Carbon Tetrachloride and Risk Assessment in Chemical Industries
JUL 31, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CCl4 Background and Objectives
Carbon tetrachloride (CCl4) has been a significant concern in the chemical industry for decades due to its potential environmental and health risks. This compound, once widely used as a solvent, cleaning agent, and refrigerant, has undergone a dramatic shift in its perception and regulation over the years. The evolution of CCl4 usage and its associated risks provides a compelling case study for the chemical industry's ongoing challenges in balancing technological utility with safety and environmental stewardship.
The primary objective of this technical research report is to comprehensively examine the current state of CCl4 risk assessment in chemical industries. This involves tracing the historical context of CCl4 use, understanding its physicochemical properties, and analyzing the evolving regulatory landscape that has shaped its current status. By doing so, we aim to provide a foundation for informed decision-making regarding the management and potential alternatives to CCl4 in industrial processes.
A key focus of this investigation is to explore the technological advancements in risk assessment methodologies specific to CCl4. This includes evaluating cutting-edge analytical techniques for detecting and quantifying CCl4 in various environmental matrices, as well as examining innovative approaches to exposure modeling and toxicological assessments. The goal is to identify gaps in current knowledge and highlight areas where further research and development could significantly enhance our ability to mitigate CCl4-related risks.
Furthermore, this report seeks to contextualize CCl4 risk assessment within the broader framework of sustainable chemistry and green technology initiatives. As industries worldwide strive to reduce their environmental footprint and improve worker safety, understanding the challenges and opportunities presented by CCl4 can serve as a valuable case study for similar compounds. This perspective allows us to explore how lessons learned from CCl4 management can be applied to other potentially hazardous substances in the chemical industry.
Lastly, we aim to outline the future trajectory of CCl4 risk assessment, considering emerging technologies such as artificial intelligence and machine learning in predictive toxicology, as well as advancements in green chemistry that may offer safer alternatives. By anticipating these developments, this report intends to provide a roadmap for researchers, industry professionals, and policymakers to proactively address the challenges associated with CCl4 and similar compounds in the years to come.
The primary objective of this technical research report is to comprehensively examine the current state of CCl4 risk assessment in chemical industries. This involves tracing the historical context of CCl4 use, understanding its physicochemical properties, and analyzing the evolving regulatory landscape that has shaped its current status. By doing so, we aim to provide a foundation for informed decision-making regarding the management and potential alternatives to CCl4 in industrial processes.
A key focus of this investigation is to explore the technological advancements in risk assessment methodologies specific to CCl4. This includes evaluating cutting-edge analytical techniques for detecting and quantifying CCl4 in various environmental matrices, as well as examining innovative approaches to exposure modeling and toxicological assessments. The goal is to identify gaps in current knowledge and highlight areas where further research and development could significantly enhance our ability to mitigate CCl4-related risks.
Furthermore, this report seeks to contextualize CCl4 risk assessment within the broader framework of sustainable chemistry and green technology initiatives. As industries worldwide strive to reduce their environmental footprint and improve worker safety, understanding the challenges and opportunities presented by CCl4 can serve as a valuable case study for similar compounds. This perspective allows us to explore how lessons learned from CCl4 management can be applied to other potentially hazardous substances in the chemical industry.
Lastly, we aim to outline the future trajectory of CCl4 risk assessment, considering emerging technologies such as artificial intelligence and machine learning in predictive toxicology, as well as advancements in green chemistry that may offer safer alternatives. By anticipating these developments, this report intends to provide a roadmap for researchers, industry professionals, and policymakers to proactively address the challenges associated with CCl4 and similar compounds in the years to come.
Industrial Demand Analysis
The demand for carbon tetrachloride in chemical industries has significantly shifted over the past few decades due to environmental and health concerns. Historically, carbon tetrachloride was widely used as a solvent, cleaning agent, and in the production of refrigerants. However, its use has been severely restricted in many countries due to its ozone-depleting properties and potential health risks.
Despite these restrictions, there remains a limited industrial demand for carbon tetrachloride in specific applications. The chemical is still used as a feedstock in the production of certain chemicals, particularly hydrofluorocarbons (HFCs) and hydrofluoroolefins (HFOs), which are used as refrigerants and foam blowing agents. This demand is driven by the ongoing transition from ozone-depleting substances to more environmentally friendly alternatives.
In the pharmaceutical industry, carbon tetrachloride continues to find application in the synthesis of certain drugs and as a solvent in analytical processes. Its use in this sector is carefully controlled and monitored due to regulatory requirements and safety concerns. The agrochemical industry also maintains a small demand for carbon tetrachloride in the production of some pesticides and herbicides.
The global market for carbon tetrachloride has contracted significantly, with production volumes decreasing by over 90% since the 1980s. This decline is primarily attributed to the implementation of the Montreal Protocol, which phased out the production and consumption of ozone-depleting substances. As a result, the remaining demand is concentrated in a few specific industrial sectors and geographic regions where alternatives are not yet fully implemented or where exemptions exist.
The future industrial demand for carbon tetrachloride is expected to continue its downward trend as more sustainable and safer alternatives are developed and adopted. However, the rate of decline may slow as the remaining applications become more difficult to replace. Industries that still rely on carbon tetrachloride are investing in improved risk assessment and management strategies to ensure compliance with stringent regulations and to minimize potential environmental and health impacts.
Emerging markets, particularly in developing countries, may see a temporary increase in demand for carbon tetrachloride as their industrial sectors grow. However, international pressure and environmental agreements are likely to limit this growth and encourage the adoption of alternative technologies and chemicals.
Despite these restrictions, there remains a limited industrial demand for carbon tetrachloride in specific applications. The chemical is still used as a feedstock in the production of certain chemicals, particularly hydrofluorocarbons (HFCs) and hydrofluoroolefins (HFOs), which are used as refrigerants and foam blowing agents. This demand is driven by the ongoing transition from ozone-depleting substances to more environmentally friendly alternatives.
In the pharmaceutical industry, carbon tetrachloride continues to find application in the synthesis of certain drugs and as a solvent in analytical processes. Its use in this sector is carefully controlled and monitored due to regulatory requirements and safety concerns. The agrochemical industry also maintains a small demand for carbon tetrachloride in the production of some pesticides and herbicides.
The global market for carbon tetrachloride has contracted significantly, with production volumes decreasing by over 90% since the 1980s. This decline is primarily attributed to the implementation of the Montreal Protocol, which phased out the production and consumption of ozone-depleting substances. As a result, the remaining demand is concentrated in a few specific industrial sectors and geographic regions where alternatives are not yet fully implemented or where exemptions exist.
The future industrial demand for carbon tetrachloride is expected to continue its downward trend as more sustainable and safer alternatives are developed and adopted. However, the rate of decline may slow as the remaining applications become more difficult to replace. Industries that still rely on carbon tetrachloride are investing in improved risk assessment and management strategies to ensure compliance with stringent regulations and to minimize potential environmental and health impacts.
Emerging markets, particularly in developing countries, may see a temporary increase in demand for carbon tetrachloride as their industrial sectors grow. However, international pressure and environmental agreements are likely to limit this growth and encourage the adoption of alternative technologies and chemicals.
CCl4 Hazards and Challenges
Carbon tetrachloride (CCl4) poses significant hazards and challenges in chemical industries, primarily due to its toxicity and environmental impact. The compound is known for its high volatility and stability, which contribute to its persistence in the environment and potential for long-range transport. These properties make CCl4 a substance of concern for both occupational safety and environmental protection.
In industrial settings, the primary hazard associated with CCl4 is its acute and chronic toxicity. Inhalation of CCl4 vapors can cause severe respiratory irritation, central nervous system depression, and liver damage. Prolonged exposure may lead to kidney dysfunction and increased risk of cancer. The compound's ability to readily penetrate skin also presents a risk of dermal absorption, necessitating stringent personal protective equipment protocols in workplaces where CCl4 is handled.
Environmental challenges arise from CCl4's ozone-depleting potential and its contribution to global warming. Despite being phased out under the Montreal Protocol, legacy emissions and unintentional production continue to impact atmospheric ozone levels. The compound's long atmospheric lifetime, estimated at 26 years, exacerbates its environmental persistence and global distribution.
Risk assessment in chemical industries must address the multifaceted nature of CCl4 hazards. This includes evaluating exposure pathways, quantifying potential releases, and assessing the effectiveness of control measures. Challenges in risk assessment stem from the need to consider both acute and chronic health effects, as well as long-term environmental impacts.
Industrial processes involving CCl4, whether as a solvent, cleaning agent, or intermediate in chemical synthesis, require robust engineering controls and safety systems. Containment strategies, vapor recovery systems, and proper waste management are critical to minimizing releases. However, the implementation of these measures can be technically challenging and economically burdensome for industries.
Regulatory compliance presents another significant challenge. The global phase-out of CCl4 production and use under international agreements has led to complex regulatory landscapes. Industries must navigate varying national and regional regulations, which may include reporting requirements, emission limits, and restrictions on use. Ensuring compliance while maintaining operational efficiency demands continuous monitoring and adaptation of industrial practices.
In conclusion, addressing the hazards and challenges associated with CCl4 in chemical industries requires a comprehensive approach. This encompasses rigorous safety protocols, advanced engineering controls, stringent environmental management, and ongoing research into safer alternatives. The complexity of these challenges underscores the need for collaborative efforts between industry, regulatory bodies, and research institutions to develop sustainable solutions and mitigate the risks posed by CCl4.
In industrial settings, the primary hazard associated with CCl4 is its acute and chronic toxicity. Inhalation of CCl4 vapors can cause severe respiratory irritation, central nervous system depression, and liver damage. Prolonged exposure may lead to kidney dysfunction and increased risk of cancer. The compound's ability to readily penetrate skin also presents a risk of dermal absorption, necessitating stringent personal protective equipment protocols in workplaces where CCl4 is handled.
Environmental challenges arise from CCl4's ozone-depleting potential and its contribution to global warming. Despite being phased out under the Montreal Protocol, legacy emissions and unintentional production continue to impact atmospheric ozone levels. The compound's long atmospheric lifetime, estimated at 26 years, exacerbates its environmental persistence and global distribution.
Risk assessment in chemical industries must address the multifaceted nature of CCl4 hazards. This includes evaluating exposure pathways, quantifying potential releases, and assessing the effectiveness of control measures. Challenges in risk assessment stem from the need to consider both acute and chronic health effects, as well as long-term environmental impacts.
Industrial processes involving CCl4, whether as a solvent, cleaning agent, or intermediate in chemical synthesis, require robust engineering controls and safety systems. Containment strategies, vapor recovery systems, and proper waste management are critical to minimizing releases. However, the implementation of these measures can be technically challenging and economically burdensome for industries.
Regulatory compliance presents another significant challenge. The global phase-out of CCl4 production and use under international agreements has led to complex regulatory landscapes. Industries must navigate varying national and regional regulations, which may include reporting requirements, emission limits, and restrictions on use. Ensuring compliance while maintaining operational efficiency demands continuous monitoring and adaptation of industrial practices.
In conclusion, addressing the hazards and challenges associated with CCl4 in chemical industries requires a comprehensive approach. This encompasses rigorous safety protocols, advanced engineering controls, stringent environmental management, and ongoing research into safer alternatives. The complexity of these challenges underscores the need for collaborative efforts between industry, regulatory bodies, and research institutions to develop sustainable solutions and mitigate the risks posed by CCl4.
Current Risk Assessment Methods
01 Health and environmental risks
Carbon tetrachloride poses significant health and environmental risks due to its toxicity and ozone-depleting properties. Exposure can lead to liver and kidney damage, and it has been classified as a potential carcinogen. Its use has been heavily restricted or banned in many applications due to these concerns.- Health and environmental risks: Carbon tetrachloride poses significant health and environmental risks. It is known to be toxic to humans and animals, potentially causing liver and kidney damage, and is also a potent ozone-depleting substance. Long-term exposure can lead to serious health issues, including cancer. Its use in various industrial applications has been heavily restricted or banned in many countries due to these risks.
- Industrial applications and alternatives: Despite its risks, carbon tetrachloride has been widely used in various industrial applications, including as a solvent, cleaning agent, and in the production of refrigerants. However, due to its harmful effects, efforts have been made to find safer alternatives. These alternatives include other chlorinated solvents, hydrofluorocarbons, and water-based solutions, which aim to provide similar functionality with reduced environmental and health impacts.
- Detection and monitoring methods: To mitigate the risks associated with carbon tetrachloride, various detection and monitoring methods have been developed. These include advanced analytical techniques such as gas chromatography, mass spectrometry, and spectrophotometric methods. Continuous monitoring systems have also been implemented in industrial settings to detect leaks or emissions, helping to prevent environmental contamination and protect worker health.
- Remediation and treatment technologies: Given the persistent nature of carbon tetrachloride contamination, various remediation and treatment technologies have been developed. These include physical methods such as air stripping and activated carbon adsorption, as well as chemical and biological treatment processes. Advanced oxidation processes and bioremediation techniques have shown promise in degrading carbon tetrachloride in contaminated soil and groundwater.
- Regulatory measures and phase-out strategies: Due to its harmful effects, carbon tetrachloride has been subject to strict regulatory measures worldwide. Many countries have implemented phase-out strategies and banned its use in consumer products. International agreements, such as the Montreal Protocol, have played a crucial role in reducing its production and consumption globally. These measures aim to protect human health and the environment while promoting the development and adoption of safer alternatives.
02 Industrial applications and alternatives
Historically, carbon tetrachloride was widely used in various industrial applications, including as a solvent, cleaning agent, and fire extinguishing agent. However, due to its risks, safer alternatives have been developed and implemented across industries to replace carbon tetrachloride in these applications.Expand Specific Solutions03 Detection and monitoring methods
Various methods have been developed to detect and monitor carbon tetrachloride in the environment and workplace. These include advanced analytical techniques and sensors to measure its presence in air, water, and soil, helping to ensure compliance with safety regulations and environmental standards.Expand Specific Solutions04 Remediation and treatment technologies
Efforts to address carbon tetrachloride contamination have led to the development of various remediation and treatment technologies. These include chemical, biological, and physical methods to remove or neutralize carbon tetrachloride in contaminated sites, particularly in soil and groundwater.Expand Specific Solutions05 Regulatory measures and safety protocols
Stringent regulatory measures and safety protocols have been implemented globally to manage the risks associated with carbon tetrachloride. These include restrictions on its production, use, and disposal, as well as guidelines for proper handling and storage to minimize exposure risks in industrial settings where its use is still permitted.Expand Specific Solutions
Key Industry Stakeholders
The carbon tetrachloride risk assessment landscape in chemical industries is characterized by a mature market with established players and evolving regulatory frameworks. Major companies like China Petroleum & Chemical Corp., Occidental Chemical Corp., and DuPont de Nemours, Inc. are at the forefront of addressing safety and environmental concerns. The market is driven by increasing awareness of occupational health hazards and stringent environmental regulations. Technological advancements in risk assessment methodologies and monitoring tools are enhancing the industry's capability to manage carbon tetrachloride-related risks effectively. Collaboration between industry leaders, research institutions, and regulatory bodies is fostering innovation and best practices in this critical area of chemical safety management.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed a comprehensive risk assessment framework for carbon tetrachloride in chemical industries. Their approach integrates advanced modeling techniques with real-time monitoring systems to evaluate potential hazards and exposure risks. Sinopec employs a multi-tiered assessment strategy, including hazard identification, exposure assessment, and risk characterization [1]. They utilize state-of-the-art dispersion models to predict the spread of carbon tetrachloride in various environmental conditions, allowing for more accurate risk predictions [3]. Additionally, Sinopec has implemented an automated early warning system that continuously monitors carbon tetrachloride levels in air and water, triggering immediate responses when threshold values are exceeded [5].
Strengths: Comprehensive approach, advanced modeling techniques, and real-time monitoring. Weaknesses: High implementation costs and potential for false alarms in the early warning system.
Occidental Chemical Corp.
Technical Solution: Occidental Chemical Corp. has developed a proprietary risk assessment methodology specifically tailored for carbon tetrachloride in chemical industries. Their approach combines traditional risk assessment techniques with innovative machine learning algorithms to predict potential exposure scenarios and their consequences. The company utilizes a sophisticated database of historical incident data and environmental factors to inform their risk models [2]. Occidental's system incorporates real-time atmospheric and process data to continuously update risk assessments, allowing for dynamic risk management [4]. They have also implemented a novel biomonitoring program for workers potentially exposed to carbon tetrachloride, providing early detection of exposure and informing risk mitigation strategies [6].
Strengths: Advanced predictive modeling, dynamic risk assessment, and comprehensive worker monitoring. Weaknesses: Reliance on historical data may not fully capture emerging risks.
Innovative Safety Technologies
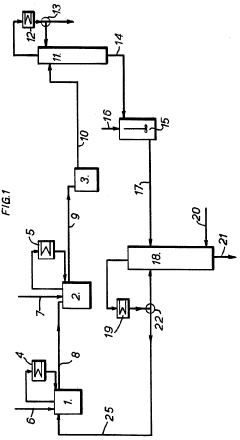
Carbon tetrachloride manufacture
PatentInactiveGB1201557A
Innovation
- A method involving passing carbon disulfide vapors through a fractionating column with a liquid phase containing a finely divided solid catalyst, such as iron salts, to react with chlorine and/or sulfur monochloride, allowing for complete reaction and efficient separation of carbon tetrachloride, reducing sulfur monochloride residues and eliminating the need for separate reactors and distillation systems.
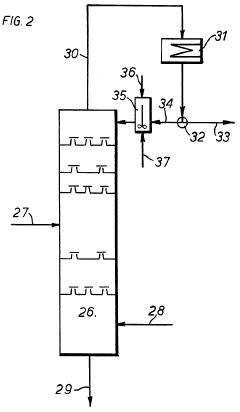
Process of preparation of monobasic lead salt of 2,4 di nitro resorcinol using trichloro ethylene as an inert
PatentInactiveIN201821045441A
Innovation
- The process involves using trichloroethylene as an inert solvent media to replace carbon tetrachloride, involving nitrosation of resorcinol followed by alkaline oxidation and subsequent purification of 2,4-dinitroso resorcinol to produce monobasic lead salt of 2,4-di nitro resorcinol, which is then used in detonator compositions and fuze applications.
Environmental Impact Assessment
The environmental impact assessment of carbon tetrachloride in chemical industries is a critical component of risk management strategies. Carbon tetrachloride, a potent ozone-depleting substance and potential carcinogen, poses significant risks to both human health and the environment. Its production and use in various industrial processes necessitate a comprehensive evaluation of its ecological footprint.
Atmospheric emissions of carbon tetrachloride contribute to stratospheric ozone depletion, which can lead to increased ultraviolet radiation reaching the Earth's surface. This phenomenon has far-reaching consequences for terrestrial and aquatic ecosystems, potentially disrupting food chains and biodiversity. Moreover, the persistence of carbon tetrachloride in the atmosphere exacerbates its long-term environmental impact, with a atmospheric lifetime of approximately 26 years.
Water contamination is another major concern associated with carbon tetrachloride. Its high solubility and mobility in groundwater systems make it a significant threat to aquatic environments and drinking water sources. Once released into water bodies, carbon tetrachloride can persist for extended periods, potentially bioaccumulating in aquatic organisms and entering the food chain.
Soil contamination from carbon tetrachloride spills or improper disposal practices can lead to long-lasting environmental damage. The compound's ability to volatilize from soil and migrate through soil pores poses risks to soil microorganisms and plant life, potentially affecting soil fertility and ecosystem balance.
The assessment of carbon tetrachloride's environmental impact must also consider its potential for secondary pollutant formation. When released into the atmosphere, it can undergo photochemical reactions, contributing to the formation of other harmful compounds and exacerbating air quality issues.
To mitigate these environmental risks, comprehensive monitoring and control measures are essential. This includes implementing advanced air pollution control technologies, enhancing wastewater treatment processes, and developing robust soil remediation techniques. Additionally, the assessment should encompass the evaluation of alternative substances or processes that could replace carbon tetrachloride in industrial applications, thereby reducing its overall environmental footprint.
The environmental impact assessment of carbon tetrachloride must also consider the compound's global transport mechanisms. Its long atmospheric lifetime allows for long-range transport, potentially affecting regions far from its point of release. This necessitates international cooperation and coordinated efforts to monitor and regulate its use and emissions on a global scale.
Atmospheric emissions of carbon tetrachloride contribute to stratospheric ozone depletion, which can lead to increased ultraviolet radiation reaching the Earth's surface. This phenomenon has far-reaching consequences for terrestrial and aquatic ecosystems, potentially disrupting food chains and biodiversity. Moreover, the persistence of carbon tetrachloride in the atmosphere exacerbates its long-term environmental impact, with a atmospheric lifetime of approximately 26 years.
Water contamination is another major concern associated with carbon tetrachloride. Its high solubility and mobility in groundwater systems make it a significant threat to aquatic environments and drinking water sources. Once released into water bodies, carbon tetrachloride can persist for extended periods, potentially bioaccumulating in aquatic organisms and entering the food chain.
Soil contamination from carbon tetrachloride spills or improper disposal practices can lead to long-lasting environmental damage. The compound's ability to volatilize from soil and migrate through soil pores poses risks to soil microorganisms and plant life, potentially affecting soil fertility and ecosystem balance.
The assessment of carbon tetrachloride's environmental impact must also consider its potential for secondary pollutant formation. When released into the atmosphere, it can undergo photochemical reactions, contributing to the formation of other harmful compounds and exacerbating air quality issues.
To mitigate these environmental risks, comprehensive monitoring and control measures are essential. This includes implementing advanced air pollution control technologies, enhancing wastewater treatment processes, and developing robust soil remediation techniques. Additionally, the assessment should encompass the evaluation of alternative substances or processes that could replace carbon tetrachloride in industrial applications, thereby reducing its overall environmental footprint.
The environmental impact assessment of carbon tetrachloride must also consider the compound's global transport mechanisms. Its long atmospheric lifetime allows for long-range transport, potentially affecting regions far from its point of release. This necessitates international cooperation and coordinated efforts to monitor and regulate its use and emissions on a global scale.
Occupational Health Protocols
Occupational health protocols for managing carbon tetrachloride exposure in chemical industries are critical for ensuring worker safety and regulatory compliance. These protocols typically involve a multi-faceted approach, combining engineering controls, administrative measures, and personal protective equipment (PPE).
Engineering controls form the first line of defense against carbon tetrachloride exposure. These include closed-system operations, local exhaust ventilation, and process isolation. Implementing robust ventilation systems with appropriate air exchange rates is crucial for maintaining safe atmospheric conditions in work areas where carbon tetrachloride is used or produced.
Administrative controls complement engineering measures by establishing safe work practices and procedures. This includes developing and enforcing standard operating procedures (SOPs) for handling carbon tetrachloride, implementing job rotation to limit individual exposure times, and conducting regular safety training sessions for all personnel working with or near the chemical.
Personal protective equipment serves as the last line of defense against carbon tetrachloride exposure. Workers must be provided with and trained in the proper use of appropriate PPE, including chemical-resistant gloves, protective clothing, and respiratory protection. The selection of PPE should be based on a thorough risk assessment and comply with relevant occupational safety standards.
Regular environmental monitoring is a crucial component of occupational health protocols. This involves periodic air sampling and analysis to ensure that carbon tetrachloride concentrations in the workplace remain below permissible exposure limits. Biological monitoring of workers, such as blood or urine tests, may also be implemented to assess individual exposure levels and health impacts.
Emergency response procedures are an essential element of occupational health protocols. These procedures should outline steps for handling spills, leaks, or accidental exposures to carbon tetrachloride. This includes providing easily accessible emergency shower and eyewash stations, as well as training workers in first aid procedures specific to carbon tetrachloride exposure.
Medical surveillance programs should be established to monitor the health of workers potentially exposed to carbon tetrachloride. These programs typically include pre-employment medical examinations, periodic health assessments, and specialized tests to detect early signs of carbon tetrachloride-related health effects, particularly focusing on liver and kidney function.
Documentation and record-keeping are vital aspects of occupational health protocols. Detailed records of exposure monitoring results, medical surveillance data, training sessions, and incident reports should be maintained. These records not only assist in regulatory compliance but also provide valuable data for ongoing risk assessment and improvement of safety measures.
Engineering controls form the first line of defense against carbon tetrachloride exposure. These include closed-system operations, local exhaust ventilation, and process isolation. Implementing robust ventilation systems with appropriate air exchange rates is crucial for maintaining safe atmospheric conditions in work areas where carbon tetrachloride is used or produced.
Administrative controls complement engineering measures by establishing safe work practices and procedures. This includes developing and enforcing standard operating procedures (SOPs) for handling carbon tetrachloride, implementing job rotation to limit individual exposure times, and conducting regular safety training sessions for all personnel working with or near the chemical.
Personal protective equipment serves as the last line of defense against carbon tetrachloride exposure. Workers must be provided with and trained in the proper use of appropriate PPE, including chemical-resistant gloves, protective clothing, and respiratory protection. The selection of PPE should be based on a thorough risk assessment and comply with relevant occupational safety standards.
Regular environmental monitoring is a crucial component of occupational health protocols. This involves periodic air sampling and analysis to ensure that carbon tetrachloride concentrations in the workplace remain below permissible exposure limits. Biological monitoring of workers, such as blood or urine tests, may also be implemented to assess individual exposure levels and health impacts.
Emergency response procedures are an essential element of occupational health protocols. These procedures should outline steps for handling spills, leaks, or accidental exposures to carbon tetrachloride. This includes providing easily accessible emergency shower and eyewash stations, as well as training workers in first aid procedures specific to carbon tetrachloride exposure.
Medical surveillance programs should be established to monitor the health of workers potentially exposed to carbon tetrachloride. These programs typically include pre-employment medical examinations, periodic health assessments, and specialized tests to detect early signs of carbon tetrachloride-related health effects, particularly focusing on liver and kidney function.
Documentation and record-keeping are vital aspects of occupational health protocols. Detailed records of exposure monitoring results, medical surveillance data, training sessions, and incident reports should be maintained. These records not only assist in regulatory compliance but also provide valuable data for ongoing risk assessment and improvement of safety measures.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!