Switching From Batch To Continuous For Multistep Synthesis: Roadmap
SEP 3, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Continuous Flow Synthesis Background and Objectives
Continuous flow synthesis represents a paradigm shift in chemical manufacturing, evolving from traditional batch processes that have dominated the industry for centuries. This technological approach enables reactions to occur in a flowing stream rather than in stationary vessels, offering unprecedented control over reaction parameters. The evolution of continuous flow technology can be traced back to the early 2000s when researchers began exploring microreactor technology for pharmaceutical applications, but its roots extend to earlier industrial continuous processes in petrochemical industries.
The trajectory of continuous flow synthesis has accelerated significantly in the past decade, driven by advances in microfluidic technology, automation systems, and real-time analytical methods. This convergence of technologies has transformed continuous flow from an academic curiosity to a viable industrial solution for complex multistep syntheses.
The primary objective of transitioning from batch to continuous processing for multistep synthesis is to enhance manufacturing efficiency while maintaining or improving product quality. Continuous flow systems offer precise control over reaction parameters such as temperature, pressure, and mixing, which can lead to improved yield, selectivity, and reproducibility. Additionally, the reduced reactor volumes inherent to flow systems minimize safety risks associated with handling hazardous materials.
Another critical goal is to enable more sustainable chemical manufacturing processes. Continuous flow systems typically require less solvent, generate less waste, and consume less energy compared to traditional batch processes. This alignment with green chemistry principles makes continuous flow an attractive option for industries facing increasing regulatory and market pressure to reduce environmental impact.
The technology aims to address the scalability challenges that often plague pharmaceutical and fine chemical manufacturing. Rather than traditional scale-up, continuous flow enables "scale-out" or "numbering up" approaches, where production capacity is increased by adding parallel processing units rather than designing larger reactors. This approach potentially reduces the time and cost associated with transferring processes from laboratory to commercial scale.
Looking forward, the field is moving toward fully integrated continuous manufacturing platforms capable of executing complex multistep syntheses with minimal human intervention. These systems incorporate in-line purification, real-time monitoring, and feedback control mechanisms to ensure consistent product quality. The ultimate vision is to develop modular, reconfigurable platforms that can rapidly adapt to produce different chemical entities, enabling more agile and responsive manufacturing capabilities.
The trajectory of continuous flow synthesis has accelerated significantly in the past decade, driven by advances in microfluidic technology, automation systems, and real-time analytical methods. This convergence of technologies has transformed continuous flow from an academic curiosity to a viable industrial solution for complex multistep syntheses.
The primary objective of transitioning from batch to continuous processing for multistep synthesis is to enhance manufacturing efficiency while maintaining or improving product quality. Continuous flow systems offer precise control over reaction parameters such as temperature, pressure, and mixing, which can lead to improved yield, selectivity, and reproducibility. Additionally, the reduced reactor volumes inherent to flow systems minimize safety risks associated with handling hazardous materials.
Another critical goal is to enable more sustainable chemical manufacturing processes. Continuous flow systems typically require less solvent, generate less waste, and consume less energy compared to traditional batch processes. This alignment with green chemistry principles makes continuous flow an attractive option for industries facing increasing regulatory and market pressure to reduce environmental impact.
The technology aims to address the scalability challenges that often plague pharmaceutical and fine chemical manufacturing. Rather than traditional scale-up, continuous flow enables "scale-out" or "numbering up" approaches, where production capacity is increased by adding parallel processing units rather than designing larger reactors. This approach potentially reduces the time and cost associated with transferring processes from laboratory to commercial scale.
Looking forward, the field is moving toward fully integrated continuous manufacturing platforms capable of executing complex multistep syntheses with minimal human intervention. These systems incorporate in-line purification, real-time monitoring, and feedback control mechanisms to ensure consistent product quality. The ultimate vision is to develop modular, reconfigurable platforms that can rapidly adapt to produce different chemical entities, enabling more agile and responsive manufacturing capabilities.
Market Demand Analysis for Continuous Manufacturing
The pharmaceutical industry is witnessing a significant shift from traditional batch manufacturing to continuous manufacturing processes, driven by increasing demand for more efficient, cost-effective, and sustainable production methods. Market research indicates that the global continuous manufacturing market in pharmaceuticals is projected to grow substantially, with estimates suggesting a compound annual growth rate of over 13% through 2028. This growth is primarily fueled by the industry's need to reduce production costs while maintaining high-quality standards.
Healthcare providers and patients are demanding more affordable medications, putting pressure on pharmaceutical companies to optimize their manufacturing processes. Continuous manufacturing offers a solution by reducing production time by up to 90% compared to batch processes, significantly decreasing the time-to-market for new drugs. This efficiency gain translates directly to cost savings, with some companies reporting reductions in manufacturing costs by 15-30%.
Regulatory bodies, including the FDA and EMA, have shown strong support for continuous manufacturing adoption, recognizing its potential to enhance product quality and consistency. This regulatory endorsement has created a favorable market environment, encouraging pharmaceutical companies to invest in continuous manufacturing technologies. The FDA's Emerging Technology Program specifically facilitates the implementation of innovative approaches like continuous manufacturing.
Contract manufacturing organizations (CMOs) are experiencing increased demand for continuous manufacturing capabilities, as pharmaceutical companies seek partners with advanced technological expertise. This trend is particularly evident in the production of high-value, low-volume drugs where production efficiency is crucial for maintaining competitive pricing.
Geographically, North America currently leads the continuous manufacturing market, followed by Europe. However, Asia-Pacific regions are showing the fastest growth rates, driven by the expansion of pharmaceutical manufacturing in countries like China and India. These emerging markets are increasingly adopting continuous manufacturing to meet international quality standards and compete globally.
The market for equipment and technologies supporting continuous manufacturing is also expanding rapidly. Vendors offering integrated solutions that combine process analytical technology (PAT) with continuous manufacturing systems are seeing particularly strong demand. This integration enables real-time quality monitoring, which is essential for regulatory compliance and process optimization.
Environmental considerations are becoming increasingly important market drivers. Continuous manufacturing typically requires smaller facility footprints, consumes less energy, and generates less waste compared to batch processes. These sustainability benefits align with corporate environmental goals and regulatory requirements, further stimulating market demand.
Healthcare providers and patients are demanding more affordable medications, putting pressure on pharmaceutical companies to optimize their manufacturing processes. Continuous manufacturing offers a solution by reducing production time by up to 90% compared to batch processes, significantly decreasing the time-to-market for new drugs. This efficiency gain translates directly to cost savings, with some companies reporting reductions in manufacturing costs by 15-30%.
Regulatory bodies, including the FDA and EMA, have shown strong support for continuous manufacturing adoption, recognizing its potential to enhance product quality and consistency. This regulatory endorsement has created a favorable market environment, encouraging pharmaceutical companies to invest in continuous manufacturing technologies. The FDA's Emerging Technology Program specifically facilitates the implementation of innovative approaches like continuous manufacturing.
Contract manufacturing organizations (CMOs) are experiencing increased demand for continuous manufacturing capabilities, as pharmaceutical companies seek partners with advanced technological expertise. This trend is particularly evident in the production of high-value, low-volume drugs where production efficiency is crucial for maintaining competitive pricing.
Geographically, North America currently leads the continuous manufacturing market, followed by Europe. However, Asia-Pacific regions are showing the fastest growth rates, driven by the expansion of pharmaceutical manufacturing in countries like China and India. These emerging markets are increasingly adopting continuous manufacturing to meet international quality standards and compete globally.
The market for equipment and technologies supporting continuous manufacturing is also expanding rapidly. Vendors offering integrated solutions that combine process analytical technology (PAT) with continuous manufacturing systems are seeing particularly strong demand. This integration enables real-time quality monitoring, which is essential for regulatory compliance and process optimization.
Environmental considerations are becoming increasingly important market drivers. Continuous manufacturing typically requires smaller facility footprints, consumes less energy, and generates less waste compared to batch processes. These sustainability benefits align with corporate environmental goals and regulatory requirements, further stimulating market demand.
Technical Barriers in Batch-to-Continuous Transition
The transition from batch to continuous processing in multistep synthesis faces significant technical barriers that must be addressed for successful implementation. One of the primary challenges is the integration of multiple reaction steps with different kinetics and conditions into a seamless continuous flow system. Traditional batch processes often involve intermediate isolation, purification, and characterization steps that are difficult to translate directly into continuous operations without substantial process redesign.
Equipment compatibility presents another major obstacle. Many existing manufacturing facilities are designed specifically for batch operations, with reactors, separation units, and control systems optimized for discrete processing. Retrofitting these facilities for continuous operation requires significant capital investment and engineering expertise, creating economic barriers to adoption, particularly for smaller manufacturers or those with limited resources.
Reaction control and monitoring in continuous systems demand more sophisticated approaches than batch counterparts. In continuous processing, real-time analytical techniques must be implemented to ensure consistent product quality and process stability. The development of robust Process Analytical Technology (PAT) tools capable of providing immediate feedback for process control remains challenging, especially for complex multistep syntheses involving multiple phase transitions or heterogeneous reactions.
Heat and mass transfer limitations constitute significant technical hurdles. While continuous flow systems generally offer improved heat transfer characteristics due to higher surface-to-volume ratios, certain reactions with extreme exothermic or endothermic profiles may still present challenges. Similarly, mass transfer limitations in multiphase reactions can lead to reduced efficiency or incomplete conversions in continuous systems if not properly addressed through specialized reactor designs.
Solid handling represents one of the most formidable barriers in continuous processing. Many chemical syntheses involve solid intermediates, catalysts, or products that can cause clogging, fouling, or uneven flow in continuous systems. Developing reliable methods for continuous crystallization, filtration, and solids transport remains an active area of research with significant technical challenges.
Scale-up considerations add another layer of complexity. While batch processes follow relatively straightforward scale-up principles based on geometric similarity, continuous processes often require different approaches focused on maintaining residence time distributions, mixing patterns, and heat transfer characteristics across scales. This necessitates sophisticated modeling and simulation capabilities that may not be readily available to all organizations.
Regulatory compliance and validation present additional barriers, particularly in highly regulated industries like pharmaceuticals. Continuous manufacturing requires different validation approaches and control strategies compared to batch processing, and regulatory frameworks are still evolving to accommodate these differences.
Equipment compatibility presents another major obstacle. Many existing manufacturing facilities are designed specifically for batch operations, with reactors, separation units, and control systems optimized for discrete processing. Retrofitting these facilities for continuous operation requires significant capital investment and engineering expertise, creating economic barriers to adoption, particularly for smaller manufacturers or those with limited resources.
Reaction control and monitoring in continuous systems demand more sophisticated approaches than batch counterparts. In continuous processing, real-time analytical techniques must be implemented to ensure consistent product quality and process stability. The development of robust Process Analytical Technology (PAT) tools capable of providing immediate feedback for process control remains challenging, especially for complex multistep syntheses involving multiple phase transitions or heterogeneous reactions.
Heat and mass transfer limitations constitute significant technical hurdles. While continuous flow systems generally offer improved heat transfer characteristics due to higher surface-to-volume ratios, certain reactions with extreme exothermic or endothermic profiles may still present challenges. Similarly, mass transfer limitations in multiphase reactions can lead to reduced efficiency or incomplete conversions in continuous systems if not properly addressed through specialized reactor designs.
Solid handling represents one of the most formidable barriers in continuous processing. Many chemical syntheses involve solid intermediates, catalysts, or products that can cause clogging, fouling, or uneven flow in continuous systems. Developing reliable methods for continuous crystallization, filtration, and solids transport remains an active area of research with significant technical challenges.
Scale-up considerations add another layer of complexity. While batch processes follow relatively straightforward scale-up principles based on geometric similarity, continuous processes often require different approaches focused on maintaining residence time distributions, mixing patterns, and heat transfer characteristics across scales. This necessitates sophisticated modeling and simulation capabilities that may not be readily available to all organizations.
Regulatory compliance and validation present additional barriers, particularly in highly regulated industries like pharmaceuticals. Continuous manufacturing requires different validation approaches and control strategies compared to batch processing, and regulatory frameworks are still evolving to accommodate these differences.
Current Batch-to-Continuous Conversion Methodologies
01 Flow chemistry for continuous multistep synthesis
Flow chemistry techniques enable continuous processing of multistep chemical syntheses by connecting reaction steps in sequence without isolation of intermediates. This approach improves process efficiency through better heat and mass transfer, precise residence time control, and reduced reaction volumes. Flow reactors can be designed with integrated mixing, heating/cooling, and monitoring capabilities to optimize reaction conditions in real-time, resulting in higher yields and product quality while reducing waste generation.- Flow chemistry for continuous multistep synthesis: Flow chemistry techniques enable continuous processing of chemical reactions in a series of connected reactors, allowing for efficient multistep synthesis without isolation of intermediates. This approach provides better control over reaction parameters, improved heat and mass transfer, and reduced reaction times compared to batch processes. The continuous flow systems can be integrated with in-line monitoring and automated control systems to optimize reaction conditions in real-time, leading to higher yields and process efficiency.
- Process intensification and integration strategies: Process intensification combines multiple unit operations into fewer, more efficient steps to enhance overall manufacturing efficiency. This includes techniques such as reactive distillation, membrane reactors, and microreactor technology that integrate reaction and separation processes. By reducing the number of discrete steps and equipment pieces, these approaches minimize energy consumption, reduce waste generation, and improve space utilization while maintaining or enhancing product quality and yield in multistep synthesis.
- Real-time monitoring and process analytical technology: Implementation of real-time monitoring systems and process analytical technology (PAT) enables continuous assessment of critical process parameters during multistep synthesis. These technologies allow for immediate detection of deviations from optimal conditions and facilitate automated adjustments to maintain product quality. Spectroscopic methods, such as near-infrared and Raman spectroscopy, coupled with advanced data analytics, provide insights into reaction progress without interrupting the manufacturing process, thereby improving efficiency and consistency.
- Modular and reconfigurable manufacturing systems: Modular manufacturing platforms offer flexibility in process design and operation for multistep synthesis. These systems consist of standardized, interchangeable process units that can be rapidly reconfigured to accommodate different synthesis routes or product specifications. The modular approach facilitates scale-up, technology transfer, and implementation of process improvements without significant downtime. This versatility is particularly valuable for pharmaceutical and fine chemical manufacturing where product portfolios and market demands frequently change.
- Green chemistry principles for sustainable continuous processing: Integration of green chemistry principles into continuous manufacturing processes enhances sustainability and efficiency in multistep synthesis. These approaches include solvent-free or reduced-solvent reactions, use of renewable feedstocks, catalytic instead of stoichiometric reagents, and energy-efficient reaction conditions. Continuous processing inherently supports these principles by enabling precise control over reaction parameters, minimizing waste generation, and reducing energy consumption compared to traditional batch methods, while maintaining or improving product quality and process economics.
02 Process intensification and integration strategies
Process intensification combines multiple unit operations into fewer, more efficient steps to enhance overall manufacturing efficiency. This includes telescoping reactions (conducting sequential reactions without intermediate isolation), using multifunctional catalysts, and implementing hybrid separation-reaction systems. Integration strategies focus on connecting process steps seamlessly, optimizing material flows, and reducing downtime between operations, which collectively minimize waste, energy consumption, and production time while maximizing resource utilization.Expand Specific Solutions03 Advanced control systems and process automation
Implementation of advanced control systems and automation technologies enables real-time monitoring and adjustment of continuous manufacturing processes. These systems utilize sensors, data analytics, and machine learning algorithms to maintain optimal reaction conditions, detect deviations, and make automatic adjustments. Process analytical technology (PAT) provides continuous quality verification rather than end-point testing, while digital twins and simulation models help predict process behavior and optimize operating parameters, resulting in improved consistency and reduced variability.Expand Specific Solutions04 Novel reactor designs for multistep synthesis
Specialized reactor designs facilitate efficient multistep synthesis in continuous manufacturing. These include microreactors with enhanced mixing and heat transfer capabilities, modular reactor systems that can be reconfigured for different synthesis pathways, and cascade reactor arrangements that accommodate sequential transformations. Some designs incorporate in-line purification, separation, or workup steps between reaction zones, while others feature compartmentalized reaction environments with controlled interfaces for incompatible reaction conditions, enabling more complex syntheses to be performed continuously.Expand Specific Solutions05 Sustainable and green chemistry approaches
Continuous manufacturing processes for multistep synthesis can incorporate sustainable chemistry principles to improve efficiency while reducing environmental impact. These approaches include solvent-free or reduced-solvent reactions, use of renewable feedstocks, catalytic processes that minimize waste generation, and energy-efficient operations. Continuous processing inherently supports green chemistry through precise control of reaction parameters, which leads to higher selectivity, fewer side products, and reduced purification requirements. Additionally, process intensification in continuous systems typically results in smaller equipment footprints and lower resource consumption compared to batch processes.Expand Specific Solutions
Key Industry Players in Continuous Flow Chemistry
The continuous manufacturing landscape for multistep synthesis is evolving rapidly, transitioning from early-stage development to commercial implementation. The market is experiencing significant growth, projected to reach several billion dollars by 2025, driven by pharmaceutical and chemical industries seeking improved efficiency and sustainability. Technologically, the field shows varying maturity levels across players: established companies like Bayer AG, Bristol Myers Squibb, and Illumina demonstrate advanced capabilities with commercial implementations; academic institutions including Harvard College and ETH Zurich provide fundamental research breakthroughs; while specialized firms such as Econic Technologies and OPX Biotechnologies focus on niche applications. The integration of digital technologies by companies like Sartorius Stedim Data Analytics is accelerating development, though regulatory frameworks and scale-up challenges remain significant barriers to widespread adoption.
President & Fellows of Harvard College
Technical Solution: Harvard College has pioneered continuous flow synthesis technologies through their development of integrated microfluidic systems for multistep organic synthesis. Their approach involves miniaturized flow reactors that enable precise control over reaction parameters including temperature, pressure, and residence time. Harvard researchers have developed modular flow chemistry platforms that can perform sequential reactions without intermediate purification steps, significantly improving efficiency in multistep synthesis. Their systems incorporate in-line analytical monitoring (using spectroscopic techniques like IR, UV, and NMR) that provides real-time reaction feedback, allowing for automated optimization and quality control. Harvard has also developed machine learning algorithms that can predict optimal flow conditions based on reaction kinetics data, reducing the time required for process development from weeks to days.
Strengths: Superior reaction control with enhanced safety profiles for hazardous reagents; significant reduction in solvent usage (up to 90% in some processes); ability to rapidly scale processes from lab to production without extensive reoptimization. Weaknesses: Higher initial capital investment compared to batch systems; requires specialized expertise in flow chemistry; some reactions with long residence times remain challenging to implement.
Evonik Operations GmbH
Technical Solution: Evonik has developed an advanced continuous flow manufacturing platform called "Continuous Process Excellence" specifically designed for multistep chemical synthesis. Their technology integrates multiple reaction modules with automated control systems that enable seamless transition between synthesis steps. Evonik's approach utilizes specialized reactor designs including microreactors, tube reactors, and continuous stirred tank reactors (CSTRs) that can be configured in series to accommodate different reaction requirements within a single production line. Their systems incorporate proprietary mixing technologies that achieve millisecond mixing times, enabling precise control over highly exothermic reactions and improving selectivity. Evonik has successfully implemented continuous crystallization processes that integrate directly with their synthesis modules, eliminating batch crystallization bottlenecks. The company has demonstrated up to 30% yield improvements in pharmaceutical intermediate production compared to traditional batch processes through better control of reaction parameters and reduced side reactions.
Strengths: Modular design allows flexible configuration for different synthesis routes; demonstrated success in industrial-scale implementation; significant improvements in product quality consistency with reduced batch-to-batch variation. Weaknesses: Higher complexity in system design and maintenance; challenges in handling solids and highly viscous materials in continuous flow; requires significant process redesign when converting existing batch processes.
Critical Process Parameters and Control Strategies
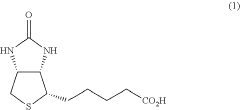
Full continuous-flow preparation method of (+)-biotin
PatentPendingUS20230183260A1
Innovation
- A full continuous-flow preparation method using a multi-stage micro-reaction system involving asymmetric ring-opening, reduction, cyclization, sulfenylation, Fukuyama coupling, and hydrolysis reactions in micro-channel reactors to produce (+)-biotin, optimizing reaction conditions and reagents for high yield and safety.
Convertible batch/continuous reactor and use of the same
PatentInactiveEP1889659B1
Innovation
- A convertible reactor design that operates both in batch and continuous modes, featuring a specific geometry with a mixture volume, straight vertical sections, and a separation control volume, allowing for phase separation while maintaining contact between phases, and is suitable for reactions where reagents are extracted into product phases, enabling efficient conversion and decantation.
Scale-up Considerations and Equipment Requirements
Scaling up continuous multistep synthesis processes requires careful consideration of equipment selection and operational parameters to maintain process efficiency and product quality. When transitioning from batch to continuous manufacturing, organizations must evaluate their existing infrastructure against the specialized requirements of continuous flow systems.
The equipment needed for continuous multistep synthesis differs significantly from batch processing equipment. Flow reactors, typically constructed from materials like stainless steel, glass, or specialized polymers, must withstand specific chemical environments while providing excellent heat transfer capabilities. Microreactors and tubular reactors represent common choices, with selection depending on reaction kinetics, mixing requirements, and throughput targets.
Pumping systems constitute another critical component, with high-precision pumps necessary to ensure consistent flow rates across all synthesis stages. HPLC pumps, syringe pumps, and peristaltic pumps each offer distinct advantages depending on the viscosity of process fluids and required flow precision. Organizations must evaluate pump durability under continuous operation conditions, as equipment reliability becomes paramount when processes run uninterrupted for extended periods.
Heat management systems require particular attention during scale-up. Continuous processes generate heat differently than batch operations, necessitating efficient temperature control mechanisms. Plate heat exchangers, jacketed reactors, and microwave heating systems may be employed depending on reaction exothermicity and temperature sensitivity. The selection must account for heat transfer efficiency at increased throughput levels.
Inline monitoring and analytical equipment represent a significant investment but are essential for maintaining process control. Real-time analysis using spectroscopic methods (NIR, Raman, UV-Vis) enables immediate detection of process deviations, while pressure sensors and flow meters provide critical operational data. The integration of these monitoring systems with process control software creates opportunities for automated adjustments and quality assurance.
Space requirements also differ substantially between batch and continuous operations. While continuous systems generally require less physical space per production volume, they demand more sophisticated facility design to accommodate uninterrupted material flow. Considerations include strategic placement of feed vessels, appropriate spacing for maintenance access, and integration with downstream processing equipment.
Capital expenditure planning must account for both initial equipment costs and long-term operational considerations. Though continuous equipment often carries higher upfront costs, organizations should evaluate total cost of ownership, including reduced labor requirements, improved yield consistency, and energy efficiency gains that typically favor continuous processing at scale.
The equipment needed for continuous multistep synthesis differs significantly from batch processing equipment. Flow reactors, typically constructed from materials like stainless steel, glass, or specialized polymers, must withstand specific chemical environments while providing excellent heat transfer capabilities. Microreactors and tubular reactors represent common choices, with selection depending on reaction kinetics, mixing requirements, and throughput targets.
Pumping systems constitute another critical component, with high-precision pumps necessary to ensure consistent flow rates across all synthesis stages. HPLC pumps, syringe pumps, and peristaltic pumps each offer distinct advantages depending on the viscosity of process fluids and required flow precision. Organizations must evaluate pump durability under continuous operation conditions, as equipment reliability becomes paramount when processes run uninterrupted for extended periods.
Heat management systems require particular attention during scale-up. Continuous processes generate heat differently than batch operations, necessitating efficient temperature control mechanisms. Plate heat exchangers, jacketed reactors, and microwave heating systems may be employed depending on reaction exothermicity and temperature sensitivity. The selection must account for heat transfer efficiency at increased throughput levels.
Inline monitoring and analytical equipment represent a significant investment but are essential for maintaining process control. Real-time analysis using spectroscopic methods (NIR, Raman, UV-Vis) enables immediate detection of process deviations, while pressure sensors and flow meters provide critical operational data. The integration of these monitoring systems with process control software creates opportunities for automated adjustments and quality assurance.
Space requirements also differ substantially between batch and continuous operations. While continuous systems generally require less physical space per production volume, they demand more sophisticated facility design to accommodate uninterrupted material flow. Considerations include strategic placement of feed vessels, appropriate spacing for maintenance access, and integration with downstream processing equipment.
Capital expenditure planning must account for both initial equipment costs and long-term operational considerations. Though continuous equipment often carries higher upfront costs, organizations should evaluate total cost of ownership, including reduced labor requirements, improved yield consistency, and energy efficiency gains that typically favor continuous processing at scale.
Regulatory Compliance for Continuous Manufacturing
The transition from batch to continuous manufacturing in pharmaceutical and chemical industries necessitates comprehensive understanding of regulatory frameworks. Regulatory bodies worldwide have been adapting their guidelines to accommodate continuous manufacturing technologies while ensuring product quality and safety remain paramount.
The U.S. Food and Drug Administration (FDA) has been at the forefront of supporting continuous manufacturing adoption through initiatives like the Emerging Technology Program. This program provides a pathway for companies to engage with regulators early in the development process, addressing potential compliance issues before formal submission. The FDA's 2019 guidance document specifically addresses quality considerations for continuous manufacturing, outlining expectations for process validation, control strategies, and real-time release testing.
Similarly, the European Medicines Agency (EMA) has developed frameworks supporting continuous manufacturing implementation. Their guidelines emphasize the importance of process analytical technology (PAT) integration and quality-by-design principles when transitioning from batch to continuous processes. The International Council for Harmonisation (ICH) guidelines, particularly ICH Q8-Q11, provide additional regulatory context applicable to continuous manufacturing systems.
Regulatory compliance for continuous manufacturing requires robust demonstration of process understanding and control. Companies must establish clear definitions of critical quality attributes (CQAs) and critical process parameters (CPPs), supported by appropriate control strategies. The concept of a "state of control" becomes particularly important, requiring manufacturers to demonstrate consistent product quality throughout extended production runs.
Validation approaches differ significantly between batch and continuous processes. While batch validation typically focuses on discrete lots, continuous process validation requires demonstrating consistent quality across the manufacturing timeline. This includes validation of start-up and shutdown procedures, which present unique challenges in continuous operations. Regulatory expectations include comprehensive process monitoring strategies with defined sampling plans and statistical approaches for trend analysis.
Data integrity and management represent another critical regulatory consideration. The large volumes of real-time data generated by continuous manufacturing systems require robust data handling protocols, secure storage solutions, and appropriate audit trail mechanisms. Regulatory agencies expect manufacturers to implement systems capable of detecting process deviations promptly and initiating appropriate corrective actions.
Global regulatory harmonization remains an ongoing challenge. Companies implementing continuous manufacturing for products intended for multiple markets must navigate varying regulatory expectations. Industry consortia and regulatory science initiatives are working to develop standardized approaches and shared understanding of continuous manufacturing requirements across different jurisdictions, facilitating more streamlined global implementation.
The U.S. Food and Drug Administration (FDA) has been at the forefront of supporting continuous manufacturing adoption through initiatives like the Emerging Technology Program. This program provides a pathway for companies to engage with regulators early in the development process, addressing potential compliance issues before formal submission. The FDA's 2019 guidance document specifically addresses quality considerations for continuous manufacturing, outlining expectations for process validation, control strategies, and real-time release testing.
Similarly, the European Medicines Agency (EMA) has developed frameworks supporting continuous manufacturing implementation. Their guidelines emphasize the importance of process analytical technology (PAT) integration and quality-by-design principles when transitioning from batch to continuous processes. The International Council for Harmonisation (ICH) guidelines, particularly ICH Q8-Q11, provide additional regulatory context applicable to continuous manufacturing systems.
Regulatory compliance for continuous manufacturing requires robust demonstration of process understanding and control. Companies must establish clear definitions of critical quality attributes (CQAs) and critical process parameters (CPPs), supported by appropriate control strategies. The concept of a "state of control" becomes particularly important, requiring manufacturers to demonstrate consistent product quality throughout extended production runs.
Validation approaches differ significantly between batch and continuous processes. While batch validation typically focuses on discrete lots, continuous process validation requires demonstrating consistent quality across the manufacturing timeline. This includes validation of start-up and shutdown procedures, which present unique challenges in continuous operations. Regulatory expectations include comprehensive process monitoring strategies with defined sampling plans and statistical approaches for trend analysis.
Data integrity and management represent another critical regulatory consideration. The large volumes of real-time data generated by continuous manufacturing systems require robust data handling protocols, secure storage solutions, and appropriate audit trail mechanisms. Regulatory agencies expect manufacturers to implement systems capable of detecting process deviations promptly and initiating appropriate corrective actions.
Global regulatory harmonization remains an ongoing challenge. Companies implementing continuous manufacturing for products intended for multiple markets must navigate varying regulatory expectations. Industry consortia and regulatory science initiatives are working to develop standardized approaches and shared understanding of continuous manufacturing requirements across different jurisdictions, facilitating more streamlined global implementation.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!