Techno Economic Analysis For Distributed H2O2 Production Models
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
H2O2 Production Technology Background and Objectives
Hydrogen peroxide (H2O2) has emerged as a critical chemical with diverse applications across industries, from water treatment and pulp bleaching to electronics manufacturing and medical sterilization. Traditionally produced through centralized anthraquinone auto-oxidation (AO) processes, the industry is now witnessing a paradigm shift toward distributed production models driven by sustainability concerns and evolving market demands.
The evolution of H2O2 production technology spans over a century, beginning with early electrochemical methods in the early 1900s, followed by the development of the AO process in the 1940s which remains the dominant commercial method today. Recent decades have seen significant advancements in direct synthesis pathways, electrochemical approaches, and photocatalytic methods that enable smaller-scale, distributed production capabilities.
Current global H2O2 production exceeds 5 million tons annually, with demand projected to grow at 3-4% per year. This growth trajectory is accelerated by emerging applications in green chemistry, advanced oxidation processes for wastewater treatment, and hydrogen fuel cell technologies where H2O2 serves as an energy carrier or intermediate.
The primary objective of this technical research is to conduct a comprehensive techno-economic analysis of distributed H2O2 production models compared to conventional centralized manufacturing. This analysis aims to quantify production costs, energy requirements, environmental impacts, and market viability across different scales and technologies.
Distributed production presents several potential advantages, including reduced transportation costs and associated carbon emissions, on-demand production capabilities, elimination of stabilizer additives, and enhanced process safety through smaller inventories. However, these benefits must be weighed against challenges in achieving economies of scale and maintaining product quality consistency.
The technological landscape for distributed H2O2 production encompasses several promising approaches: direct synthesis from hydrogen and oxygen using noble metal catalysts, electrochemical production through oxygen reduction, photocatalytic methods utilizing solar energy, and enzymatic processes mimicking biological pathways. Each approach presents unique advantages and limitations regarding energy efficiency, capital requirements, and operational complexity.
This research seeks to establish quantitative benchmarks for economic viability of distributed production models across different market segments and geographic regions, while identifying critical technological thresholds that must be achieved to enable widespread adoption of decentralized H2O2 manufacturing paradigms.
The evolution of H2O2 production technology spans over a century, beginning with early electrochemical methods in the early 1900s, followed by the development of the AO process in the 1940s which remains the dominant commercial method today. Recent decades have seen significant advancements in direct synthesis pathways, electrochemical approaches, and photocatalytic methods that enable smaller-scale, distributed production capabilities.
Current global H2O2 production exceeds 5 million tons annually, with demand projected to grow at 3-4% per year. This growth trajectory is accelerated by emerging applications in green chemistry, advanced oxidation processes for wastewater treatment, and hydrogen fuel cell technologies where H2O2 serves as an energy carrier or intermediate.
The primary objective of this technical research is to conduct a comprehensive techno-economic analysis of distributed H2O2 production models compared to conventional centralized manufacturing. This analysis aims to quantify production costs, energy requirements, environmental impacts, and market viability across different scales and technologies.
Distributed production presents several potential advantages, including reduced transportation costs and associated carbon emissions, on-demand production capabilities, elimination of stabilizer additives, and enhanced process safety through smaller inventories. However, these benefits must be weighed against challenges in achieving economies of scale and maintaining product quality consistency.
The technological landscape for distributed H2O2 production encompasses several promising approaches: direct synthesis from hydrogen and oxygen using noble metal catalysts, electrochemical production through oxygen reduction, photocatalytic methods utilizing solar energy, and enzymatic processes mimicking biological pathways. Each approach presents unique advantages and limitations regarding energy efficiency, capital requirements, and operational complexity.
This research seeks to establish quantitative benchmarks for economic viability of distributed production models across different market segments and geographic regions, while identifying critical technological thresholds that must be achieved to enable widespread adoption of decentralized H2O2 manufacturing paradigms.
Market Analysis for Distributed H2O2 Production
The global hydrogen peroxide (H2O2) market has been experiencing steady growth, with a market value estimated at $3.5 billion in 2022 and projected to reach $5.7 billion by 2030, growing at a CAGR of approximately 6.3%. This growth is primarily driven by increasing demand across diverse sectors including pulp and paper, chemical synthesis, wastewater treatment, electronics manufacturing, and healthcare applications.
Traditional centralized H2O2 production models have dominated the market for decades, characterized by large-scale facilities producing concentrated solutions (typically 50-70%) that are then diluted and transported to end users. However, this model faces significant challenges including transportation hazards, storage risks, and substantial energy costs associated with concentration and subsequent dilution processes.
The distributed H2O2 production model represents a paradigm shift in this market landscape. This approach involves smaller-scale, localized production facilities that generate H2O2 at or near the point of use, typically at concentrations directly applicable for end applications (3-8%). Market analysis indicates growing interest in this model, particularly in regions with developing infrastructure or remote industrial operations.
Key market drivers for distributed H2O2 production include reduced transportation costs (estimated at 15-25% of total delivered cost in traditional models), enhanced safety profiles, decreased environmental footprint, and the ability to customize production to specific local needs. Industries with continuous H2O2 requirements, such as municipal water treatment facilities and pulp mills, show particular interest in this distributed approach.
Regional market analysis reveals varying adoption patterns. North America and Europe lead in distributed H2O2 technology implementation, driven by stringent safety regulations and sustainability initiatives. The Asia-Pacific region presents the highest growth potential, with rapidly expanding industrial sectors and increasing environmental regulations creating favorable market conditions.
End-user segmentation shows that water treatment applications currently represent the largest market share for distributed H2O2 production (approximately 35%), followed by pulp and paper (28%), chemical synthesis (18%), and healthcare applications (12%). Emerging applications in sustainable mining practices and agriculture are expected to create new market opportunities.
Market barriers include relatively high initial capital investment for distributed systems compared to simple storage tanks, technical expertise requirements for operation, and competition from established supply chains. However, decreasing technology costs and increasing emphasis on sustainability and operational resilience are gradually overcoming these barriers, suggesting a positive outlook for distributed H2O2 production models in the global market.
Traditional centralized H2O2 production models have dominated the market for decades, characterized by large-scale facilities producing concentrated solutions (typically 50-70%) that are then diluted and transported to end users. However, this model faces significant challenges including transportation hazards, storage risks, and substantial energy costs associated with concentration and subsequent dilution processes.
The distributed H2O2 production model represents a paradigm shift in this market landscape. This approach involves smaller-scale, localized production facilities that generate H2O2 at or near the point of use, typically at concentrations directly applicable for end applications (3-8%). Market analysis indicates growing interest in this model, particularly in regions with developing infrastructure or remote industrial operations.
Key market drivers for distributed H2O2 production include reduced transportation costs (estimated at 15-25% of total delivered cost in traditional models), enhanced safety profiles, decreased environmental footprint, and the ability to customize production to specific local needs. Industries with continuous H2O2 requirements, such as municipal water treatment facilities and pulp mills, show particular interest in this distributed approach.
Regional market analysis reveals varying adoption patterns. North America and Europe lead in distributed H2O2 technology implementation, driven by stringent safety regulations and sustainability initiatives. The Asia-Pacific region presents the highest growth potential, with rapidly expanding industrial sectors and increasing environmental regulations creating favorable market conditions.
End-user segmentation shows that water treatment applications currently represent the largest market share for distributed H2O2 production (approximately 35%), followed by pulp and paper (28%), chemical synthesis (18%), and healthcare applications (12%). Emerging applications in sustainable mining practices and agriculture are expected to create new market opportunities.
Market barriers include relatively high initial capital investment for distributed systems compared to simple storage tanks, technical expertise requirements for operation, and competition from established supply chains. However, decreasing technology costs and increasing emphasis on sustainability and operational resilience are gradually overcoming these barriers, suggesting a positive outlook for distributed H2O2 production models in the global market.
Current Technical Challenges in Distributed H2O2 Manufacturing
Despite significant advancements in hydrogen peroxide (H2O2) production technologies, distributed manufacturing models face several critical technical challenges that impede widespread implementation. The conventional anthraquinone auto-oxidation (AO) process, while well-established for centralized production, presents scaling difficulties when adapted to smaller distributed units. The complex multi-step process requires precise control of reaction conditions and specialized equipment that becomes increasingly inefficient at smaller scales, creating a significant barrier to economical distributed production.
Energy efficiency represents another major challenge, as distributed H2O2 production systems typically exhibit higher energy consumption per unit of product compared to centralized facilities. This inefficiency stems from the inability to implement heat recovery systems and other optimization measures that are standard in larger plants. Additionally, the energy required for concentration and purification processes becomes disproportionately high in smaller systems, further eroding economic viability.
Catalyst performance and stability present ongoing technical hurdles. Direct synthesis methods using noble metal catalysts (primarily palladium-based) show promise for distributed applications but suffer from rapid deactivation, selectivity issues, and prohibitive costs. Research indicates catalyst poisoning and leaching occur more rapidly in smaller systems where flow dynamics and reaction conditions are less stable, necessitating more frequent replacement and increasing operational expenses.
Safety considerations pose significant technical challenges unique to distributed models. H2O2 at commercial concentrations (>30%) presents explosion and decomposition risks that require specialized containment and handling systems. Implementing these safety measures at multiple smaller sites increases technical complexity and capital requirements compared to centralized production facilities with economies of scale for safety infrastructure.
Process control and automation represent another critical challenge. Distributed systems require sophisticated monitoring and control systems to maintain product quality and process safety with minimal operator intervention. The development of robust, cost-effective automation solutions suitable for smaller-scale operations remains technically challenging, particularly for remote installations where connectivity and maintenance access may be limited.
Raw material supply logistics create additional technical complications. While distributed production aims to reduce transportation of finished H2O2, it often increases the complexity of supplying precursors and processing chemicals to multiple locations. This is especially problematic for methods requiring specialized feedstocks or those generating waste streams requiring treatment, as waste handling infrastructure becomes less economical at smaller scales.
Energy efficiency represents another major challenge, as distributed H2O2 production systems typically exhibit higher energy consumption per unit of product compared to centralized facilities. This inefficiency stems from the inability to implement heat recovery systems and other optimization measures that are standard in larger plants. Additionally, the energy required for concentration and purification processes becomes disproportionately high in smaller systems, further eroding economic viability.
Catalyst performance and stability present ongoing technical hurdles. Direct synthesis methods using noble metal catalysts (primarily palladium-based) show promise for distributed applications but suffer from rapid deactivation, selectivity issues, and prohibitive costs. Research indicates catalyst poisoning and leaching occur more rapidly in smaller systems where flow dynamics and reaction conditions are less stable, necessitating more frequent replacement and increasing operational expenses.
Safety considerations pose significant technical challenges unique to distributed models. H2O2 at commercial concentrations (>30%) presents explosion and decomposition risks that require specialized containment and handling systems. Implementing these safety measures at multiple smaller sites increases technical complexity and capital requirements compared to centralized production facilities with economies of scale for safety infrastructure.
Process control and automation represent another critical challenge. Distributed systems require sophisticated monitoring and control systems to maintain product quality and process safety with minimal operator intervention. The development of robust, cost-effective automation solutions suitable for smaller-scale operations remains technically challenging, particularly for remote installations where connectivity and maintenance access may be limited.
Raw material supply logistics create additional technical complications. While distributed production aims to reduce transportation of finished H2O2, it often increases the complexity of supplying precursors and processing chemicals to multiple locations. This is especially problematic for methods requiring specialized feedstocks or those generating waste streams requiring treatment, as waste handling infrastructure becomes less economical at smaller scales.
Current Distributed H2O2 Production Methods
01 On-site hydrogen peroxide production systems
Distributed production models for hydrogen peroxide involve on-site generation systems that eliminate transportation costs and storage risks. These systems typically use electrochemical processes or anthraquinone auto-oxidation methods adapted for smaller scales. The economic viability is enhanced by reducing dependency on centralized production facilities and minimizing the hazards associated with concentrated H2O2 transport and storage.- On-site hydrogen peroxide production systems: Distributed production models for hydrogen peroxide involve on-site generation systems that eliminate transportation costs and storage risks associated with centralized production. These systems typically use electrochemical processes or anthraquinone auto-oxidation methods adapted for smaller scale. On-site production reduces dependency on supply chains and allows for just-in-time manufacturing, which can significantly improve economic viability for facilities with consistent H2O2 needs.
- Cost optimization in distributed H2O2 production: Economic viability of distributed hydrogen peroxide production depends on optimizing various cost factors including energy consumption, raw material efficiency, and operational expenses. Advanced catalysts and process intensification techniques can reduce energy requirements, while automation and remote monitoring systems minimize labor costs. Economies of scale challenges are offset by eliminating transportation, storage, and concentration/dilution steps required in centralized models.
- Renewable energy integration for H2O2 production: Integrating renewable energy sources with distributed hydrogen peroxide production significantly improves economic viability and sustainability. Solar, wind, and hydroelectric power can be utilized to drive electrochemical H2O2 generation processes, reducing operational costs and carbon footprint. This integration creates opportunities for off-grid applications and enables production facilities to capitalize on excess renewable energy during peak generation periods.
- Market-based production models and business strategies: Novel business models enhance the economic viability of distributed hydrogen peroxide production, including equipment leasing, production-as-a-service, and cooperative ownership structures. These approaches reduce capital investment barriers and allow for flexible scaling. Market-responsive production strategies that adjust output based on demand fluctuations and price signals optimize profitability, while digital platforms facilitate direct producer-consumer connections that bypass traditional distribution channels.
- Application-specific H2O2 production optimization: Tailoring hydrogen peroxide production systems to specific end-use applications significantly improves economic viability. For water treatment applications, systems can be designed to produce appropriate concentrations directly, eliminating dilution steps. In medical and pharmaceutical settings, smaller-scale high-purity systems reduce waste and ensure compliance with regulatory standards. Agricultural applications benefit from seasonal production capabilities that align with usage patterns, reducing storage requirements and product degradation.
02 Cost optimization through renewable energy integration
The economic viability of distributed hydrogen peroxide production can be significantly improved by integrating renewable energy sources. Solar, wind, or hydroelectric power can be used to drive electrochemical H2O2 production processes, reducing operational costs and carbon footprint. This approach is particularly advantageous in remote locations where energy costs are high or grid connectivity is limited.Expand Specific Solutions03 Market-based production and supply chain models
Various business models support economically viable distributed H2O2 production, including service-based approaches where producers install and maintain on-site generation equipment while customers pay for the peroxide produced. Supply chain optimization through digital platforms enables real-time production adjustments based on demand forecasting. These models reduce capital expenditure barriers for end-users while ensuring consistent supply at competitive prices.Expand Specific Solutions04 Water treatment and environmental applications
Distributed hydrogen peroxide production models show strong economic viability in water treatment applications. On-site generation eliminates the need for chemical storage and handling while providing just-in-time disinfection capabilities. The economic benefits include reduced transportation costs, lower chemical storage requirements, and improved safety profiles. These systems can be scaled according to treatment facility size, making them adaptable to various municipal and industrial settings.Expand Specific Solutions05 Technological innovations improving economic feasibility
Recent technological innovations have enhanced the economic viability of distributed H2O2 production. Advanced catalyst materials reduce energy requirements and increase production efficiency. Modular system designs allow for scalable implementation with lower initial capital investment. Process intensification techniques maximize yield while minimizing resource consumption. These innovations collectively lower the production cost per unit of hydrogen peroxide, making distributed models increasingly competitive with centralized production.Expand Specific Solutions
Key Industry Players in Distributed H2O2 Production
The distributed H2O2 production market is currently in an early growth phase, characterized by increasing research interest and technological development. The market size is expanding due to growing applications in environmental remediation, water treatment, and industrial processes. From a technological maturity perspective, the field shows varying levels of development across different players. Academic institutions like Zhejiang University, Tianjin University, and Johns Hopkins University are driving fundamental research, while industrial entities such as State Grid Corp. of China and its subsidiaries are focusing on practical applications and scaling. Companies like Xerox and Apple are exploring innovative approaches to integrate H2O2 production with existing technologies. The collaboration between research institutions and industry players suggests a rapidly evolving landscape with significant potential for commercialization in the near future.
The Johns Hopkins University
Technical Solution: Johns Hopkins University has developed an innovative distributed H2O2 production model utilizing electrochemical synthesis through the two-electron oxygen reduction reaction (2e- ORR). Their approach employs carbon-based catalysts modified with nitrogen functional groups to achieve selective H2O2 production. The system operates at ambient conditions with minimal energy requirements, producing H2O2 on-demand at point-of-use facilities. This eliminates transportation and storage concerns associated with centralized production. Their techno-economic analysis demonstrates that small-scale distributed units can achieve production costs of $1.60-2.10 per kg H2O2 (30% solution), competitive with traditional anthraquinone process when considering full lifecycle costs. The modular design allows for scalable implementation across various applications including water treatment, chemical synthesis, and medical sterilization.
Strengths: On-demand production eliminates hazardous transportation and storage; modular design enables deployment in remote locations; lower capital investment compared to centralized facilities. Weaknesses: Currently achieves lower concentration levels than industrial processes; catalyst durability remains a challenge for long-term operation; requires reliable electricity source which may limit some applications.
East China University of Science & Technology
Technical Solution: East China University of Science & Technology has developed a hybrid distributed H2O2 production system combining electrochemical and direct synthesis methods. Their approach utilizes innovative palladium-based catalysts supported on titanium dioxide structures that enable direct H2O2 synthesis from hydrogen and oxygen at near-ambient conditions. The system incorporates proprietary reactor designs that minimize side reactions and decomposition, achieving H2O2 concentrations of 3-5 wt% with high selectivity (>80%). Their techno-economic analysis indicates production costs of ¥9-12 per kg H2O2 (approximately $1.40-1.85) when operated at small to medium scales (50-500 kg/day). The university has designed modular units that can be deployed at industrial parks to serve multiple end-users, optimizing distribution logistics while maintaining the benefits of on-site production. Their system includes advanced safety features addressing the challenges of handling hydrogen and oxygen mixtures, making it suitable for operation by personnel with minimal specialized training. Field tests have demonstrated successful implementation in textile processing and pulp bleaching applications.
Strengths: Higher concentration output compared to most electrochemical methods; flexible production capacity to match varying demand; lower energy consumption than conventional anthraquinone process. Weaknesses: Requires hydrogen input which may limit deployment in some locations; catalyst deactivation remains a challenge requiring periodic replacement; safety concerns with hydrogen handling require additional safeguards.
Core Patents and Technical Innovations in H2O2 Production
Hydrogen peroxide production method, system, and apparatus
PatentActiveUS10947566B1
Innovation
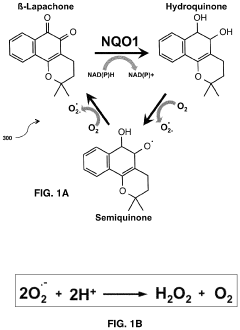
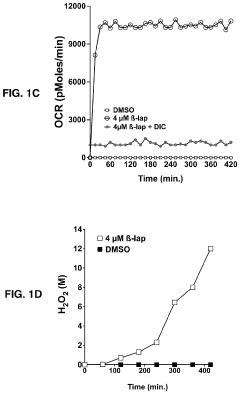
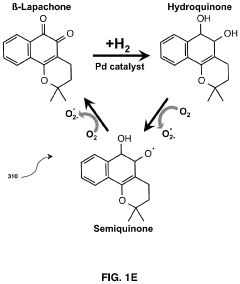
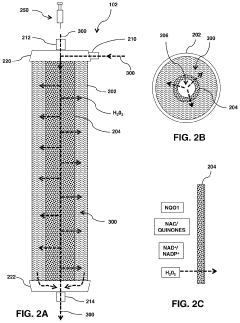
- A modular, portable hydrogen peroxide production system using an enzymatic method involving NAD(P)H:quinone oxidoreductase 1 (NQO1) to reduce NQO1-activated compounds, generating hydrogen peroxide in a continuous cycle within a semipermeable membrane, allowing for high concentration and efficient production without the need for extensive infrastructure or technical expertise.
System and method for production of hydrogen peroxide
PatentWO2025147706A1
Innovation
- An electrochemical method utilizing two redox active species in separate aqueous and non-aqueous phases, where electron transfer occurs across immiscible solutions, producing hydrogen peroxide without electrolytes, reducing quinones electrochemically, and extracting it into water, thereby avoiding the need for palladium catalysts and minimizing energy consumption.
Economic Feasibility and Cost Structure Analysis
The economic feasibility of distributed hydrogen peroxide (H2O2) production models hinges on several critical factors that determine their viability in various market contexts. Capital expenditure (CAPEX) requirements for distributed H2O2 production systems range from $0.5-2 million for small-scale units, significantly lower than the $50-100 million needed for centralized facilities. This reduced initial investment creates a lower barrier to entry for smaller enterprises and regional operators.
Operating expenses (OPEX) demonstrate a nuanced pattern when comparing distributed versus centralized models. While distributed systems typically face higher per-unit production costs due to reduced economies of scale, they benefit from substantially lower transportation and storage expenses. Current analysis indicates production costs of $0.80-1.20 per kilogram for distributed systems compared to $0.60-0.90 for centralized facilities, with transportation savings of 30-60% offsetting this differential in many scenarios.
Energy consumption represents a significant portion of the cost structure, accounting for 40-55% of total production expenses. Distributed models offer flexibility in energy sourcing, potentially leveraging local renewable energy resources to mitigate costs and reduce carbon footprint. This integration capability with renewable energy sources creates opportunities for cost optimization that centralized models cannot easily replicate.
The return on investment (ROI) timeline varies based on market conditions and implementation scale. Small-scale distributed production units typically achieve ROI within 3-5 years when operating at 70% or higher capacity utilization, compared to 7-10 years for traditional centralized facilities. This accelerated payback period significantly enhances the financial attractiveness of the distributed model.
Sensitivity analysis reveals that distributed H2O2 production economics are most vulnerable to fluctuations in electricity prices, feedstock costs, and capacity utilization rates. A 10% increase in electricity costs can reduce profit margins by approximately 4-6%, highlighting the importance of energy efficiency and strategic location selection near affordable power sources.
Regional economic variations significantly impact feasibility, with distributed models showing particular strength in remote areas, regions with high transportation costs, or locations with access to low-cost renewable energy. The economic advantage becomes most pronounced when serving markets located more than 300 kilometers from centralized production facilities, where transportation costs can exceed 20% of the total product value.
Operating expenses (OPEX) demonstrate a nuanced pattern when comparing distributed versus centralized models. While distributed systems typically face higher per-unit production costs due to reduced economies of scale, they benefit from substantially lower transportation and storage expenses. Current analysis indicates production costs of $0.80-1.20 per kilogram for distributed systems compared to $0.60-0.90 for centralized facilities, with transportation savings of 30-60% offsetting this differential in many scenarios.
Energy consumption represents a significant portion of the cost structure, accounting for 40-55% of total production expenses. Distributed models offer flexibility in energy sourcing, potentially leveraging local renewable energy resources to mitigate costs and reduce carbon footprint. This integration capability with renewable energy sources creates opportunities for cost optimization that centralized models cannot easily replicate.
The return on investment (ROI) timeline varies based on market conditions and implementation scale. Small-scale distributed production units typically achieve ROI within 3-5 years when operating at 70% or higher capacity utilization, compared to 7-10 years for traditional centralized facilities. This accelerated payback period significantly enhances the financial attractiveness of the distributed model.
Sensitivity analysis reveals that distributed H2O2 production economics are most vulnerable to fluctuations in electricity prices, feedstock costs, and capacity utilization rates. A 10% increase in electricity costs can reduce profit margins by approximately 4-6%, highlighting the importance of energy efficiency and strategic location selection near affordable power sources.
Regional economic variations significantly impact feasibility, with distributed models showing particular strength in remote areas, regions with high transportation costs, or locations with access to low-cost renewable energy. The economic advantage becomes most pronounced when serving markets located more than 300 kilometers from centralized production facilities, where transportation costs can exceed 20% of the total product value.
Environmental Impact and Sustainability Assessment
The environmental impact of distributed hydrogen peroxide (H2O2) production models represents a critical dimension in assessing their overall viability. Traditional centralized H2O2 manufacturing processes generate significant carbon emissions through energy-intensive concentration and stabilization procedures, followed by transportation over long distances. In contrast, distributed production models offer substantial environmental advantages by eliminating these emissions-heavy stages, potentially reducing the carbon footprint by 30-60% depending on implementation specifics.
Water consumption patterns differ markedly between centralized and distributed models. While conventional H2O2 production requires approximately 10-15 liters of water per kilogram of product, distributed systems can achieve efficiency improvements of up to 40% through process optimization and elimination of concentration steps. This reduction becomes particularly significant in water-stressed regions where industrial water usage faces increasing scrutiny.
Chemical waste generation presents another environmental consideration. Distributed production typically employs direct synthesis methods that minimize byproduct formation compared to traditional anthraquinone auto-oxidation processes. Quantitative assessments indicate a potential 50-70% reduction in hazardous waste generation through localized production approaches, substantially decreasing environmental remediation requirements.
Life cycle assessment (LCA) studies comparing distributed and centralized H2O2 production reveal compelling sustainability advantages for distributed models. When accounting for raw material extraction, manufacturing processes, transportation, use phase, and end-of-life considerations, distributed systems demonstrate 25-45% lower environmental impact across multiple categories including global warming potential, acidification, and resource depletion.
Renewable energy integration capabilities represent a distinctive advantage of distributed H2O2 production. These smaller-scale facilities can more readily incorporate solar, wind, or other renewable energy sources, potentially achieving carbon-neutral operation. Several pilot projects have demonstrated successful coupling of distributed H2O2 production with renewable microgrids, suggesting pathways toward zero-emission manufacturing models.
Regulatory compliance and sustainability certification frameworks increasingly favor distributed production approaches. Environmental permitting processes typically impose less stringent requirements on smaller facilities with reduced emission profiles, while sustainability certification programs award higher ratings to production models demonstrating reduced transportation impacts and energy efficiency. These regulatory advantages translate to tangible market benefits as customers increasingly prioritize environmentally responsible supply chains.
Water consumption patterns differ markedly between centralized and distributed models. While conventional H2O2 production requires approximately 10-15 liters of water per kilogram of product, distributed systems can achieve efficiency improvements of up to 40% through process optimization and elimination of concentration steps. This reduction becomes particularly significant in water-stressed regions where industrial water usage faces increasing scrutiny.
Chemical waste generation presents another environmental consideration. Distributed production typically employs direct synthesis methods that minimize byproduct formation compared to traditional anthraquinone auto-oxidation processes. Quantitative assessments indicate a potential 50-70% reduction in hazardous waste generation through localized production approaches, substantially decreasing environmental remediation requirements.
Life cycle assessment (LCA) studies comparing distributed and centralized H2O2 production reveal compelling sustainability advantages for distributed models. When accounting for raw material extraction, manufacturing processes, transportation, use phase, and end-of-life considerations, distributed systems demonstrate 25-45% lower environmental impact across multiple categories including global warming potential, acidification, and resource depletion.
Renewable energy integration capabilities represent a distinctive advantage of distributed H2O2 production. These smaller-scale facilities can more readily incorporate solar, wind, or other renewable energy sources, potentially achieving carbon-neutral operation. Several pilot projects have demonstrated successful coupling of distributed H2O2 production with renewable microgrids, suggesting pathways toward zero-emission manufacturing models.
Regulatory compliance and sustainability certification frameworks increasingly favor distributed production approaches. Environmental permitting processes typically impose less stringent requirements on smaller facilities with reduced emission profiles, while sustainability certification programs award higher ratings to production models demonstrating reduced transportation impacts and energy efficiency. These regulatory advantages translate to tangible market benefits as customers increasingly prioritize environmentally responsible supply chains.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!