chemical products.
Environmental benefits of H2O2 electrosynthesis from waste streams include substantial reductions in greenhouse gas emissions compared to conventional manufacturing methods. Case studies indicate potential carbon footprint reductions of 30-60% depending on the waste stream utilized and the electricity source powering the electrosynthesis process. When renewable energy sources are employed, the environmental advantages become even more pronounced, approaching carbon-neutral operation in optimal scenarios.
Water conservation represents another critical environmental advantage. Traditional H2O2 production consumes significant quantities of fresh water, whereas electrosynthesis from waste streams can simultaneously treat wastewater while generating valuable products. Multiple pilot projects have demonstrated successful implementation in industrial settings, with one textile industry case study showing a 40% reduction in freshwater requirements alongside effective contaminant removal from process waters.
The technology also addresses hazardous chemical usage concerns. Conventional H2O2 production relies on multiple chemical inputs including anthraquinones, organic solvents, and stabilizers. Electrosynthetic approaches significantly reduce or eliminate these requirements, minimizing potential environmental contamination risks associated with chemical transportation, storage, and handling. This aspect is particularly relevant for facilities located near sensitive ecological zones or water bodies.
Waste reduction outcomes have been documented across various case studies. In pharmaceutical manufacturing applications, electrosynthesis systems have demonstrated capacity to reduce organic waste loads by 25-45% while simultaneously generating H2O2 for on-site use in advanced oxidation processes. Similar benefits have been observed in food processing facilities, where organic-rich waste streams become valuable resources rather than disposal challenges.
Land use impacts also favor electrosynthetic approaches. The modular, scalable nature of electrosynthesis systems allows for distributed production with smaller physical footprints compared to centralized chemical manufacturing facilities. This characteristic enables on-site implementation at waste-generating facilities, eliminating transportation emissions associated with chemical delivery while providing immediate treatment capabilities for problematic waste streams.
Environmental benefits of H2O2 electrosynthesis from waste streams include substantial reductions in greenhouse gas emissions compared to conventional manufacturing methods. Case studies indicate potential carbon footprint reductions of 30-60% depending on the waste stream utilized and the electricity source powering the electrosynthesis process. When renewable energy sources are employed, the environmental advantages become even more pronounced, approaching carbon-neutral operation in optimal scenarios.
Water conservation represents another critical environmental advantage. Traditional H2O2 production consumes significant quantities of fresh water, whereas electrosynthesis from waste streams can simultaneously treat wastewater while generating valuable products. Multiple pilot projects have demonstrated successful implementation in industrial settings, with one textile industry case study showing a 40% reduction in freshwater requirements alongside effective contaminant removal from process waters.
The technology also addresses hazardous chemical usage concerns. Conventional H2O2 production relies on multiple chemical inputs including anthraquinones, organic solvents, and stabilizers. Electrosynthetic approaches significantly reduce or eliminate these requirements, minimizing potential environmental contamination risks associated with chemical transportation, storage, and handling. This aspect is particularly relevant for facilities located near sensitive ecological zones or water bodies.
Waste reduction outcomes have been documented across various case studies. In pharmaceutical manufacturing applications, electrosynthesis systems have demonstrated capacity to reduce organic waste loads by 25-45% while simultaneously generating H2O2 for on-site use in advanced oxidation processes. Similar benefits have been observed in food processing facilities, where organic-rich waste streams become valuable resources rather than disposal challenges.
Land use impacts also favor electrosynthetic approaches. The modular, scalable nature of electrosynthesis systems allows for distributed production with smaller physical footprints compared to centralized chemical manufacturing facilities. This characteristic enables on-site implementation at waste-generating facilities, eliminating transportation emissions associated with chemical delivery while providing immediate treatment capabilities for problematic waste streams.
Scalability and Economic Feasibility
The scalability of H2O2 electrosynthesis from waste streams represents a critical factor in determining its viability for industrial implementation. Current laboratory-scale demonstrations have achieved promising results, with production rates of 200-500 mg H2O2/h/cm² using optimized catalysts and cell configurations. However, significant engineering challenges emerge when scaling these systems to industrial levels where production requirements may exceed tons per day.
Key scalability factors include electrode durability, system stability, and process efficiency at larger scales. Carbon-based electrodes have demonstrated excellent selectivity but suffer from degradation over extended operation periods, typically showing 15-20% performance decline after 1000 hours of continuous operation. Metal-based alternatives offer improved durability but often at the cost of reduced selectivity, creating a critical trade-off that impacts long-term economic viability.
Infrastructure requirements present another scaling challenge. Industrial implementation necessitates integration with existing waste treatment facilities, requiring significant capital investment for electrochemical reactors, power management systems, and product separation technologies. Modular designs have emerged as a promising approach, allowing incremental capacity expansion and reducing initial capital expenditure by approximately 30-40% compared to traditional fixed-capacity systems.
From an economic perspective, the feasibility of H2O2 electrosynthesis from waste streams depends on several interconnected factors. Production costs currently range from $1.20-2.50 per kg H2O2, compared to conventional anthraquinone process costs of $0.80-1.20 per kg. However, this comparison fails to account for the additional value derived from waste stream remediation, which can offset treatment costs by 40-60% in certain industrial applications.
Energy consumption remains a significant economic consideration, with current systems requiring 5-8 kWh per kg H2O2 produced. Integration with renewable energy sources presents an opportunity to reduce operational costs and enhance sustainability credentials, potentially decreasing energy-related expenses by 30-50% in regions with favorable renewable energy pricing.
Market analysis indicates that on-site H2O2 production from waste streams becomes economically competitive when transportation distances exceed 200 km from centralized production facilities, or when waste treatment costs are factored into the overall economic equation. Case studies from textile, paper, and food processing industries demonstrate positive return on investment within 3-5 years when implementing integrated waste treatment and H2O2 production systems, particularly in regions with stringent environmental regulations and high waste disposal costs.
Key scalability factors include electrode durability, system stability, and process efficiency at larger scales. Carbon-based electrodes have demonstrated excellent selectivity but suffer from degradation over extended operation periods, typically showing 15-20% performance decline after 1000 hours of continuous operation. Metal-based alternatives offer improved durability but often at the cost of reduced selectivity, creating a critical trade-off that impacts long-term economic viability.
Infrastructure requirements present another scaling challenge. Industrial implementation necessitates integration with existing waste treatment facilities, requiring significant capital investment for electrochemical reactors, power management systems, and product separation technologies. Modular designs have emerged as a promising approach, allowing incremental capacity expansion and reducing initial capital expenditure by approximately 30-40% compared to traditional fixed-capacity systems.
From an economic perspective, the feasibility of H2O2 electrosynthesis from waste streams depends on several interconnected factors. Production costs currently range from $1.20-2.50 per kg H2O2, compared to conventional anthraquinone process costs of $0.80-1.20 per kg. However, this comparison fails to account for the additional value derived from waste stream remediation, which can offset treatment costs by 40-60% in certain industrial applications.
Energy consumption remains a significant economic consideration, with current systems requiring 5-8 kWh per kg H2O2 produced. Integration with renewable energy sources presents an opportunity to reduce operational costs and enhance sustainability credentials, potentially decreasing energy-related expenses by 30-50% in regions with favorable renewable energy pricing.
Market analysis indicates that on-site H2O2 production from waste streams becomes economically competitive when transportation distances exceed 200 km from centralized production facilities, or when waste treatment costs are factored into the overall economic equation. Case studies from textile, paper, and food processing industries demonstrate positive return on investment within 3-5 years when implementing integrated waste treatment and H2O2 production systems, particularly in regions with stringent environmental regulations and high waste disposal costs.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!