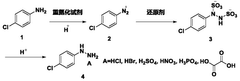Case Study: Continuous Flow Synthesis Of A High-Potency API
SEP 3, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Continuous Flow API Synthesis Background and Objectives
Continuous flow synthesis represents a paradigm shift in pharmaceutical manufacturing, moving away from traditional batch processes toward more efficient, controlled, and sustainable production methods. This approach has evolved significantly over the past two decades, driven by increasing demands for safer handling of high-potency active pharmaceutical ingredients (HPAPIs) and more economical production processes. The evolution began with simple microreactor systems in the early 2000s and has progressed to sophisticated integrated continuous manufacturing platforms capable of multi-step syntheses with in-line purification and real-time analysis.
The technological trajectory clearly points toward fully automated, AI-optimized continuous flow systems that can adapt process parameters in real-time to maintain optimal product quality. This advancement is particularly crucial for HPAPIs, which often require handling under stringent containment conditions due to their pharmacological potency at extremely low concentrations, typically below 10 μg/kg of body weight.
The primary objectives of implementing continuous flow synthesis for HPAPIs include minimizing operator exposure to hazardous compounds, reducing environmental impact through decreased solvent usage and waste generation, and improving process efficiency. Additionally, continuous flow systems aim to enhance product quality through precise control of reaction parameters such as temperature, pressure, and residence time, leading to more consistent impurity profiles and higher yields.
From a regulatory perspective, continuous manufacturing aligns with the FDA's Quality by Design (QbD) initiative, which encourages pharmaceutical companies to build quality into products through thorough understanding and control of manufacturing processes. The FDA's 2019 guidance document specifically addressing continuous manufacturing demonstrates regulatory support for this technological transition.
Economic considerations also drive the adoption of continuous flow synthesis, with potential benefits including reduced capital expenditure for facility construction (smaller footprint), lower operational costs, and faster time-to-market for new drugs. The ability to scale production by extending operation time rather than increasing reactor size (numbering-up versus scaling-up) represents a fundamental advantage over batch processing.
Current technical challenges include addressing solid handling in continuous systems, developing robust control strategies for long-duration runs, and integrating multiple reaction steps with varying optimal conditions. The industry is actively working to overcome these limitations through innovations in reactor design, advanced process analytical technology (PAT), and novel approaches to crystallization and filtration operations in continuous mode.
This case study examines the implementation of continuous flow synthesis for a specific high-potency API, exploring how these general principles and objectives translate into practical application within pharmaceutical manufacturing environments.
The technological trajectory clearly points toward fully automated, AI-optimized continuous flow systems that can adapt process parameters in real-time to maintain optimal product quality. This advancement is particularly crucial for HPAPIs, which often require handling under stringent containment conditions due to their pharmacological potency at extremely low concentrations, typically below 10 μg/kg of body weight.
The primary objectives of implementing continuous flow synthesis for HPAPIs include minimizing operator exposure to hazardous compounds, reducing environmental impact through decreased solvent usage and waste generation, and improving process efficiency. Additionally, continuous flow systems aim to enhance product quality through precise control of reaction parameters such as temperature, pressure, and residence time, leading to more consistent impurity profiles and higher yields.
From a regulatory perspective, continuous manufacturing aligns with the FDA's Quality by Design (QbD) initiative, which encourages pharmaceutical companies to build quality into products through thorough understanding and control of manufacturing processes. The FDA's 2019 guidance document specifically addressing continuous manufacturing demonstrates regulatory support for this technological transition.
Economic considerations also drive the adoption of continuous flow synthesis, with potential benefits including reduced capital expenditure for facility construction (smaller footprint), lower operational costs, and faster time-to-market for new drugs. The ability to scale production by extending operation time rather than increasing reactor size (numbering-up versus scaling-up) represents a fundamental advantage over batch processing.
Current technical challenges include addressing solid handling in continuous systems, developing robust control strategies for long-duration runs, and integrating multiple reaction steps with varying optimal conditions. The industry is actively working to overcome these limitations through innovations in reactor design, advanced process analytical technology (PAT), and novel approaches to crystallization and filtration operations in continuous mode.
This case study examines the implementation of continuous flow synthesis for a specific high-potency API, exploring how these general principles and objectives translate into practical application within pharmaceutical manufacturing environments.
Market Demand Analysis for High-Potency API Production
The global market for high-potency active pharmaceutical ingredients (HPAPIs) has been experiencing robust growth, driven primarily by the increasing prevalence of cancer and other chronic diseases. The demand for targeted therapies with enhanced efficacy at lower doses has positioned HPAPIs as critical components in modern pharmaceutical development. Current market valuations place the HPAPI sector at approximately $26.8 billion in 2023, with projections indicating growth to reach $41.5 billion by 2028, representing a compound annual growth rate (CAGR) of 9.2%.
Oncology remains the dominant therapeutic application for HPAPIs, accounting for nearly 45% of market share. This dominance reflects the inherent characteristics of cancer treatments, which often require highly potent compounds to target rapidly dividing cells while minimizing damage to healthy tissue. The rising global cancer burden, with an estimated 19.3 million new cases annually, continues to fuel demand for innovative HPAPI-based therapies.
Beyond oncology, HPAPIs are gaining significant traction in hormonal treatments, cardiovascular medications, and central nervous system therapies. This diversification of application areas is expanding the potential market reach and creating new opportunities for pharmaceutical manufacturers. The trend toward personalized medicine is further accelerating demand for specialized, high-potency compounds tailored to specific patient populations or genetic profiles.
From a manufacturing perspective, the pharmaceutical industry is increasingly recognizing the limitations of traditional batch processing for HPAPI production. Conventional methods present significant challenges related to operator safety, environmental containment, and process efficiency. These concerns are particularly acute given that HPAPIs typically have occupational exposure limits below 10 μg/m³, with some compounds requiring handling at nanogram levels.
Continuous flow synthesis represents a paradigm shift in addressing these manufacturing challenges. Market analysis indicates that pharmaceutical companies implementing continuous manufacturing technologies for HPAPIs can achieve cost reductions of 15-30% compared to batch processes, primarily through improved yield, reduced waste generation, and decreased energy consumption. Additionally, the enhanced safety profile of closed continuous systems is becoming increasingly attractive as regulatory scrutiny of containment measures intensifies.
Contract manufacturing organizations (CMOs) and contract development and manufacturing organizations (CDMOs) are responding to this market demand by significantly expanding their HPAPI capabilities. Investment in continuous flow technology for HPAPI production has increased by approximately 22% annually since 2020, reflecting industry recognition of its strategic importance. This trend is particularly pronounced in established pharmaceutical markets like North America and Europe, though emerging economies in Asia-Pacific are rapidly developing comparable capabilities.
Oncology remains the dominant therapeutic application for HPAPIs, accounting for nearly 45% of market share. This dominance reflects the inherent characteristics of cancer treatments, which often require highly potent compounds to target rapidly dividing cells while minimizing damage to healthy tissue. The rising global cancer burden, with an estimated 19.3 million new cases annually, continues to fuel demand for innovative HPAPI-based therapies.
Beyond oncology, HPAPIs are gaining significant traction in hormonal treatments, cardiovascular medications, and central nervous system therapies. This diversification of application areas is expanding the potential market reach and creating new opportunities for pharmaceutical manufacturers. The trend toward personalized medicine is further accelerating demand for specialized, high-potency compounds tailored to specific patient populations or genetic profiles.
From a manufacturing perspective, the pharmaceutical industry is increasingly recognizing the limitations of traditional batch processing for HPAPI production. Conventional methods present significant challenges related to operator safety, environmental containment, and process efficiency. These concerns are particularly acute given that HPAPIs typically have occupational exposure limits below 10 μg/m³, with some compounds requiring handling at nanogram levels.
Continuous flow synthesis represents a paradigm shift in addressing these manufacturing challenges. Market analysis indicates that pharmaceutical companies implementing continuous manufacturing technologies for HPAPIs can achieve cost reductions of 15-30% compared to batch processes, primarily through improved yield, reduced waste generation, and decreased energy consumption. Additionally, the enhanced safety profile of closed continuous systems is becoming increasingly attractive as regulatory scrutiny of containment measures intensifies.
Contract manufacturing organizations (CMOs) and contract development and manufacturing organizations (CDMOs) are responding to this market demand by significantly expanding their HPAPI capabilities. Investment in continuous flow technology for HPAPI production has increased by approximately 22% annually since 2020, reflecting industry recognition of its strategic importance. This trend is particularly pronounced in established pharmaceutical markets like North America and Europe, though emerging economies in Asia-Pacific are rapidly developing comparable capabilities.
Technical Challenges in Continuous Flow Synthesis
Continuous flow synthesis for high-potency APIs presents several significant technical challenges that must be addressed to ensure successful implementation. The primary challenge lies in the handling of hazardous materials, as many high-potency APIs involve toxic, highly reactive, or explosive intermediates. Traditional batch processes often require extensive safety measures, but continuous flow systems must incorporate these safety considerations directly into the equipment design and process controls.
Temperature control represents another critical challenge, particularly for reactions requiring precise thermal management. Unlike batch reactors where temperature gradients can be problematic, continuous flow systems offer superior heat transfer capabilities due to high surface-area-to-volume ratios. However, this advantage comes with the technical challenge of designing microreactors with appropriate materials and geometries to maintain uniform temperature profiles throughout the reaction pathway.
Mixing efficiency presents unique difficulties in continuous flow synthesis. The laminar flow regime typical in microreactors limits conventional turbulent mixing, necessitating specialized mixing structures such as split-and-recombine units or static mixers. For high-potency APIs, achieving homogeneous mixing without cross-contamination or product degradation requires sophisticated engineering solutions.
Scalability remains one of the most significant hurdles in continuous flow technology. While laboratory-scale demonstrations may prove successful, scaling to production volumes introduces complexities in maintaining consistent residence times, pressure drops, and mixing efficiencies. The "numbering up" approach (adding parallel reactors) rather than traditional "scaling up" presents its own challenges in ensuring uniform flow distribution and consistent product quality across multiple channels.
Solid handling represents a particularly difficult challenge for continuous flow systems. Precipitation of intermediates or products can lead to clogging, pressure buildup, and system failure. For high-potency APIs, where solid formation is common, innovative solutions such as ultrasonic irradiation, specialized reactor geometries, or solvent selection strategies must be implemented to prevent system blockages.
Process analytical technology (PAT) integration presents technical difficulties unique to continuous systems. Real-time monitoring and control require specialized sensors capable of operating under flow conditions with sufficient sensitivity to detect process deviations rapidly. For high-potency APIs, where product purity requirements are stringent, developing robust analytical methods that can function in-line without compromising sample integrity is technically demanding.
Regulatory compliance adds another layer of complexity, as continuous manufacturing for pharmaceuticals must meet stringent validation requirements. Demonstrating consistent quality, establishing appropriate control strategies, and defining process boundaries requires sophisticated process understanding and control systems beyond those typically needed for batch processes.
Temperature control represents another critical challenge, particularly for reactions requiring precise thermal management. Unlike batch reactors where temperature gradients can be problematic, continuous flow systems offer superior heat transfer capabilities due to high surface-area-to-volume ratios. However, this advantage comes with the technical challenge of designing microreactors with appropriate materials and geometries to maintain uniform temperature profiles throughout the reaction pathway.
Mixing efficiency presents unique difficulties in continuous flow synthesis. The laminar flow regime typical in microreactors limits conventional turbulent mixing, necessitating specialized mixing structures such as split-and-recombine units or static mixers. For high-potency APIs, achieving homogeneous mixing without cross-contamination or product degradation requires sophisticated engineering solutions.
Scalability remains one of the most significant hurdles in continuous flow technology. While laboratory-scale demonstrations may prove successful, scaling to production volumes introduces complexities in maintaining consistent residence times, pressure drops, and mixing efficiencies. The "numbering up" approach (adding parallel reactors) rather than traditional "scaling up" presents its own challenges in ensuring uniform flow distribution and consistent product quality across multiple channels.
Solid handling represents a particularly difficult challenge for continuous flow systems. Precipitation of intermediates or products can lead to clogging, pressure buildup, and system failure. For high-potency APIs, where solid formation is common, innovative solutions such as ultrasonic irradiation, specialized reactor geometries, or solvent selection strategies must be implemented to prevent system blockages.
Process analytical technology (PAT) integration presents technical difficulties unique to continuous systems. Real-time monitoring and control require specialized sensors capable of operating under flow conditions with sufficient sensitivity to detect process deviations rapidly. For high-potency APIs, where product purity requirements are stringent, developing robust analytical methods that can function in-line without compromising sample integrity is technically demanding.
Regulatory compliance adds another layer of complexity, as continuous manufacturing for pharmaceuticals must meet stringent validation requirements. Demonstrating consistent quality, establishing appropriate control strategies, and defining process boundaries requires sophisticated process understanding and control systems beyond those typically needed for batch processes.
Current Continuous Flow Synthesis Methodologies
01 Continuous flow reactors for high-potency API synthesis
Continuous flow reactors provide a controlled environment for synthesizing high-potency active pharmaceutical ingredients (APIs). These systems offer advantages such as precise temperature control, improved mixing, and reduced exposure risks to operators handling hazardous compounds. The enclosed nature of continuous flow systems is particularly beneficial for high-potency compound synthesis, minimizing contamination risks and enhancing safety protocols during production.- Continuous flow reactors for high-potency API synthesis: Continuous flow reactors provide a controlled environment for synthesizing high-potency active pharmaceutical ingredients (APIs). These systems offer advantages such as precise temperature control, improved mixing, and reduced exposure risks to operators. The enclosed nature of continuous flow systems is particularly beneficial for handling hazardous or highly potent compounds, minimizing contamination risks and enhancing safety during the manufacturing process.
- Microreactor technology for potent compound synthesis: Microreactor technology enables the synthesis of high-potency compounds with enhanced efficiency and safety. These miniaturized reaction systems provide excellent heat and mass transfer properties, allowing for precise control over reaction parameters. Microreactors facilitate rapid mixing, controlled residence times, and improved yield of potent compounds while reducing waste generation. The small internal volumes also minimize potential exposure to hazardous materials during synthesis operations.
- Process intensification techniques for high-potency synthesis: Process intensification techniques in continuous flow synthesis enhance the production of high-potency compounds through improved efficiency and control. These approaches include multistep reactions in sequence, in-line purification, and real-time monitoring systems. By integrating multiple operations into a continuous process, manufacturers can achieve higher yields, greater purity, and reduced processing times while maintaining containment of potent materials throughout the production cycle.
- Automated control systems for continuous flow synthesis: Automated control systems are essential for continuous flow synthesis of high-potency compounds, providing precise regulation of critical process parameters. These systems incorporate sensors, feedback loops, and advanced algorithms to maintain optimal reaction conditions. Automation reduces human intervention, minimizing exposure risks while ensuring consistent product quality. Real-time monitoring capabilities allow for immediate adjustments to process variables, enhancing both safety and efficiency in high-potency manufacturing.
- Containment strategies for high-potency continuous manufacturing: Specialized containment strategies are implemented in continuous flow synthesis to safely handle high-potency compounds. These include closed processing systems, isolator technology, and engineered controls that prevent operator exposure and environmental contamination. Advanced sealing mechanisms, pressure differentials, and dedicated waste handling systems ensure potent compounds remain contained throughout the manufacturing process. These containment approaches enable the safe production of highly potent materials while meeting stringent occupational exposure limits.
02 Microreactor technology for potent compound synthesis
Microreactor technology enables the synthesis of high-potency compounds through enhanced heat and mass transfer capabilities. These miniaturized reaction systems provide better control over reaction parameters, allowing for improved yield and purity of potent compounds. The small reaction volumes reduce the amount of hazardous materials present at any given time, thereby increasing safety while maintaining production efficiency for highly active pharmaceutical ingredients.Expand Specific Solutions03 Process intensification techniques for high-potency synthesis
Process intensification techniques in continuous flow synthesis involve optimizing reaction conditions to enhance productivity while maintaining safety for high-potency compounds. These approaches include the use of novel catalysts, alternative solvents, and innovative reactor designs that enable faster reactions with improved selectivity. By intensifying the process, manufacturers can achieve higher throughput of potent compounds while reducing waste generation and energy consumption.Expand Specific Solutions04 Automated control systems for continuous flow synthesis
Automated control systems are essential for continuous flow synthesis of high-potency compounds, providing real-time monitoring and adjustment of critical process parameters. These systems incorporate sensors, feedback loops, and advanced algorithms to maintain optimal reaction conditions throughout the synthesis process. Automation reduces human intervention, minimizing exposure risks while ensuring consistent quality of high-potency products through precise control of flow rates, temperatures, and residence times.Expand Specific Solutions05 Integrated purification in continuous flow systems
Integrated purification techniques within continuous flow systems enable the direct processing of high-potency compounds without intermediate isolation steps. These approaches combine reaction and purification operations in a single continuous process, reducing handling of potent materials and minimizing contamination risks. In-line purification methods such as extraction, crystallization, and chromatography can be incorporated into the flow system, allowing for the production of high-purity potent compounds with enhanced safety and efficiency.Expand Specific Solutions
Key Industry Players in Continuous API Manufacturing
Continuous flow synthesis of high-potency APIs is currently in a growth phase, with the market expanding rapidly due to increasing demand for efficient, scalable pharmaceutical manufacturing processes. The global market size for continuous flow technology in API synthesis is projected to reach significant value as pharmaceutical companies seek cost-effective and environmentally sustainable production methods. From a technological maturity perspective, companies like AstaTech BioPharmaceutical and Amgen are leading implementation in commercial settings, while Industrial Technology Research Institute and Lunan Pharmaceutical Group are advancing research applications. Academic-industry partnerships involving institutions like New Jersey Institute of Technology are accelerating innovation, particularly in handling complex, high-potency compounds. The technology is transitioning from early adoption to mainstream implementation, with regulatory frameworks evolving to accommodate continuous manufacturing approaches.
AstaTech (Chengdu) BioPharmaceutical Corp.
Technical Solution: AstaTech has developed an integrated continuous flow synthesis platform specifically designed for high-potency APIs. Their approach utilizes microreactor technology with precise temperature control systems that enable reactions to occur in milliseconds rather than hours. The platform incorporates real-time analytical monitoring through inline FTIR and HPLC systems, allowing for immediate quality assessment and process adjustments. AstaTech's system features automated pressure regulation mechanisms that safely handle hazardous reagents at elevated pressures, significantly reducing operator exposure to toxic compounds. Their modular design allows for rapid reconfiguration between different synthesis routes, with specialized catalyst immobilization techniques that extend catalyst lifetime and reduce precious metal consumption by up to 60% compared to batch processes. The company has successfully implemented this technology for several oncology drug candidates, achieving consistent impurity profiles below 0.1% and reducing manufacturing footprint by approximately 75% compared to traditional batch methods.
Strengths: Superior handling of hazardous reagents with minimal operator exposure; exceptional process control leading to higher purity products; significantly reduced manufacturing footprint and waste generation. Weaknesses: Higher initial capital investment compared to batch equipment; requires specialized operator training; more complex validation procedures for regulatory approval.
Avadel Ireland Ltd.
Technical Solution: Avadel has pioneered a proprietary continuous flow synthesis platform called "MicroFlow" specifically optimized for high-potency API production. Their system employs a cascade of microreactors with precise residence time distribution control, allowing for multi-step syntheses without intermediate isolation. The platform incorporates patented mixing technology that achieves mixing times under 50 milliseconds, enabling reactions with highly unstable intermediates that would be impossible in batch processes. Avadel's system features cryogenic capability down to -80°C with minimal temperature gradients (<1°C across reaction zones), critical for stereoselective transformations in API synthesis. Their platform integrates PAT (Process Analytical Technology) tools including online Raman spectroscopy and mass spectrometry for real-time reaction monitoring and automated control. The company has documented yield improvements of 15-30% and impurity reductions of up to 70% compared to batch processes for several commercial high-potency compounds. Avadel's technology also incorporates containment solutions achieving OEL (Occupational Exposure Limit) levels below 10 ng/m³, essential for handling cytotoxic compounds safely.
Strengths: Exceptional containment capabilities for highly potent compounds; superior control of reaction parameters leading to improved stereoselectivity; significant reduction in solvent usage (typically 40-60% less than batch). Weaknesses: Limited throughput for certain reaction types requiring extended residence times; challenges with solids handling in continuous mode; higher complexity in scale-up validation compared to traditional methods.
Critical Process Parameters and Control Strategies
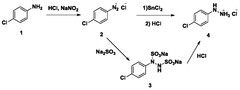
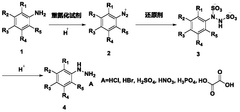
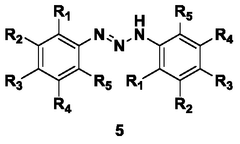
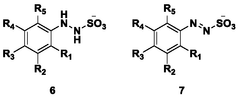
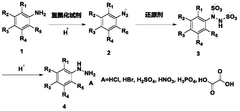
Process for continuous flow synthesis of 4-chlorophenylhydrazine salt
PatentWO2018019249A1
Innovation
- Adopting a new continuous flow synthesis process, through the three-step reaction of diazotization, reduction and acidolysis to form salt continuously in an integrated reactor, a full continuous flow process is realized, the process flow is simplified, and the reaction efficiency and product quality are improved.
Continuous flow synthesis process for phenylhydrazine salt and substituted phenylhydrazine salt
PatentWO2018019250A1
Innovation
- Adopting a new continuous flow synthesis process, through the three-step reaction of diazotization, reduction and acidolysis to salt in an integrated reactor, using aniline or substituted aniline acidic liquid, diazotization reagent and acid as raw materials, It achieves full continuous reaction, avoids intermittent operations and multiple purification steps in traditional processes, and improves reaction efficiency and product quality.
Regulatory Compliance for Continuous Manufacturing
Regulatory compliance represents a critical challenge in the implementation of continuous manufacturing processes for high-potency active pharmaceutical ingredients (HPAPIs). The pharmaceutical industry faces stringent requirements from regulatory bodies worldwide, with the FDA, EMA, and ICH providing frameworks that manufacturers must adhere to when transitioning from batch to continuous processing methods.
For the continuous flow synthesis of high-potency APIs, regulatory considerations begin with risk assessment and management strategies. These must address the unique challenges posed by continuous manufacturing, including real-time release testing, process analytical technology (PAT) implementation, and validation protocols. The FDA's guidance on Process Validation emphasizes a lifecycle approach that is particularly relevant for continuous processes, requiring robust design, qualification, and ongoing verification.
Quality by Design (QbD) principles have become fundamental to regulatory compliance in continuous manufacturing. This approach necessitates thorough understanding of critical quality attributes (CQAs), critical process parameters (CPPs), and their relationships. For high-potency APIs, these considerations are amplified by safety requirements related to containment and cross-contamination prevention.
Regulatory bodies increasingly recognize the advantages of continuous manufacturing for consistent quality and reduced variability. The FDA's Emerging Technology Program and similar initiatives by other agencies provide pathways for manufacturers to engage with regulators early in development. This collaborative approach helps address compliance challenges proactively rather than reactively.
Data integrity presents another significant regulatory consideration. Continuous processes generate substantial volumes of data that must be managed according to ALCOA+ principles (Attributable, Legible, Contemporaneous, Original, Accurate, plus Complete, Consistent, Enduring, and Available). Automated systems must be validated according to GAMP 5 guidelines, with appropriate audit trails and data security measures.
Change management protocols are essential when implementing continuous manufacturing for high-potency APIs. Regulatory filings must clearly document process changes, control strategies, and comparability assessments between batch and continuous processes. Post-approval change management protocols (PACMPs) can facilitate smoother regulatory pathways for future modifications.
Environmental and occupational safety regulations add another layer of compliance requirements. High-potency APIs require specialized containment strategies, with continuous manufacturing potentially offering advantages through closed systems that minimize operator exposure and environmental release.
For the continuous flow synthesis of high-potency APIs, regulatory considerations begin with risk assessment and management strategies. These must address the unique challenges posed by continuous manufacturing, including real-time release testing, process analytical technology (PAT) implementation, and validation protocols. The FDA's guidance on Process Validation emphasizes a lifecycle approach that is particularly relevant for continuous processes, requiring robust design, qualification, and ongoing verification.
Quality by Design (QbD) principles have become fundamental to regulatory compliance in continuous manufacturing. This approach necessitates thorough understanding of critical quality attributes (CQAs), critical process parameters (CPPs), and their relationships. For high-potency APIs, these considerations are amplified by safety requirements related to containment and cross-contamination prevention.
Regulatory bodies increasingly recognize the advantages of continuous manufacturing for consistent quality and reduced variability. The FDA's Emerging Technology Program and similar initiatives by other agencies provide pathways for manufacturers to engage with regulators early in development. This collaborative approach helps address compliance challenges proactively rather than reactively.
Data integrity presents another significant regulatory consideration. Continuous processes generate substantial volumes of data that must be managed according to ALCOA+ principles (Attributable, Legible, Contemporaneous, Original, Accurate, plus Complete, Consistent, Enduring, and Available). Automated systems must be validated according to GAMP 5 guidelines, with appropriate audit trails and data security measures.
Change management protocols are essential when implementing continuous manufacturing for high-potency APIs. Regulatory filings must clearly document process changes, control strategies, and comparability assessments between batch and continuous processes. Post-approval change management protocols (PACMPs) can facilitate smoother regulatory pathways for future modifications.
Environmental and occupational safety regulations add another layer of compliance requirements. High-potency APIs require specialized containment strategies, with continuous manufacturing potentially offering advantages through closed systems that minimize operator exposure and environmental release.
Scale-up Considerations and Process Economics
The scale-up of continuous flow synthesis for high-potency APIs presents unique economic and technical challenges compared to traditional batch processes. When transitioning from laboratory to production scale, maintaining consistent flow dynamics becomes critical. The Reynolds number must be preserved across different reactor dimensions to ensure similar mixing patterns and heat transfer characteristics, which directly impacts reaction efficiency and product quality.
Equipment selection represents a significant capital investment consideration. While microreactors offer excellent heat and mass transfer at lab scale, they may face throughput limitations in commercial production. Meso-scale flow reactors often provide the optimal balance between transfer efficiency and production capacity. The modular nature of flow systems allows for "numbering up" rather than traditional "scaling up," reducing scale-up risks but potentially increasing equipment costs.
Material compatibility becomes increasingly important at larger scales, particularly with corrosive reagents or high-pressure conditions. Advanced materials like Hastelloy or silicon carbide may be necessary, substantially affecting capital expenditure calculations. Additionally, control systems complexity increases with scale, requiring sophisticated process analytical technology (PAT) integration for real-time monitoring and control.
From an economic perspective, continuous flow synthesis of high-potency APIs typically demonstrates superior cost efficiency despite higher initial capital investment. Operating costs benefit from reduced solvent usage (typically 20-50% less than batch processes), improved yield consistency, and minimized waste generation. Energy efficiency gains of 30-40% are commonly reported due to better heat transfer characteristics and the elimination of heating/cooling cycles inherent to batch operations.
The economic analysis must account for regulatory considerations specific to continuous manufacturing. While regulatory bodies increasingly support continuous processing, validation requirements may differ from traditional approaches. The ability to implement Quality by Design (QbD) principles through continuous monitoring represents both a regulatory advantage and an economic benefit through reduced testing and release times.
Risk assessment for scale-up must evaluate supply chain resilience, as continuous processes require uninterrupted material flow. Implementing buffer systems and redundant critical components adds cost but mitigates production interruption risks. The economic model should incorporate these reliability factors alongside traditional metrics like net present value (NPV) and return on investment (ROI).
Ultimately, successful scale-up of continuous flow synthesis for high-potency APIs requires balancing technical parameters with economic considerations, creating a robust process that maintains the safety and quality advantages of continuous manufacturing while achieving commercial viability.
Equipment selection represents a significant capital investment consideration. While microreactors offer excellent heat and mass transfer at lab scale, they may face throughput limitations in commercial production. Meso-scale flow reactors often provide the optimal balance between transfer efficiency and production capacity. The modular nature of flow systems allows for "numbering up" rather than traditional "scaling up," reducing scale-up risks but potentially increasing equipment costs.
Material compatibility becomes increasingly important at larger scales, particularly with corrosive reagents or high-pressure conditions. Advanced materials like Hastelloy or silicon carbide may be necessary, substantially affecting capital expenditure calculations. Additionally, control systems complexity increases with scale, requiring sophisticated process analytical technology (PAT) integration for real-time monitoring and control.
From an economic perspective, continuous flow synthesis of high-potency APIs typically demonstrates superior cost efficiency despite higher initial capital investment. Operating costs benefit from reduced solvent usage (typically 20-50% less than batch processes), improved yield consistency, and minimized waste generation. Energy efficiency gains of 30-40% are commonly reported due to better heat transfer characteristics and the elimination of heating/cooling cycles inherent to batch operations.
The economic analysis must account for regulatory considerations specific to continuous manufacturing. While regulatory bodies increasingly support continuous processing, validation requirements may differ from traditional approaches. The ability to implement Quality by Design (QbD) principles through continuous monitoring represents both a regulatory advantage and an economic benefit through reduced testing and release times.
Risk assessment for scale-up must evaluate supply chain resilience, as continuous processes require uninterrupted material flow. Implementing buffer systems and redundant critical components adds cost but mitigates production interruption risks. The economic model should incorporate these reliability factors alongside traditional metrics like net present value (NPV) and return on investment (ROI).
Ultimately, successful scale-up of continuous flow synthesis for high-potency APIs requires balancing technical parameters with economic considerations, creating a robust process that maintains the safety and quality advantages of continuous manufacturing while achieving commercial viability.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!