Carbon Tetrachloride: Top Applications in Modern Chemistry Solutions
JUL 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CCl4 History and Objectives
Carbon tetrachloride (CCl4) has a rich history in chemistry, dating back to its first synthesis in 1839 by French chemist Henri Victor Regnault. Initially, it was primarily used as a solvent and cleaning agent due to its non-flammable properties. The compound gained significant industrial importance in the early 20th century, particularly in the production of refrigerants and as a precursor for chlorofluorocarbons (CFCs).
Throughout the mid-20th century, CCl4 found widespread applications in various sectors, including dry cleaning, fire extinguishers, and as a fumigant in agriculture. Its versatility and effectiveness led to its extensive use in both industrial and consumer products. However, the 1970s marked a turning point in the compound's history when its harmful effects on the ozone layer and human health became apparent.
The Montreal Protocol, signed in 1987, initiated the phase-out of ozone-depleting substances, including CCl4. This international agreement significantly impacted the production and use of carbon tetrachloride, leading to a dramatic decline in its global consumption. The protocol's implementation has been successful in reducing atmospheric concentrations of CCl4, although its long atmospheric lifetime means that its effects persist.
In recent years, the focus on CCl4 has shifted towards understanding its continued presence in the atmosphere and identifying potential sources of emissions. Researchers have discovered unexpected levels of CCl4 in the atmosphere, suggesting ongoing production or release from legacy sources. This has led to renewed interest in monitoring and controlling CCl4 emissions globally.
The objectives of current CCl4 research and applications are multifaceted. Firstly, there is a continued effort to develop alternative compounds and technologies to replace CCl4 in its remaining applications, particularly in industrial processes where substitutes have been challenging to implement. Secondly, environmental scientists are working to improve detection and measurement techniques for atmospheric CCl4 to better understand its global distribution and sources.
Another key objective is to explore potential beneficial applications of CCl4 that do not result in environmental release. For instance, researchers are investigating its use in controlled laboratory settings for specific chemical reactions where its unique properties can be advantageous. Additionally, there is ongoing work to develop more efficient methods for the destruction and disposal of existing CCl4 stockpiles to prevent future emissions.
The evolution of CCl4 from a widely used industrial chemical to a controlled substance exemplifies the dynamic nature of chemical applications and environmental regulations. As we move forward, the goals surrounding CCl4 continue to balance the need for effective chemical solutions with the imperative of environmental protection and human health safety.
Throughout the mid-20th century, CCl4 found widespread applications in various sectors, including dry cleaning, fire extinguishers, and as a fumigant in agriculture. Its versatility and effectiveness led to its extensive use in both industrial and consumer products. However, the 1970s marked a turning point in the compound's history when its harmful effects on the ozone layer and human health became apparent.
The Montreal Protocol, signed in 1987, initiated the phase-out of ozone-depleting substances, including CCl4. This international agreement significantly impacted the production and use of carbon tetrachloride, leading to a dramatic decline in its global consumption. The protocol's implementation has been successful in reducing atmospheric concentrations of CCl4, although its long atmospheric lifetime means that its effects persist.
In recent years, the focus on CCl4 has shifted towards understanding its continued presence in the atmosphere and identifying potential sources of emissions. Researchers have discovered unexpected levels of CCl4 in the atmosphere, suggesting ongoing production or release from legacy sources. This has led to renewed interest in monitoring and controlling CCl4 emissions globally.
The objectives of current CCl4 research and applications are multifaceted. Firstly, there is a continued effort to develop alternative compounds and technologies to replace CCl4 in its remaining applications, particularly in industrial processes where substitutes have been challenging to implement. Secondly, environmental scientists are working to improve detection and measurement techniques for atmospheric CCl4 to better understand its global distribution and sources.
Another key objective is to explore potential beneficial applications of CCl4 that do not result in environmental release. For instance, researchers are investigating its use in controlled laboratory settings for specific chemical reactions where its unique properties can be advantageous. Additionally, there is ongoing work to develop more efficient methods for the destruction and disposal of existing CCl4 stockpiles to prevent future emissions.
The evolution of CCl4 from a widely used industrial chemical to a controlled substance exemplifies the dynamic nature of chemical applications and environmental regulations. As we move forward, the goals surrounding CCl4 continue to balance the need for effective chemical solutions with the imperative of environmental protection and human health safety.
Industrial Demand Analysis
Carbon tetrachloride (CCl4) continues to play a significant role in various industrial applications, despite environmental concerns and regulatory restrictions. The global demand for carbon tetrachloride has shown a steady decline over the past decades due to its ozone-depleting properties and potential health hazards. However, certain industries still rely on its unique chemical properties for specific processes.
The pharmaceutical sector remains one of the primary consumers of carbon tetrachloride. It is used as a solvent in the synthesis of various drugs and as a reagent in analytical procedures. The industry's demand is driven by the need for high-purity solvents in drug manufacturing processes, where alternatives may not provide the same level of effectiveness or purity.
In the chemical manufacturing industry, carbon tetrachloride finds applications as a feedstock for the production of other chlorinated compounds. While efforts have been made to replace it with more environmentally friendly alternatives, some processes still require its use due to its specific chemical properties and reactivity.
The agrochemical sector also contributes to the demand for carbon tetrachloride. It is used in the production of certain pesticides and herbicides, although this application has been significantly reduced in recent years due to the development of alternative synthesis routes and more sustainable agricultural practices.
In the field of materials science and engineering, carbon tetrachloride is utilized in the production of advanced materials such as carbon nanotubes and graphene. These materials have growing applications in electronics, energy storage, and composite materials, potentially driving a niche demand for high-purity carbon tetrachloride.
The cleaning and degreasing industry, particularly in specialized applications where alternatives are less effective, continues to use carbon tetrachloride. However, this usage has dramatically decreased due to stringent regulations and the availability of safer substitutes.
Market analysis indicates that the Asia-Pacific region, particularly China and India, accounts for a significant portion of the global carbon tetrachloride consumption. This is attributed to the rapid industrialization and the presence of a large chemical manufacturing base in these countries.
Despite the overall declining trend, the global market for carbon tetrachloride is expected to maintain a certain level of stability in the coming years. This is primarily due to its irreplaceable role in specific chemical processes and the lack of equally effective alternatives in certain applications. However, ongoing research and development efforts are focused on finding sustainable substitutes, which may further impact the future demand for carbon tetrachloride across various industries.
The pharmaceutical sector remains one of the primary consumers of carbon tetrachloride. It is used as a solvent in the synthesis of various drugs and as a reagent in analytical procedures. The industry's demand is driven by the need for high-purity solvents in drug manufacturing processes, where alternatives may not provide the same level of effectiveness or purity.
In the chemical manufacturing industry, carbon tetrachloride finds applications as a feedstock for the production of other chlorinated compounds. While efforts have been made to replace it with more environmentally friendly alternatives, some processes still require its use due to its specific chemical properties and reactivity.
The agrochemical sector also contributes to the demand for carbon tetrachloride. It is used in the production of certain pesticides and herbicides, although this application has been significantly reduced in recent years due to the development of alternative synthesis routes and more sustainable agricultural practices.
In the field of materials science and engineering, carbon tetrachloride is utilized in the production of advanced materials such as carbon nanotubes and graphene. These materials have growing applications in electronics, energy storage, and composite materials, potentially driving a niche demand for high-purity carbon tetrachloride.
The cleaning and degreasing industry, particularly in specialized applications where alternatives are less effective, continues to use carbon tetrachloride. However, this usage has dramatically decreased due to stringent regulations and the availability of safer substitutes.
Market analysis indicates that the Asia-Pacific region, particularly China and India, accounts for a significant portion of the global carbon tetrachloride consumption. This is attributed to the rapid industrialization and the presence of a large chemical manufacturing base in these countries.
Despite the overall declining trend, the global market for carbon tetrachloride is expected to maintain a certain level of stability in the coming years. This is primarily due to its irreplaceable role in specific chemical processes and the lack of equally effective alternatives in certain applications. However, ongoing research and development efforts are focused on finding sustainable substitutes, which may further impact the future demand for carbon tetrachloride across various industries.
CCl4 Challenges and Status
Carbon tetrachloride (CCl4) has been a subject of significant concern in modern chemistry due to its environmental and health impacts. Despite its historical importance in various industrial applications, the use of CCl4 has been severely restricted globally. The current status of CCl4 is characterized by a complex interplay of regulatory measures, environmental challenges, and ongoing research efforts.
One of the primary challenges associated with CCl4 is its ozone-depleting potential. As a potent ozone-depleting substance, CCl4 has been phased out under the Montreal Protocol, leading to a dramatic reduction in its production and use. However, atmospheric measurements indicate that emissions of CCl4 are higher than expected, suggesting ongoing production or unidentified sources. This discrepancy presents a significant challenge for environmental scientists and policymakers.
The persistence of CCl4 in the environment is another major concern. With a long atmospheric lifetime of approximately 26 years, CCl4 continues to impact the ozone layer and contribute to global warming long after its release. This persistence complicates efforts to mitigate its effects and necessitates long-term monitoring and remediation strategies.
In terms of human health, CCl4 poses serious risks. It is classified as a probable human carcinogen and can cause liver, kidney, and central nervous system damage. Occupational exposure remains a concern in industries where CCl4 is still used or where it may be present as a contaminant. Developing safe handling protocols and effective personal protective equipment is an ongoing challenge.
The current status of CCl4 in chemistry solutions is largely focused on finding alternatives and developing remediation technologies. Many industries have successfully transitioned to safer substitutes, but some niche applications still rely on CCl4. Research is ongoing to identify environmentally friendly alternatives that can match the performance of CCl4 in these specialized uses.
Remediation efforts for CCl4 contamination in soil and groundwater present significant technical challenges. Traditional pump-and-treat methods are often ineffective due to CCl4's low solubility and tendency to form dense non-aqueous phase liquids (DNAPLs). Advanced remediation techniques, such as in-situ chemical oxidation and bioremediation, are being developed and refined to address these issues more effectively.
The global distribution of CCl4 emissions and its long-range transport capabilities add another layer of complexity to its management. International cooperation is crucial for addressing the CCl4 challenge, as emissions in one region can have far-reaching effects. Improving global monitoring networks and enhancing data sharing among nations are ongoing efforts to better understand and control CCl4 emissions.
One of the primary challenges associated with CCl4 is its ozone-depleting potential. As a potent ozone-depleting substance, CCl4 has been phased out under the Montreal Protocol, leading to a dramatic reduction in its production and use. However, atmospheric measurements indicate that emissions of CCl4 are higher than expected, suggesting ongoing production or unidentified sources. This discrepancy presents a significant challenge for environmental scientists and policymakers.
The persistence of CCl4 in the environment is another major concern. With a long atmospheric lifetime of approximately 26 years, CCl4 continues to impact the ozone layer and contribute to global warming long after its release. This persistence complicates efforts to mitigate its effects and necessitates long-term monitoring and remediation strategies.
In terms of human health, CCl4 poses serious risks. It is classified as a probable human carcinogen and can cause liver, kidney, and central nervous system damage. Occupational exposure remains a concern in industries where CCl4 is still used or where it may be present as a contaminant. Developing safe handling protocols and effective personal protective equipment is an ongoing challenge.
The current status of CCl4 in chemistry solutions is largely focused on finding alternatives and developing remediation technologies. Many industries have successfully transitioned to safer substitutes, but some niche applications still rely on CCl4. Research is ongoing to identify environmentally friendly alternatives that can match the performance of CCl4 in these specialized uses.
Remediation efforts for CCl4 contamination in soil and groundwater present significant technical challenges. Traditional pump-and-treat methods are often ineffective due to CCl4's low solubility and tendency to form dense non-aqueous phase liquids (DNAPLs). Advanced remediation techniques, such as in-situ chemical oxidation and bioremediation, are being developed and refined to address these issues more effectively.
The global distribution of CCl4 emissions and its long-range transport capabilities add another layer of complexity to its management. International cooperation is crucial for addressing the CCl4 challenge, as emissions in one region can have far-reaching effects. Improving global monitoring networks and enhancing data sharing among nations are ongoing efforts to better understand and control CCl4 emissions.
Current CCl4 Solutions
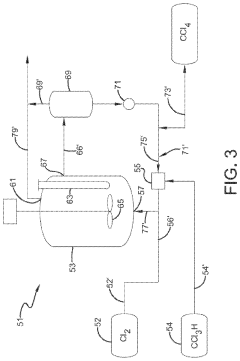
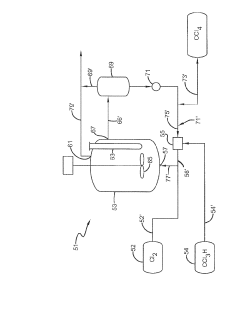
01 Production and purification of carbon tetrachloride
Various methods for producing and purifying carbon tetrachloride are described. These include chemical synthesis processes, distillation techniques, and purification methods to obtain high-quality carbon tetrachloride for industrial and laboratory use.- Production and purification of carbon tetrachloride: Various methods for producing and purifying carbon tetrachloride are described. These include chemical synthesis processes, distillation techniques, and purification methods to obtain high-quality carbon tetrachloride for industrial and laboratory use.
- Applications of carbon tetrachloride in chemical processes: Carbon tetrachloride is utilized in various chemical processes, including as a solvent, reagent, or intermediate in the production of other chemicals. Its applications span across different industries, such as pharmaceuticals, plastics, and agrochemicals.
- Environmental and safety considerations: Due to its environmental impact and health hazards, methods for detecting, monitoring, and safely handling carbon tetrachloride are developed. This includes techniques for environmental remediation and workplace safety measures to minimize exposure risks.
- Alternatives and substitutes for carbon tetrachloride: Research into alternatives and substitutes for carbon tetrachloride is ongoing, aiming to replace its use in various applications with less harmful substances. This includes developing new compounds or processes that can perform similar functions without the associated environmental and health risks.
- Analytical and testing methods involving carbon tetrachloride: Carbon tetrachloride is used in various analytical and testing methods, including as a solvent in spectroscopy, a standard in chemical analysis, and a component in certain laboratory procedures. Techniques for its detection and quantification in different matrices are also developed.
02 Applications of carbon tetrachloride in chemical processes
Carbon tetrachloride is utilized in various chemical processes, including as a solvent, reagent, or intermediate in the production of other chemicals. Its applications span across different industries, such as pharmaceuticals, plastics, and agrochemicals.Expand Specific Solutions03 Environmental and safety considerations
Due to its environmental impact and health hazards, methods for detecting, monitoring, and safely handling carbon tetrachloride are developed. This includes techniques for environmental remediation and workplace safety measures to minimize exposure risks.Expand Specific Solutions04 Alternatives and substitutes for carbon tetrachloride
Research into alternatives and substitutes for carbon tetrachloride is ongoing, aiming to replace its use in various applications with safer and more environmentally friendly options. This includes developing new compounds or processes that can perform similar functions without the associated risks.Expand Specific Solutions05 Historical uses and developments
Carbon tetrachloride has a long history of industrial and commercial use. Patents describe its early applications, manufacturing methods, and gradual phase-out in certain areas due to environmental concerns. This point covers the evolution of carbon tetrachloride's use over time.Expand Specific Solutions
Key Industry Players
The carbon tetrachloride market is in a mature phase, with a relatively stable global demand. The market size is estimated to be in the range of $100-200 million annually. Technologically, carbon tetrachloride production is well-established, with most major chemical companies having the capability to manufacture it. Key players in this field include Occidental Chemical Corp., Bayer AG, and Wacker Chemie AG, who possess advanced production facilities and extensive experience in chlorinated solvents. However, due to environmental concerns and regulatory restrictions, the industry is focusing on developing alternative applications and safer production methods. Companies like Teijin Chemicals Ltd. and Spolek pro chemickou a hutní výrobu are investing in research to explore new uses in specialty chemicals and pharmaceuticals.
Occidental Chemical Corp.
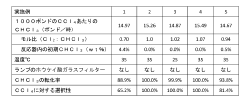
Technical Solution: Occidental Chemical Corp. has developed advanced purification techniques for carbon tetrachloride production, focusing on minimizing impurities and enhancing product quality. Their process involves multi-stage distillation and adsorption methods, resulting in ultra-high purity CCl4 (99.9%+) suitable for semiconductor manufacturing and analytical chemistry applications[1]. The company has also implemented a closed-loop recycling system to capture and reuse CCl4 byproducts, significantly reducing waste and environmental impact[2]. Additionally, Occidental has invested in safer handling and transportation methods, including specialized containers with leak detection systems and remote monitoring capabilities[3].
Strengths: High-purity product, environmentally responsible practices, advanced safety measures. Weaknesses: High production costs, regulatory challenges due to CCl4's environmental concerns.
Bayer AG
Technical Solution: Bayer AG has focused on developing alternative solutions to replace carbon tetrachloride in various applications, addressing environmental and health concerns. Their research has led to the creation of safer, more sustainable substitutes for CCl4 in pharmaceutical synthesis and agrochemical production[7]. Bayer has successfully implemented green chemistry principles to redesign processes that traditionally relied on CCl4, such as chlorination reactions and solvent extraction. The company has also invested in advanced analytical techniques that minimize the use of CCl4 in quality control laboratories[8]. Additionally, Bayer is exploring the potential of CCl4 in carbon capture and utilization technologies, aiming to transform this problematic compound into a valuable resource for combating climate change[9].
Strengths: Focus on sustainable alternatives, diverse application areas, strong R&D capabilities. Weaknesses: Transition costs, potential performance trade-offs in some applications.
CCl4 Innovations Review
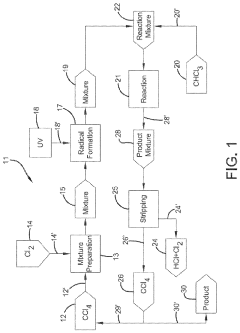
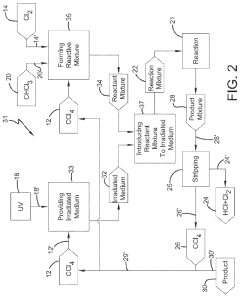
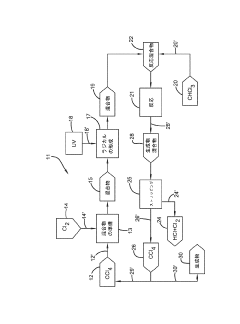
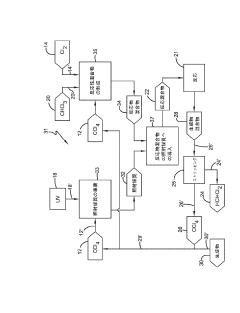
Photochlorination of partially-chlorinated chloromethanes to carbon tetrachloride
PatentActiveUS20240025823A1
Innovation
- A method involving the photochlorination of a chloromethanes stream containing chloroform, methyl chloride, and methylene chloride, combined with chlorine and additional carbon tetrachloride, and subjected to electromagnetic radiation to form carbon tetrachloride, achieving high conversion rates with reduced levels of unwanted chlorinated hydrocarbons.
Producing carbon tetrachloride by photochlorination of chloroform
PatentActiveJP2024069268A
Innovation
- A method involving the photochlorination of chloroform with chlorine in the presence of electromagnetic radiation, maintaining a low concentration of chloroform and a stoichiometric concentration of chlorine, to produce carbon tetrachloride with high selectivity and minimize the formation of hexachloroethane.
Environmental Regulations
Carbon tetrachloride, once widely used in various industrial and consumer applications, has been subject to increasingly stringent environmental regulations due to its harmful effects on human health and the environment. The Montreal Protocol, an international treaty designed to protect the ozone layer, has played a crucial role in phasing out the production and consumption of carbon tetrachloride since 1987.
In the United States, the Environmental Protection Agency (EPA) has implemented strict regulations on carbon tetrachloride under the Clean Air Act and the Toxic Substances Control Act. These regulations have effectively banned the use of carbon tetrachloride in consumer products and significantly limited its industrial applications. The EPA has also established stringent emission standards for facilities that still use or produce carbon tetrachloride.
The European Union has taken similar measures through the REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation. Under REACH, carbon tetrachloride is classified as a substance of very high concern (SVHC) due to its carcinogenic properties and ozone-depleting potential. This classification imposes strict controls on its manufacture, import, and use within the EU.
Globally, many countries have adopted regulations aligned with the Montreal Protocol, implementing phase-out schedules for ozone-depleting substances, including carbon tetrachloride. These regulations typically involve a combination of import/export controls, production quotas, and restrictions on specific uses.
Despite these regulations, carbon tetrachloride continues to be used in certain essential applications, such as feedstock for the production of other chemicals. In these cases, strict containment and emission control measures are required to minimize environmental release.
The enforcement of these regulations has led to a significant decline in global carbon tetrachloride emissions. However, recent atmospheric measurements have revealed higher-than-expected levels, suggesting potential unreported sources or emissions. This has prompted renewed efforts to identify and address these sources through enhanced monitoring and enforcement mechanisms.
As environmental concerns continue to grow, it is likely that regulations surrounding carbon tetrachloride will become even more stringent in the future. This may include further restrictions on remaining uses, increased reporting requirements, and enhanced monitoring of atmospheric concentrations to ensure compliance with international agreements and national regulations.
In the United States, the Environmental Protection Agency (EPA) has implemented strict regulations on carbon tetrachloride under the Clean Air Act and the Toxic Substances Control Act. These regulations have effectively banned the use of carbon tetrachloride in consumer products and significantly limited its industrial applications. The EPA has also established stringent emission standards for facilities that still use or produce carbon tetrachloride.
The European Union has taken similar measures through the REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation. Under REACH, carbon tetrachloride is classified as a substance of very high concern (SVHC) due to its carcinogenic properties and ozone-depleting potential. This classification imposes strict controls on its manufacture, import, and use within the EU.
Globally, many countries have adopted regulations aligned with the Montreal Protocol, implementing phase-out schedules for ozone-depleting substances, including carbon tetrachloride. These regulations typically involve a combination of import/export controls, production quotas, and restrictions on specific uses.
Despite these regulations, carbon tetrachloride continues to be used in certain essential applications, such as feedstock for the production of other chemicals. In these cases, strict containment and emission control measures are required to minimize environmental release.
The enforcement of these regulations has led to a significant decline in global carbon tetrachloride emissions. However, recent atmospheric measurements have revealed higher-than-expected levels, suggesting potential unreported sources or emissions. This has prompted renewed efforts to identify and address these sources through enhanced monitoring and enforcement mechanisms.
As environmental concerns continue to grow, it is likely that regulations surrounding carbon tetrachloride will become even more stringent in the future. This may include further restrictions on remaining uses, increased reporting requirements, and enhanced monitoring of atmospheric concentrations to ensure compliance with international agreements and national regulations.
Safety Protocols for CCl4
Carbon tetrachloride (CCl4) is a highly toxic and potentially dangerous chemical compound that requires strict safety protocols for handling and use. These protocols are essential to protect workers, the environment, and the general public from the harmful effects of CCl4 exposure.
Personal protective equipment (PPE) is crucial when working with CCl4. Workers must wear chemical-resistant gloves, protective eyewear, and respiratory protection appropriate for the concentration and duration of exposure. Full-body protective suits may be necessary for certain applications or in case of potential splashes or spills.
Proper ventilation is paramount in areas where CCl4 is used or stored. Local exhaust ventilation systems should be installed to capture and remove vapors at the source. General room ventilation should also be maintained to ensure adequate air exchange and prevent the accumulation of CCl4 vapors.
Storage of CCl4 requires special considerations. It should be kept in tightly sealed containers in a cool, dry, and well-ventilated area away from sources of heat, ignition, and direct sunlight. Incompatible materials, such as strong oxidizers and alkali metals, must be stored separately to prevent dangerous reactions.
Spill response procedures must be established and communicated to all personnel working with CCl4. This includes having appropriate spill containment materials readily available, such as absorbent pads or vermiculite, and proper disposal methods for contaminated materials.
Regular monitoring of air quality in areas where CCl4 is used is essential to ensure that exposure levels remain below permissible limits. This may involve the use of personal sampling devices or fixed-point air monitoring systems.
Training is a critical component of CCl4 safety protocols. All personnel who may come into contact with the substance must receive comprehensive training on its hazards, proper handling techniques, emergency procedures, and the use of PPE.
Emergency response plans should be developed and regularly practiced. These plans should include procedures for evacuation, first aid, and decontamination in case of accidental exposure or release.
Proper labeling and signage are necessary to communicate the hazards of CCl4 and the required precautions. This includes clear labeling on all containers and prominent display of safety data sheets in areas where the chemical is used or stored.
Waste management protocols for CCl4 must adhere to strict environmental regulations. Proper disposal methods, such as incineration or chemical treatment, should be employed to prevent environmental contamination.
Regular safety audits and inspections should be conducted to ensure compliance with established protocols and to identify areas for improvement in CCl4 handling and storage practices.
Personal protective equipment (PPE) is crucial when working with CCl4. Workers must wear chemical-resistant gloves, protective eyewear, and respiratory protection appropriate for the concentration and duration of exposure. Full-body protective suits may be necessary for certain applications or in case of potential splashes or spills.
Proper ventilation is paramount in areas where CCl4 is used or stored. Local exhaust ventilation systems should be installed to capture and remove vapors at the source. General room ventilation should also be maintained to ensure adequate air exchange and prevent the accumulation of CCl4 vapors.
Storage of CCl4 requires special considerations. It should be kept in tightly sealed containers in a cool, dry, and well-ventilated area away from sources of heat, ignition, and direct sunlight. Incompatible materials, such as strong oxidizers and alkali metals, must be stored separately to prevent dangerous reactions.
Spill response procedures must be established and communicated to all personnel working with CCl4. This includes having appropriate spill containment materials readily available, such as absorbent pads or vermiculite, and proper disposal methods for contaminated materials.
Regular monitoring of air quality in areas where CCl4 is used is essential to ensure that exposure levels remain below permissible limits. This may involve the use of personal sampling devices or fixed-point air monitoring systems.
Training is a critical component of CCl4 safety protocols. All personnel who may come into contact with the substance must receive comprehensive training on its hazards, proper handling techniques, emergency procedures, and the use of PPE.
Emergency response plans should be developed and regularly practiced. These plans should include procedures for evacuation, first aid, and decontamination in case of accidental exposure or release.
Proper labeling and signage are necessary to communicate the hazards of CCl4 and the required precautions. This includes clear labeling on all containers and prominent display of safety data sheets in areas where the chemical is used or stored.
Waste management protocols for CCl4 must adhere to strict environmental regulations. Proper disposal methods, such as incineration or chemical treatment, should be employed to prevent environmental contamination.
Regular safety audits and inspections should be conducted to ensure compliance with established protocols and to identify areas for improvement in CCl4 handling and storage practices.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!