Continuous Flow Microreactor Design Principles For API Synthesis
SEP 3, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Microreactor Technology Evolution and Objectives
Microreactor technology has evolved significantly over the past three decades, transforming from laboratory curiosities to essential tools in pharmaceutical manufacturing. The initial development in the 1990s focused primarily on proof-of-concept designs with simple channel structures and limited material options. These early microreactors demonstrated enhanced mixing and heat transfer capabilities but lacked the sophistication required for complex API synthesis.
The early 2000s marked a pivotal transition as researchers began exploring more advanced fabrication techniques, including photolithography, laser ablation, and precision CNC machining. This period saw the introduction of multi-layer designs and the first integrated sensors, enabling real-time monitoring of reaction parameters. Material science advancements expanded options beyond glass and silicon to include various polymers, metals, and ceramics, each offering specific advantages for different reaction conditions.
By 2010, microreactor technology had matured significantly with the development of modular systems that could be reconfigured for different synthesis pathways. The integration of automation and digital control systems represented another major advancement, allowing for precise manipulation of reaction parameters and the implementation of feedback control loops. These developments dramatically improved reproducibility and process reliability, critical factors for pharmaceutical manufacturing.
Recent years have witnessed the emergence of 3D-printed microreactors, offering unprecedented geometric complexity and customization possibilities. Parallel processing architectures have been developed to address throughput limitations, while advances in surface functionalization have enabled better control of catalyst immobilization and fouling prevention. The integration of artificial intelligence and machine learning algorithms has further enhanced process optimization capabilities.
The primary objectives driving microreactor development for API synthesis include intensifying production processes to reduce equipment footprint while maintaining or increasing throughput. Enhancing reaction selectivity and yield through superior mixing and precise temperature control remains a central goal. Improving safety profiles by minimizing reagent volumes and containing hazardous intermediates has become increasingly important in pharmaceutical manufacturing contexts.
Additional objectives include reducing time-to-market through rapid process development and scale-up, as well as enabling continuous manufacturing paradigms that align with regulatory initiatives like FDA's Quality by Design approach. Environmental sustainability goals are driving research into more energy-efficient designs and reduced solvent usage. The ultimate vision is to develop plug-and-play microreactor platforms that can rapidly adapt to different API synthesis requirements, supporting the pharmaceutical industry's need for agility in responding to market demands.
The early 2000s marked a pivotal transition as researchers began exploring more advanced fabrication techniques, including photolithography, laser ablation, and precision CNC machining. This period saw the introduction of multi-layer designs and the first integrated sensors, enabling real-time monitoring of reaction parameters. Material science advancements expanded options beyond glass and silicon to include various polymers, metals, and ceramics, each offering specific advantages for different reaction conditions.
By 2010, microreactor technology had matured significantly with the development of modular systems that could be reconfigured for different synthesis pathways. The integration of automation and digital control systems represented another major advancement, allowing for precise manipulation of reaction parameters and the implementation of feedback control loops. These developments dramatically improved reproducibility and process reliability, critical factors for pharmaceutical manufacturing.
Recent years have witnessed the emergence of 3D-printed microreactors, offering unprecedented geometric complexity and customization possibilities. Parallel processing architectures have been developed to address throughput limitations, while advances in surface functionalization have enabled better control of catalyst immobilization and fouling prevention. The integration of artificial intelligence and machine learning algorithms has further enhanced process optimization capabilities.
The primary objectives driving microreactor development for API synthesis include intensifying production processes to reduce equipment footprint while maintaining or increasing throughput. Enhancing reaction selectivity and yield through superior mixing and precise temperature control remains a central goal. Improving safety profiles by minimizing reagent volumes and containing hazardous intermediates has become increasingly important in pharmaceutical manufacturing contexts.
Additional objectives include reducing time-to-market through rapid process development and scale-up, as well as enabling continuous manufacturing paradigms that align with regulatory initiatives like FDA's Quality by Design approach. Environmental sustainability goals are driving research into more energy-efficient designs and reduced solvent usage. The ultimate vision is to develop plug-and-play microreactor platforms that can rapidly adapt to different API synthesis requirements, supporting the pharmaceutical industry's need for agility in responding to market demands.
Market Demand Analysis for Continuous API Manufacturing
The global pharmaceutical industry is experiencing a significant shift towards continuous manufacturing processes, driven by increasing pressure to reduce costs, improve efficiency, and enhance product quality. The market for continuous API (Active Pharmaceutical Ingredient) manufacturing is projected to grow substantially, with estimates suggesting a market value exceeding $2 billion by 2025, representing a compound annual growth rate of approximately 13%.
This growth is primarily fueled by the pharmaceutical industry's need to address several critical challenges. Rising healthcare costs worldwide have intensified scrutiny on drug pricing, compelling manufacturers to seek more cost-effective production methods. Continuous flow microreactor technology offers potential cost reductions of 15-30% compared to traditional batch processing through decreased labor requirements, reduced waste generation, and improved space utilization.
Quality considerations are equally driving market demand. Regulatory bodies, including the FDA and EMA, have actively encouraged the adoption of continuous manufacturing through various initiatives and guidance documents. These agencies recognize continuous processing's superior quality control capabilities, which enable real-time monitoring and adjustment, resulting in more consistent product quality and fewer batch rejections.
Environmental sustainability represents another significant market driver. Continuous API manufacturing typically reduces solvent usage by 50-80% compared to batch processes, while also decreasing energy consumption by 30-50%. This aligns with increasingly stringent environmental regulations and corporate sustainability goals across the pharmaceutical sector.
The COVID-19 pandemic has further accelerated interest in continuous manufacturing technologies, highlighting vulnerabilities in pharmaceutical supply chains. The ability to rapidly scale production, reduce facility footprint, and enable localized manufacturing has become strategically important for ensuring medication availability during global disruptions.
Market segmentation reveals particularly strong demand in oncology, diabetes, and cardiovascular therapeutic areas, where high-value, complex APIs benefit most from continuous processing advantages. Geographically, North America currently leads market adoption, followed by Europe, though Asia-Pacific regions are expected to show the fastest growth rate as manufacturing capabilities expand in countries like India and China.
Contract manufacturing organizations (CMOs) represent a rapidly growing market segment, as they invest in continuous manufacturing capabilities to differentiate their service offerings and attract clients seeking advanced production technologies without capital-intensive in-house investments.
This growth is primarily fueled by the pharmaceutical industry's need to address several critical challenges. Rising healthcare costs worldwide have intensified scrutiny on drug pricing, compelling manufacturers to seek more cost-effective production methods. Continuous flow microreactor technology offers potential cost reductions of 15-30% compared to traditional batch processing through decreased labor requirements, reduced waste generation, and improved space utilization.
Quality considerations are equally driving market demand. Regulatory bodies, including the FDA and EMA, have actively encouraged the adoption of continuous manufacturing through various initiatives and guidance documents. These agencies recognize continuous processing's superior quality control capabilities, which enable real-time monitoring and adjustment, resulting in more consistent product quality and fewer batch rejections.
Environmental sustainability represents another significant market driver. Continuous API manufacturing typically reduces solvent usage by 50-80% compared to batch processes, while also decreasing energy consumption by 30-50%. This aligns with increasingly stringent environmental regulations and corporate sustainability goals across the pharmaceutical sector.
The COVID-19 pandemic has further accelerated interest in continuous manufacturing technologies, highlighting vulnerabilities in pharmaceutical supply chains. The ability to rapidly scale production, reduce facility footprint, and enable localized manufacturing has become strategically important for ensuring medication availability during global disruptions.
Market segmentation reveals particularly strong demand in oncology, diabetes, and cardiovascular therapeutic areas, where high-value, complex APIs benefit most from continuous processing advantages. Geographically, North America currently leads market adoption, followed by Europe, though Asia-Pacific regions are expected to show the fastest growth rate as manufacturing capabilities expand in countries like India and China.
Contract manufacturing organizations (CMOs) represent a rapidly growing market segment, as they invest in continuous manufacturing capabilities to differentiate their service offerings and attract clients seeking advanced production technologies without capital-intensive in-house investments.
Current Challenges in Microreactor Technology for API Synthesis
Despite significant advancements in continuous flow microreactor technology for API synthesis, several critical challenges persist that limit widespread industrial adoption. Scale-up difficulties represent a primary obstacle, as the transition from laboratory-scale microreactors to production-level systems often encounters issues with maintaining consistent flow dynamics, heat transfer efficiency, and mixing characteristics across different scales. This fundamental challenge undermines one of the core advantages of microreactor technology—process consistency regardless of scale.
Material compatibility presents another significant hurdle, particularly when handling corrosive reagents common in API synthesis. Many advanced catalysts and reagents can damage reactor components, leading to contamination issues and reduced reactor lifespan. The pharmaceutical industry's stringent purity requirements make this challenge especially problematic, necessitating the development of more chemically resistant materials that maintain performance under aggressive reaction conditions.
Clogging and fouling remain persistent operational challenges in microreactor systems. The narrow channels that enable excellent heat and mass transfer also make these systems vulnerable to blockages from solid formation, precipitation, or particle accumulation. This is particularly problematic for multiphase reactions involving solids, which are common in API synthesis pathways.
Control system integration represents a technological gap that limits the potential of continuous flow systems. Current monitoring technologies often lack the sensitivity and response time needed for real-time process adjustments, particularly for fast reactions. The integration of advanced sensors capable of providing immediate feedback on reaction parameters within microchannels remains underdeveloped.
Regulatory hurdles constitute a significant non-technical barrier. Pharmaceutical manufacturing is heavily regulated, and continuous processing represents a paradigm shift from traditional batch processing. Regulatory frameworks are still adapting to accommodate continuous manufacturing approaches, creating uncertainty for companies considering technology adoption.
Cost justification challenges persist, particularly for smaller pharmaceutical companies. The initial capital investment for continuous flow systems can be substantial, and demonstrating return on investment requires comprehensive analysis of long-term benefits versus established batch processes. This economic barrier is compounded by the need for specialized expertise to design and operate these advanced systems.
Knowledge gaps in reaction engineering for continuous flow systems further complicate implementation. Many established synthetic routes were developed for batch processes and require significant re-engineering for continuous flow, demanding expertise that bridges chemistry, engineering, and process design—a combination not readily available in many organizations.
Material compatibility presents another significant hurdle, particularly when handling corrosive reagents common in API synthesis. Many advanced catalysts and reagents can damage reactor components, leading to contamination issues and reduced reactor lifespan. The pharmaceutical industry's stringent purity requirements make this challenge especially problematic, necessitating the development of more chemically resistant materials that maintain performance under aggressive reaction conditions.
Clogging and fouling remain persistent operational challenges in microreactor systems. The narrow channels that enable excellent heat and mass transfer also make these systems vulnerable to blockages from solid formation, precipitation, or particle accumulation. This is particularly problematic for multiphase reactions involving solids, which are common in API synthesis pathways.
Control system integration represents a technological gap that limits the potential of continuous flow systems. Current monitoring technologies often lack the sensitivity and response time needed for real-time process adjustments, particularly for fast reactions. The integration of advanced sensors capable of providing immediate feedback on reaction parameters within microchannels remains underdeveloped.
Regulatory hurdles constitute a significant non-technical barrier. Pharmaceutical manufacturing is heavily regulated, and continuous processing represents a paradigm shift from traditional batch processing. Regulatory frameworks are still adapting to accommodate continuous manufacturing approaches, creating uncertainty for companies considering technology adoption.
Cost justification challenges persist, particularly for smaller pharmaceutical companies. The initial capital investment for continuous flow systems can be substantial, and demonstrating return on investment requires comprehensive analysis of long-term benefits versus established batch processes. This economic barrier is compounded by the need for specialized expertise to design and operate these advanced systems.
Knowledge gaps in reaction engineering for continuous flow systems further complicate implementation. Many established synthetic routes were developed for batch processes and require significant re-engineering for continuous flow, demanding expertise that bridges chemistry, engineering, and process design—a combination not readily available in many organizations.
Current Microreactor Design Solutions for API Synthesis
01 Microreactor channel design and geometry
The design of microreactor channels significantly impacts flow characteristics and reaction efficiency. Optimal channel geometries can enhance mixing, heat transfer, and residence time distribution. Various designs include serpentine channels, split-and-recombine structures, and tapered configurations that create controlled turbulence. These geometries can be optimized to prevent clogging, reduce pressure drop, and ensure uniform flow distribution across parallel channels, which is crucial for scaling up microreactor systems.- Microreactor channel design and geometry: The design of microreactor channels significantly impacts flow characteristics and reaction efficiency. Optimal channel geometries can enhance mixing, heat transfer, and residence time distribution. Features such as zigzag patterns, spiral configurations, and varying cross-sectional areas are employed to create controlled turbulence and improve mass transfer. Channel dimensions typically range from tens to hundreds of micrometers, with surface-to-volume ratios carefully calculated to maximize reaction efficiency while minimizing pressure drop.
- Integration of mixing and heat transfer elements: Effective mixing and heat transfer are critical aspects of microreactor design. Various mixing elements such as static mixers, impinging jets, and interdigitated structures can be incorporated to enhance mass transfer without increasing reactor size. Heat transfer elements including embedded cooling channels, thermal jackets, and integrated heat exchangers allow precise temperature control, enabling highly exothermic or endothermic reactions to be conducted safely and efficiently. These elements are designed to maintain isothermal conditions throughout the reaction pathway.
- Materials selection and fabrication techniques: The selection of appropriate materials for microreactor construction depends on chemical compatibility, thermal conductivity, and manufacturing considerations. Common materials include glass, silicon, stainless steel, ceramics, and polymers like PDMS. Advanced fabrication techniques such as photolithography, etching, laser ablation, 3D printing, and micromachining enable the creation of complex microstructures with high precision. Surface modifications can be applied to enhance catalytic activity or prevent fouling during continuous operation.
- Flow control and monitoring systems: Precise control of fluid flow is essential for consistent microreactor performance. This includes the integration of micropumps, valves, pressure regulators, and flow sensors to maintain stable flow rates and residence times. Real-time monitoring systems incorporating optical, electrochemical, or spectroscopic sensors enable continuous analysis of reaction progress and product quality. Advanced control algorithms can adjust process parameters automatically in response to measured variables, ensuring optimal reaction conditions are maintained throughout operation.
- Scale-out strategies and modular design: Rather than traditional scale-up, microreactors employ scale-out strategies where production capacity is increased by operating multiple reactors in parallel. This approach maintains the advantageous characteristics of microscale processing while achieving industrial production volumes. Modular designs facilitate maintenance, allow for flexible reconfiguration of process flows, and enable gradual capacity expansion. Standardized interfaces between modules ensure compatibility and simplify system integration, while distributed control architectures manage the increased complexity of parallelized systems.
02 Integration of mixing and heat transfer elements
Effective mixing and heat transfer are critical aspects of microreactor design. Specialized elements such as micromixers, static mixers, and heat exchange structures can be integrated into the flow path. These elements enable rapid mixing of reactants and precise temperature control, which are essential for reactions with high exothermicity or those requiring specific thermal conditions. Advanced designs incorporate passive mixing structures that function without moving parts, relying instead on flow patterns to achieve efficient mixing.Expand Specific Solutions03 Materials selection and fabrication techniques
The choice of materials for microreactor construction depends on chemical compatibility, thermal conductivity, and mechanical properties. Common materials include glass, silicon, metals, and polymers, each offering different advantages. Fabrication techniques such as photolithography, etching, 3D printing, and precision machining enable the creation of complex microstructures with high precision. The selection of appropriate materials and fabrication methods is crucial for ensuring reactor durability, performance, and cost-effectiveness.Expand Specific Solutions04 Process control and monitoring systems
Advanced control and monitoring systems are essential for optimizing microreactor performance. These systems include integrated sensors for real-time measurement of temperature, pressure, flow rate, and concentration. Feedback control mechanisms allow for precise adjustment of reaction parameters to maintain optimal conditions. Modern microreactor designs incorporate automation and digital control interfaces that enable continuous monitoring and adjustment of process variables, enhancing reproducibility and safety.Expand Specific Solutions05 Scaling and parallelization strategies
Scaling up microreactor technology often involves parallelization rather than increasing reactor dimensions. This approach, known as numbering-up, maintains the advantageous characteristics of microreactors while increasing throughput. Design principles for effective parallelization include ensuring uniform flow distribution, minimizing pressure variations between parallel units, and developing modular systems that can be easily assembled and maintained. These strategies enable the transition from laboratory-scale to industrial-scale production while preserving the benefits of microreactor technology.Expand Specific Solutions
Leading Companies in Continuous Flow Technology
The continuous flow microreactor technology for API synthesis is currently in a growth phase, with increasing adoption across pharmaceutical manufacturing. The market is expanding rapidly, driven by demands for more efficient, sustainable, and cost-effective API production methods. Technologically, the field shows varying maturity levels among key players. Corning, Inc. and Lonza Ltd. demonstrate advanced capabilities with established commercial solutions, while YMC Co., Ltd. and Mitsubishi Gas Chemical have developed specialized microreactor systems. Academic institutions like Zhejiang University and The University of Nottingham contribute significant research innovations. Pharmaceutical companies including Jubilant Pharmova, Aurobindo Pharma, and Jiangsu Hengrui are increasingly implementing this technology to enhance their API manufacturing processes, indicating growing industry acceptance and technological readiness for broader commercial applications.
Corning, Inc.
Technical Solution: Corning has developed the Advanced-Flow™ Reactor (AFR) technology specifically designed for continuous flow chemistry in pharmaceutical API synthesis. Their glass microreactors feature unique heart-shaped channel geometry that creates Dean vortices for enhanced mixing efficiency even at low Reynolds numbers. The reactors incorporate multiple mixing zones with residence time units that allow precise control of reaction parameters. Corning's modular approach enables seamless scale-up from lab to production with flow rates ranging from 1 mL/min to over 100 L/h while maintaining consistent heat and mass transfer characteristics. Their systems include integrated temperature control modules capable of operating between -40°C to +200°C with rapid heating/cooling rates of up to 100°C/min, allowing for precise handling of highly exothermic reactions common in API synthesis.
Strengths: Superior heat transfer capabilities with thermal conductivity 3-5 times higher than metal reactors; excellent chemical compatibility with most solvents and reagents; transparent design allowing visual process monitoring; proven scalability from lab to production. Weaknesses: Higher initial capital investment compared to batch reactors; requires specialized training for operators; potential challenges with solids handling in narrow channels.
YMC Co., Ltd.
Technical Solution: YMC has developed the YMC FlowChem™ system, a comprehensive microreactor platform specifically designed for continuous API synthesis. Their technology employs modular glass and metal microreactors with channel dimensions ranging from 0.5-2.0mm, creating controlled laminar flow patterns with high surface-to-volume ratios (>5,000 m²/m³). This architecture enables exceptional heat transfer capabilities with coefficients exceeding 10 kW/m²K, allowing safe execution of highly exothermic reactions common in pharmaceutical synthesis. YMC's system incorporates proprietary mixing elements that achieve mixing times below 100 milliseconds, critical for controlling selectivity in complex transformations. Their platform features integrated temperature control modules capable of operating between -80°C to +200°C with stability of ±0.5°C, enabling precise handling of temperature-sensitive reactions. YMC has pioneered the integration of continuous separation technologies, including their patented continuous liquid-liquid extraction modules and continuous crystallization units, creating end-to-end solutions for API manufacturing. The system has demonstrated capability for multi-step API synthesis with inline purification, achieving residence times precisely controlled from seconds to hours, with documented improvements in yield (typically 8-15% higher) and purity for several commercial pharmaceutical intermediates.
Strengths: Comprehensive integration of reaction and downstream processing capabilities; excellent chemical compatibility with most pharmaceutical solvents and reagents; proven scalability from laboratory to production; sophisticated control systems with extensive data logging for regulatory compliance. Weaknesses: Higher complexity requiring specialized expertise; significant capital investment for full implementation; potential challenges with highly viscous systems or reactions producing solids.
Key Innovations in Continuous Flow Reactor Engineering
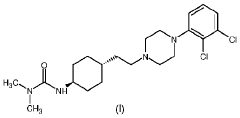
Process for consecutive continuous-flow reductions in the synthesis of medicinally relevant piperazine derivatives using a tubular reactor with alternating diameter
PatentWO2022190047A1
Innovation
- A consecutive continuous-flow process using an alternating diameter flow reactor for selective ester reduction followed by reductive amination with a 5% Pt/C catalyst in a toluene:methanol solvent mixture, optimizing reaction conditions for higher conversions and selectivity.
Regulatory Considerations for Continuous API Manufacturing
The regulatory landscape for continuous API manufacturing represents a critical consideration for pharmaceutical companies transitioning from batch to continuous processing. Regulatory agencies worldwide, including the FDA and EMA, have recognized the potential benefits of continuous manufacturing and have developed frameworks to support its implementation. The FDA's 2019 guidance document "Quality Considerations for Continuous Manufacturing" provides specific recommendations for process validation, control strategy development, and regulatory submissions for continuous manufacturing processes.
Key regulatory challenges include establishing appropriate process analytical technology (PAT) systems that can effectively monitor critical quality attributes in real-time. Regulatory bodies expect robust control strategies that demonstrate how process parameters are monitored and controlled throughout the continuous operation to ensure consistent product quality. This includes clear definitions of the state of control and appropriate responses to deviations from normal operating conditions.
Material traceability presents another significant regulatory consideration. Unlike batch processing, where discrete lots are clearly defined, continuous manufacturing requires alternative approaches to define what constitutes a "batch" or production unit. Manufacturers must implement systems that can track materials through the continuous process and establish clear criteria for product rejection in case of process excursions.
Validation protocols for continuous API manufacturing differ substantially from traditional batch validation approaches. Regulators expect comprehensive process understanding demonstrated through design space development and model-based approaches. This includes validation of residence time distributions, mixing efficiency, and heat transfer characteristics specific to microreactor designs used in API synthesis.
Regulatory submissions for continuous manufacturing processes require enhanced documentation of process development studies, control strategies, and risk assessments. Companies must demonstrate thorough understanding of how microreactor design parameters influence critical quality attributes of the API. This includes detailed characterization of flow patterns, mixing efficiency, and heat transfer capabilities across the operational range.
International harmonization of regulatory requirements remains an ongoing challenge. While major regulatory agencies support continuous manufacturing adoption, specific requirements may vary across jurisdictions. Companies implementing continuous flow microreactors for API synthesis must develop regulatory strategies that address these variations, particularly for global product distribution.
Change management protocols represent another important regulatory consideration. As continuous manufacturing technology evolves, manufacturers need clear pathways for implementing process improvements without extensive revalidation requirements. Regulatory agencies are working to develop frameworks that balance innovation with quality assurance in this rapidly evolving technological landscape.
Key regulatory challenges include establishing appropriate process analytical technology (PAT) systems that can effectively monitor critical quality attributes in real-time. Regulatory bodies expect robust control strategies that demonstrate how process parameters are monitored and controlled throughout the continuous operation to ensure consistent product quality. This includes clear definitions of the state of control and appropriate responses to deviations from normal operating conditions.
Material traceability presents another significant regulatory consideration. Unlike batch processing, where discrete lots are clearly defined, continuous manufacturing requires alternative approaches to define what constitutes a "batch" or production unit. Manufacturers must implement systems that can track materials through the continuous process and establish clear criteria for product rejection in case of process excursions.
Validation protocols for continuous API manufacturing differ substantially from traditional batch validation approaches. Regulators expect comprehensive process understanding demonstrated through design space development and model-based approaches. This includes validation of residence time distributions, mixing efficiency, and heat transfer characteristics specific to microreactor designs used in API synthesis.
Regulatory submissions for continuous manufacturing processes require enhanced documentation of process development studies, control strategies, and risk assessments. Companies must demonstrate thorough understanding of how microreactor design parameters influence critical quality attributes of the API. This includes detailed characterization of flow patterns, mixing efficiency, and heat transfer capabilities across the operational range.
International harmonization of regulatory requirements remains an ongoing challenge. While major regulatory agencies support continuous manufacturing adoption, specific requirements may vary across jurisdictions. Companies implementing continuous flow microreactors for API synthesis must develop regulatory strategies that address these variations, particularly for global product distribution.
Change management protocols represent another important regulatory consideration. As continuous manufacturing technology evolves, manufacturers need clear pathways for implementing process improvements without extensive revalidation requirements. Regulatory agencies are working to develop frameworks that balance innovation with quality assurance in this rapidly evolving technological landscape.
Scale-up Strategies for Microreactor Systems
Scaling up microreactor systems from laboratory to industrial production represents a critical challenge in continuous flow API synthesis. The transition requires careful consideration of multiple factors to maintain process efficiency and product quality. Numbering-up, a primary scale-up strategy, involves replicating identical microreactor units in parallel configurations rather than increasing individual reactor dimensions. This approach preserves the advantageous heat and mass transfer characteristics of microreactors while increasing throughput proportionally to the number of units deployed.
External numbering-up utilizes multiple complete reactor systems operating in parallel, offering operational flexibility but requiring sophisticated control systems to ensure uniform flow distribution. Internal numbering-up, conversely, incorporates multiple reaction channels within a single unit, reducing footprint and simplifying control systems while potentially introducing flow distribution challenges that must be addressed through precise engineering.
Smart scale-up methodologies incorporate computational fluid dynamics (CFD) modeling and process analytical technology (PAT) to optimize reactor designs before physical implementation. These digital tools enable prediction of flow patterns, mixing efficiency, and heat transfer characteristics across different scales, significantly reducing development time and resource expenditure. The integration of real-time monitoring systems further enhances process control during scale-up operations.
Modular design principles facilitate progressive scale-up through standardized, interchangeable reactor components. This approach allows for incremental capacity increases and rapid reconfiguration to accommodate different reaction requirements. Modular systems typically incorporate standardized connections, control interfaces, and monitoring points, enabling seamless integration of additional units as production demands increase.
Continuous manufacturing facilities implementing microreactor technology must address practical considerations including space utilization, utility requirements, and maintenance accessibility. Vertical integration strategies can minimize footprint requirements, while distributed control systems ensure synchronized operation across multiple reactor units. Material selection becomes increasingly critical at larger scales, with considerations for chemical compatibility, pressure tolerance, and long-term durability influencing design decisions.
Regulatory compliance presents additional challenges during scale-up, particularly for pharmaceutical API production. Quality by Design (QbD) principles must be incorporated throughout the scale-up process, with comprehensive documentation of process parameters, control strategies, and validation protocols. Establishing robust design spaces that accommodate operational variability while maintaining product quality is essential for successful regulatory approval of scaled microreactor systems.
External numbering-up utilizes multiple complete reactor systems operating in parallel, offering operational flexibility but requiring sophisticated control systems to ensure uniform flow distribution. Internal numbering-up, conversely, incorporates multiple reaction channels within a single unit, reducing footprint and simplifying control systems while potentially introducing flow distribution challenges that must be addressed through precise engineering.
Smart scale-up methodologies incorporate computational fluid dynamics (CFD) modeling and process analytical technology (PAT) to optimize reactor designs before physical implementation. These digital tools enable prediction of flow patterns, mixing efficiency, and heat transfer characteristics across different scales, significantly reducing development time and resource expenditure. The integration of real-time monitoring systems further enhances process control during scale-up operations.
Modular design principles facilitate progressive scale-up through standardized, interchangeable reactor components. This approach allows for incremental capacity increases and rapid reconfiguration to accommodate different reaction requirements. Modular systems typically incorporate standardized connections, control interfaces, and monitoring points, enabling seamless integration of additional units as production demands increase.
Continuous manufacturing facilities implementing microreactor technology must address practical considerations including space utilization, utility requirements, and maintenance accessibility. Vertical integration strategies can minimize footprint requirements, while distributed control systems ensure synchronized operation across multiple reactor units. Material selection becomes increasingly critical at larger scales, with considerations for chemical compatibility, pressure tolerance, and long-term durability influencing design decisions.
Regulatory compliance presents additional challenges during scale-up, particularly for pharmaceutical API production. Quality by Design (QbD) principles must be incorporated throughout the scale-up process, with comprehensive documentation of process parameters, control strategies, and validation protocols. Establishing robust design spaces that accommodate operational variability while maintaining product quality is essential for successful regulatory approval of scaled microreactor systems.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!