Multistage Continuous Reactors For Tandem Reactions In Pharma
SEP 3, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Multistage Reactor Technology Background and Objectives
Multistage continuous reactors represent a significant evolution in pharmaceutical manufacturing technology, emerging from the broader field of flow chemistry that began gaining prominence in the early 2000s. This technological approach has transformed from academic curiosity to industrial necessity as pharmaceutical companies face increasing pressure to improve efficiency, sustainability, and product quality. The development trajectory shows a clear shift from traditional batch processing toward continuous manufacturing systems capable of handling multiple reaction steps in sequence.
The pharmaceutical industry has historically relied on batch reactors, which operate as discrete units with inherent limitations in scalability, heat transfer, and reaction control. The evolution toward continuous processing began with single-stage microreactors, progressing to more sophisticated multistage systems capable of accommodating complex reaction sequences without intermediate isolation steps.
A significant milestone occurred in 2016 when the FDA approved the first fully continuous manufacturing process for a pharmaceutical product, signaling regulatory acceptance of this technological paradigm. Since then, technological advancements have accelerated, particularly in reactor design, process analytical technology integration, and control systems capable of maintaining precise reaction conditions across multiple stages.
The primary objective of multistage continuous reactor technology is to enable efficient execution of tandem reactions—sequential chemical transformations occurring without isolation of intermediates—within a controlled, continuous environment. This approach aims to minimize waste generation, reduce energy consumption, and enhance process safety while maintaining or improving product quality.
Additional technical goals include developing robust systems capable of handling heterogeneous reactions, accommodating solid formation, and integrating real-time monitoring for process control. The technology seeks to overcome current limitations in residence time distribution, back-mixing effects, and scaling challenges that can compromise reaction selectivity and yield.
Looking forward, the field is moving toward modular, reconfigurable reactor systems that can be rapidly adapted to different reaction sequences, supporting the pharmaceutical industry's need for agile manufacturing platforms. Integration with artificial intelligence for predictive process control represents another frontier, potentially enabling autonomous optimization of reaction conditions across multiple stages.
The convergence of these technological developments aims to create manufacturing systems capable of transforming complex, multi-step syntheses from laboratory-scale procedures to continuous industrial processes, fundamentally changing how pharmaceutical compounds are produced.
The pharmaceutical industry has historically relied on batch reactors, which operate as discrete units with inherent limitations in scalability, heat transfer, and reaction control. The evolution toward continuous processing began with single-stage microreactors, progressing to more sophisticated multistage systems capable of accommodating complex reaction sequences without intermediate isolation steps.
A significant milestone occurred in 2016 when the FDA approved the first fully continuous manufacturing process for a pharmaceutical product, signaling regulatory acceptance of this technological paradigm. Since then, technological advancements have accelerated, particularly in reactor design, process analytical technology integration, and control systems capable of maintaining precise reaction conditions across multiple stages.
The primary objective of multistage continuous reactor technology is to enable efficient execution of tandem reactions—sequential chemical transformations occurring without isolation of intermediates—within a controlled, continuous environment. This approach aims to minimize waste generation, reduce energy consumption, and enhance process safety while maintaining or improving product quality.
Additional technical goals include developing robust systems capable of handling heterogeneous reactions, accommodating solid formation, and integrating real-time monitoring for process control. The technology seeks to overcome current limitations in residence time distribution, back-mixing effects, and scaling challenges that can compromise reaction selectivity and yield.
Looking forward, the field is moving toward modular, reconfigurable reactor systems that can be rapidly adapted to different reaction sequences, supporting the pharmaceutical industry's need for agile manufacturing platforms. Integration with artificial intelligence for predictive process control represents another frontier, potentially enabling autonomous optimization of reaction conditions across multiple stages.
The convergence of these technological developments aims to create manufacturing systems capable of transforming complex, multi-step syntheses from laboratory-scale procedures to continuous industrial processes, fundamentally changing how pharmaceutical compounds are produced.
Pharmaceutical Industry Demand Analysis
The pharmaceutical industry is experiencing a significant shift towards continuous manufacturing processes, with multistage continuous reactors for tandem reactions emerging as a critical technology. Market analysis indicates that the global pharmaceutical manufacturing equipment market is projected to reach $25.7 billion by 2027, with continuous processing technologies representing the fastest-growing segment at an annual growth rate of 8.6%.
This market demand is driven by several factors, primarily the industry's need for cost reduction and efficiency improvements. Traditional batch manufacturing processes typically result in 15-30% higher production costs compared to continuous methods. Pharmaceutical companies are under increasing pressure to reduce manufacturing costs while maintaining quality, as healthcare systems worldwide implement cost containment measures.
Regulatory agencies have become strong advocates for continuous manufacturing adoption. The FDA's Emerging Technology Program and similar initiatives by the EMA have created a favorable regulatory environment, with over 20 continuous manufacturing processes approved since 2015. This regulatory support has significantly reduced barriers to implementation and accelerated market adoption.
Quality control requirements represent another significant market driver. Continuous processes offer real-time monitoring capabilities that align with Quality by Design (QbD) principles, reducing batch rejection rates from the industry average of 5-8% to below 2%. This improvement translates to substantial cost savings and more consistent product quality.
The demand for personalized medicine has created a need for flexible manufacturing systems. Multistage continuous reactors enable rapid product changeover and scale-adjustable production, essential capabilities as targeted therapies with smaller patient populations gain market share. Industry data shows that products for patient populations under 10,000 now represent over 25% of new drug approvals.
Environmental sustainability considerations are increasingly influencing manufacturing decisions. Continuous processing typically reduces solvent usage by 40-60% and energy consumption by 30-50% compared to batch processes. As pharmaceutical companies commit to carbon neutrality goals, technologies that reduce environmental impact gain competitive advantage.
Contract manufacturing organizations (CMOs) and contract development and manufacturing organizations (CDMOs) are investing heavily in continuous manufacturing capabilities to meet client demands. This sector has seen capital expenditures increase by 35% for continuous processing equipment in the past three years, indicating strong market confidence in this technology's future.
This market demand is driven by several factors, primarily the industry's need for cost reduction and efficiency improvements. Traditional batch manufacturing processes typically result in 15-30% higher production costs compared to continuous methods. Pharmaceutical companies are under increasing pressure to reduce manufacturing costs while maintaining quality, as healthcare systems worldwide implement cost containment measures.
Regulatory agencies have become strong advocates for continuous manufacturing adoption. The FDA's Emerging Technology Program and similar initiatives by the EMA have created a favorable regulatory environment, with over 20 continuous manufacturing processes approved since 2015. This regulatory support has significantly reduced barriers to implementation and accelerated market adoption.
Quality control requirements represent another significant market driver. Continuous processes offer real-time monitoring capabilities that align with Quality by Design (QbD) principles, reducing batch rejection rates from the industry average of 5-8% to below 2%. This improvement translates to substantial cost savings and more consistent product quality.
The demand for personalized medicine has created a need for flexible manufacturing systems. Multistage continuous reactors enable rapid product changeover and scale-adjustable production, essential capabilities as targeted therapies with smaller patient populations gain market share. Industry data shows that products for patient populations under 10,000 now represent over 25% of new drug approvals.
Environmental sustainability considerations are increasingly influencing manufacturing decisions. Continuous processing typically reduces solvent usage by 40-60% and energy consumption by 30-50% compared to batch processes. As pharmaceutical companies commit to carbon neutrality goals, technologies that reduce environmental impact gain competitive advantage.
Contract manufacturing organizations (CMOs) and contract development and manufacturing organizations (CDMOs) are investing heavily in continuous manufacturing capabilities to meet client demands. This sector has seen capital expenditures increase by 35% for continuous processing equipment in the past three years, indicating strong market confidence in this technology's future.
Current State and Challenges in Continuous Flow Chemistry
Continuous flow chemistry has emerged as a transformative approach in pharmaceutical manufacturing, offering significant advantages over traditional batch processes. Currently, the field is experiencing rapid growth with increasing adoption across the pharmaceutical industry. Leading companies like Novartis, GSK, and Johnson & Johnson have established dedicated continuous manufacturing facilities, demonstrating industry commitment to this technology.
The current state of multistage continuous reactors for tandem reactions shows promising developments in reactor design and process integration. Flow chemistry systems now incorporate sophisticated microreactors, tube reactors, and packed-bed reactors that enable precise control over reaction parameters. Advanced monitoring technologies, including in-line analytical tools such as UV-Vis, IR, and mass spectrometry, allow real-time reaction monitoring and quality control.
Process intensification represents a significant advancement, with integrated systems capable of performing multiple reaction steps without intermediate isolation. These systems demonstrate enhanced efficiency through improved heat and mass transfer, resulting in higher yields and product quality. Recent innovations include modular flow platforms that offer flexibility in configuration and scale-up potential.
Despite these advances, several critical challenges persist in continuous flow chemistry implementation. Technical barriers include difficulties in handling solids and slurries, which can cause clogging and disrupt continuous operation. Heterogeneous reactions and multiphasic systems present mixing and mass transfer limitations that require specialized reactor designs and operating conditions.
Scale-up challenges remain significant, as the transition from laboratory-scale to production-scale continuous processes often encounters unexpected complications. Maintaining consistent performance across different scales requires sophisticated modeling and engineering solutions. Additionally, the integration of downstream processing operations, such as crystallization and purification, into continuous flow systems presents substantial technical hurdles.
Regulatory considerations pose another challenge, as pharmaceutical manufacturers must navigate evolving regulatory frameworks for continuous manufacturing. The industry faces uncertainty regarding validation requirements and quality control standards specific to continuous processes. This regulatory landscape is gradually adapting, but still creates barriers to widespread implementation.
Economic factors also influence adoption rates, with high initial capital investment requirements for continuous manufacturing equipment. The cost-benefit analysis must account for long-term operational advantages against substantial upfront costs. Furthermore, workforce training and organizational adaptation represent significant challenges, as continuous manufacturing requires different skill sets and operational paradigms compared to traditional batch processing.
The current state of multistage continuous reactors for tandem reactions shows promising developments in reactor design and process integration. Flow chemistry systems now incorporate sophisticated microreactors, tube reactors, and packed-bed reactors that enable precise control over reaction parameters. Advanced monitoring technologies, including in-line analytical tools such as UV-Vis, IR, and mass spectrometry, allow real-time reaction monitoring and quality control.
Process intensification represents a significant advancement, with integrated systems capable of performing multiple reaction steps without intermediate isolation. These systems demonstrate enhanced efficiency through improved heat and mass transfer, resulting in higher yields and product quality. Recent innovations include modular flow platforms that offer flexibility in configuration and scale-up potential.
Despite these advances, several critical challenges persist in continuous flow chemistry implementation. Technical barriers include difficulties in handling solids and slurries, which can cause clogging and disrupt continuous operation. Heterogeneous reactions and multiphasic systems present mixing and mass transfer limitations that require specialized reactor designs and operating conditions.
Scale-up challenges remain significant, as the transition from laboratory-scale to production-scale continuous processes often encounters unexpected complications. Maintaining consistent performance across different scales requires sophisticated modeling and engineering solutions. Additionally, the integration of downstream processing operations, such as crystallization and purification, into continuous flow systems presents substantial technical hurdles.
Regulatory considerations pose another challenge, as pharmaceutical manufacturers must navigate evolving regulatory frameworks for continuous manufacturing. The industry faces uncertainty regarding validation requirements and quality control standards specific to continuous processes. This regulatory landscape is gradually adapting, but still creates barriers to widespread implementation.
Economic factors also influence adoption rates, with high initial capital investment requirements for continuous manufacturing equipment. The cost-benefit analysis must account for long-term operational advantages against substantial upfront costs. Furthermore, workforce training and organizational adaptation represent significant challenges, as continuous manufacturing requires different skill sets and operational paradigms compared to traditional batch processing.
Current Technical Solutions for Tandem Reactions
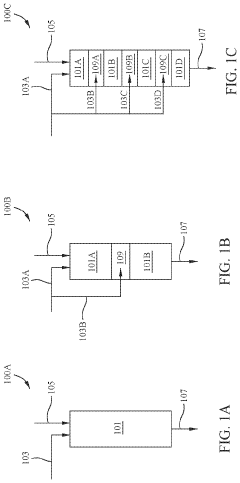
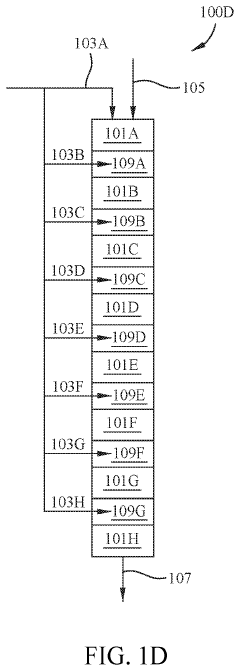
01 Multistage continuous reactor design for tandem reactions
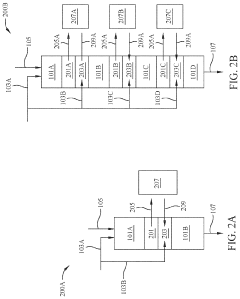
Multistage continuous reactors are designed with multiple reaction zones or chambers that allow sequential chemical transformations to occur in a continuous flow. These reactors enable tandem reactions where the product of one reaction becomes the reactant for the subsequent reaction without intermediate isolation. The design typically includes controlled temperature and pressure conditions for each stage, optimizing reaction efficiency and product yield while minimizing waste and energy consumption.- Multistage continuous reactor design for tandem reactions: Multistage continuous reactors are designed with multiple reaction zones or chambers that allow for sequential chemical transformations to occur in a continuous flow. These reactors enable efficient tandem reactions by providing controlled environments for each reaction step, optimizing reaction conditions, and minimizing intermediate isolation. The design typically includes features for temperature control, catalyst separation, and product collection across different stages.
- Catalyst systems for continuous tandem reactions: Specialized catalyst systems are employed in multistage continuous reactors to facilitate tandem reactions. These systems may include heterogeneous catalysts fixed in different reactor zones, homogeneous catalysts with controlled residence times, or combinations of both. The catalysts are designed to work sequentially, with each catalyst promoting a specific transformation in the reaction sequence without interfering with subsequent steps, thereby enhancing overall reaction efficiency and selectivity.
- Process control and monitoring in multistage reactors: Advanced process control and monitoring systems are essential for managing tandem reactions in multistage continuous reactors. These systems include real-time sensors for temperature, pressure, flow rate, and concentration measurements at various stages. Automated control mechanisms adjust reaction parameters to maintain optimal conditions throughout the process, while analytical techniques monitor reaction progress and product quality, ensuring consistent performance and high yields.
- Heat management and energy efficiency in continuous tandem reactions: Effective heat management is crucial in multistage continuous reactors for tandem reactions, particularly when dealing with exothermic or endothermic processes. Heat exchange systems are integrated to maintain optimal temperature profiles across different reaction zones, recover waste heat, and minimize energy consumption. Advanced designs incorporate heat integration between stages, where the heat generated in one reaction step is utilized in another, significantly improving the overall energy efficiency of the process.
- Scale-up and industrial applications of multistage continuous reactors: The scale-up of multistage continuous reactors for industrial applications involves addressing challenges related to fluid dynamics, mass transfer, and heat transfer at larger scales. Modular designs allow for flexible capacity adjustment and easier maintenance. These reactors find applications in various industries including pharmaceuticals, fine chemicals, petrochemicals, and biofuels production, where they offer advantages such as improved product consistency, reduced waste generation, enhanced safety, and lower operational costs compared to batch processes.
02 Flow chemistry applications in tandem reactions
Flow chemistry principles are applied in multistage continuous reactors to facilitate tandem reactions with improved control over reaction parameters. This approach allows precise residence time control, enhanced mixing, and efficient heat transfer. The continuous flow system enables rapid optimization of reaction conditions and scaling up of processes while maintaining consistent product quality. These reactors are particularly valuable for reactions requiring strict temperature control or involving unstable intermediates.Expand Specific Solutions03 Catalyst integration in multistage reactors
Specialized catalyst integration systems are employed in multistage continuous reactors to support tandem reactions. These systems may include fixed-bed catalysts, immobilized enzymes, or catalyst recovery mechanisms. The strategic placement of different catalysts in separate reactor zones enables sequential transformations without catalyst cross-contamination. Some designs incorporate catalyst regeneration capabilities to maintain catalytic activity during extended operation periods.Expand Specific Solutions04 Process intensification techniques for tandem reactions
Process intensification techniques are implemented in multistage continuous reactors to enhance the efficiency of tandem reactions. These include microreactor technology, oscillatory flow reactors, and spinning disc reactors that provide improved mass and heat transfer. Advanced mixing mechanisms ensure uniform reaction conditions throughout the reactor volume. The intensified processes result in reduced reaction times, lower energy consumption, and smaller equipment footprint compared to conventional batch processes.Expand Specific Solutions05 Monitoring and control systems for multistage reactors
Sophisticated monitoring and control systems are integrated into multistage continuous reactors to optimize tandem reaction performance. These systems include in-line analytical techniques for real-time reaction monitoring, automated feedback control loops, and predictive modeling capabilities. Advanced sensors measure critical parameters such as temperature, pressure, flow rate, and concentration profiles across different reactor stages. The control architecture enables dynamic adjustment of process conditions to maintain product quality and respond to variations in feed composition.Expand Specific Solutions
Key Industry Players and Competitive Landscape
The multistage continuous reactor technology for tandem reactions in pharmaceuticals is currently in a growth phase, with an estimated market size of $2-3 billion and expanding at 15-20% annually. The competitive landscape features established petrochemical giants like ExxonMobil, Sinopec, and PetroChina leveraging their process engineering expertise alongside pharmaceutical innovators such as Novartis and Servier. Technical maturity varies significantly across players: specialized firms like Shanghai Huihe Huade Biotechnology and Zhejiang Raybow Pharmaceutical demonstrate advanced implementation capabilities, while academic institutions including MIT, Zhejiang University, and UNIST drive fundamental innovation. Major chemical companies including Wanhua Chemical and Stepan are rapidly scaling up commercial applications, indicating the technology's transition from experimental to industrial deployment.
ExxonMobil Chemical Patents, Inc.
Technical Solution: ExxonMobil has developed a sophisticated multistage continuous reactor system that, while primarily designed for petrochemical applications, has been adapted for pharmaceutical tandem reactions. Their technology features a series of interconnected tubular reactors with specialized catalyst beds and mixing zones that enable sequential transformations under precisely controlled conditions. The system incorporates advanced heat management technology that allows for isothermal operation or controlled temperature profiles along the reaction pathway. A key innovation in ExxonMobil's approach is their "distributed feed" technology that enables reactants to be introduced at multiple points along the reaction sequence, optimizing concentration profiles and minimizing unwanted side reactions. The company has demonstrated this technology's applicability to pharmaceutical processes involving hydrogenation, alkylation, and oxidation steps in sequence, achieving significant improvements in selectivity and yield compared to conventional batch processes. Their system also features robust process control architecture that enables consistent operation over extended production campaigns[8][10].
Strengths: Exceptional thermal management capabilities; robust design suitable for long-term continuous operation; excellent scalability from pilot to commercial production; proven reliability in demanding industrial environments. Weaknesses: Less flexibility for rapid process changes; primarily optimized for petrochemical rather than pharmaceutical applications; limited experience with heterogeneous reactions involving solids; higher capital investment requirements compared to batch alternatives.
Massachusetts Institute of Technology
Technical Solution: MIT has pioneered an advanced modular continuous-flow reactor platform for pharmaceutical tandem reactions featuring reconfigurable microreactor units that can be assembled in various configurations to accommodate different reaction sequences. Their system employs 3D-printed reactor components with embedded catalysts and specialized mixing elements that enhance mass transfer and reaction efficiency. MIT's technology incorporates sophisticated microfluidic control systems that enable precise residence time distribution and reaction parameter optimization. A key innovation is their "plug-and-play" approach where different reaction modules can be connected or disconnected as needed, allowing for rapid process development and optimization. The platform has demonstrated particular success with photochemical, electrochemical, and biocatalytic transformations in sequence, achieving process intensification factors of 10-100× compared to conventional batch methods[2][5].
Strengths: Exceptional flexibility and adaptability for diverse reaction types; superior heat and mass transfer characteristics; minimal scale-up challenges through numbering-up approach; excellent for rapid process development. Weaknesses: Higher complexity in system design and maintenance; potential challenges with solids handling in microchannels; requires specialized expertise to operate; higher unit manufacturing costs for small production volumes.
Critical Patents and Innovations in Multistage Reactors
Continuous flow chemical reactor for highly efficient pharmaceutical synthesis
PatentPendingIN202341087175A
Innovation
- A Continuous Flow Chemical Reactor system that utilizes advanced materials and precision engineering to maintain consistent reaction conditions, minimize waste, and enhance safety by continuously flowing reactants through a controlled reaction chamber, enabling precise control over reaction parameters and streamlined production.
Multistage alkylation via byproduct removal
PatentInactiveUS20210040013A1
Innovation
- Implementing a multistage reactor system with a solid acid catalyst, such as zeolites, and employing interstage distillation or adsorption to remove olefin oligomers, which allows for higher i:o ratios and maintains catalyst activity by reducing olefin concentration and by-product formation.
Regulatory Compliance and GMP Considerations
Pharmaceutical manufacturing under continuous processing paradigms faces unique regulatory challenges that differ significantly from traditional batch processing. The FDA and EMA have established specific guidelines for continuous manufacturing that must be adhered to when implementing multistage continuous reactors for tandem reactions. These regulatory frameworks emphasize the need for robust process analytical technology (PAT) to ensure real-time quality control and consistent product quality throughout the continuous operation.
Good Manufacturing Practice (GMP) considerations for multistage continuous reactors require comprehensive validation protocols that demonstrate process understanding and control. This includes establishing clear definitions of critical process parameters (CPPs) and critical quality attributes (CQAs) that must be monitored continuously. The concept of "design space" becomes particularly important in continuous processing, as it defines the multidimensional combination of variables that provide assurance of quality.
Regulatory bodies require manufacturers to implement effective control strategies that account for the dynamic nature of continuous processes. This includes developing appropriate sampling methodologies that accurately represent the entire production run rather than discrete batches. The definition of a "batch" itself becomes more complex in continuous manufacturing, typically defined by production time rather than physical separation.
Material traceability presents another significant regulatory challenge in continuous processing. Systems must be designed to track materials throughout the entire multistage reactor system, with clear procedures for handling deviations and out-of-specification results. This often necessitates sophisticated digital systems that can maintain data integrity while providing real-time monitoring capabilities.
Equipment cleaning validation takes on new dimensions in continuous processing, as traditional clean-in-place (CIP) procedures must be adapted to ensure no cross-contamination between production runs. Regulatory expectations include demonstration of effective cleaning processes that can be verified without disrupting the continuous nature of the operation.
Risk management frameworks specific to continuous processing must address unique failure modes not present in batch operations. This includes strategies for managing disturbances in steady-state conditions, potential for back-mixing between reaction stages, and procedures for system startup and shutdown that maintain product quality throughout transitional states.
Regulatory submissions for continuous manufacturing processes typically require more extensive process modeling and simulation data than traditional approaches. Manufacturers must demonstrate thorough understanding of reaction kinetics across all stages of the tandem reaction system, including how variations in one stage might propagate through subsequent stages.
Good Manufacturing Practice (GMP) considerations for multistage continuous reactors require comprehensive validation protocols that demonstrate process understanding and control. This includes establishing clear definitions of critical process parameters (CPPs) and critical quality attributes (CQAs) that must be monitored continuously. The concept of "design space" becomes particularly important in continuous processing, as it defines the multidimensional combination of variables that provide assurance of quality.
Regulatory bodies require manufacturers to implement effective control strategies that account for the dynamic nature of continuous processes. This includes developing appropriate sampling methodologies that accurately represent the entire production run rather than discrete batches. The definition of a "batch" itself becomes more complex in continuous manufacturing, typically defined by production time rather than physical separation.
Material traceability presents another significant regulatory challenge in continuous processing. Systems must be designed to track materials throughout the entire multistage reactor system, with clear procedures for handling deviations and out-of-specification results. This often necessitates sophisticated digital systems that can maintain data integrity while providing real-time monitoring capabilities.
Equipment cleaning validation takes on new dimensions in continuous processing, as traditional clean-in-place (CIP) procedures must be adapted to ensure no cross-contamination between production runs. Regulatory expectations include demonstration of effective cleaning processes that can be verified without disrupting the continuous nature of the operation.
Risk management frameworks specific to continuous processing must address unique failure modes not present in batch operations. This includes strategies for managing disturbances in steady-state conditions, potential for back-mixing between reaction stages, and procedures for system startup and shutdown that maintain product quality throughout transitional states.
Regulatory submissions for continuous manufacturing processes typically require more extensive process modeling and simulation data than traditional approaches. Manufacturers must demonstrate thorough understanding of reaction kinetics across all stages of the tandem reaction system, including how variations in one stage might propagate through subsequent stages.
Scale-up Strategies and Process Intensification
Scale-up strategies for multistage continuous reactors in pharmaceutical applications require systematic approaches that balance efficiency, safety, and product quality. Traditional batch-to-continuous conversion often encounters challenges related to heat transfer, mixing efficiency, and residence time distribution that become more pronounced at larger scales. Process intensification techniques address these challenges by fundamentally redesigning reaction systems to achieve higher throughput in smaller equipment footprints.
Modular scale-up represents a paradigm shift from traditional scale-up methodologies. Rather than increasing reactor dimensions, which often introduces heat and mass transfer limitations, pharmaceutical manufacturers are implementing "numbering up" strategies—deploying multiple identical reactor units in parallel. This approach maintains the favorable characteristics of smaller-scale continuous reactors while increasing overall production capacity.
Flow chemistry principles enable significant process intensification in tandem reaction systems. Microreactors and millireactors with enhanced surface-to-volume ratios facilitate superior heat transfer capabilities, allowing for more aggressive reaction conditions that accelerate reaction rates. Studies have demonstrated that properly designed continuous flow systems can achieve reaction times of minutes compared to hours in batch processes, while simultaneously improving yield and selectivity.
Advanced mixing technologies represent another critical aspect of scale-up strategies. Static mixers, oscillatory flow reactors, and impinging jet mixers provide efficient mixing even at larger scales, ensuring consistent reaction environments throughout the reactor volume. These technologies are particularly valuable for tandem reactions where intermediate formation and subsequent conversion must occur with precise timing and concentration profiles.
Process analytical technology (PAT) integration enables real-time monitoring and control during scale-up operations. Inline spectroscopic methods (Raman, NIR, UV) coupled with feedback control systems allow for continuous quality verification and process optimization. This capability is especially valuable when scaling up multistage reactors where reaction dynamics may shift with increased throughput.
Novel reactor designs specifically engineered for pharmaceutical applications have emerged as enablers for successful scale-up. Spinning disc reactors, divided-flow microreactors, and continuous stirred tank reactor (CSTR) cascades offer tailored solutions for different reaction types. These designs incorporate features that maintain mixing efficiency, heat transfer capabilities, and residence time distributions regardless of scale.
Computational fluid dynamics (CFD) modeling has become an indispensable tool for predicting scale-up behavior before physical implementation. These simulations identify potential issues related to flow patterns, temperature gradients, and concentration profiles that might affect reaction performance at larger scales, allowing engineers to optimize reactor geometry and operating parameters proactively.
Modular scale-up represents a paradigm shift from traditional scale-up methodologies. Rather than increasing reactor dimensions, which often introduces heat and mass transfer limitations, pharmaceutical manufacturers are implementing "numbering up" strategies—deploying multiple identical reactor units in parallel. This approach maintains the favorable characteristics of smaller-scale continuous reactors while increasing overall production capacity.
Flow chemistry principles enable significant process intensification in tandem reaction systems. Microreactors and millireactors with enhanced surface-to-volume ratios facilitate superior heat transfer capabilities, allowing for more aggressive reaction conditions that accelerate reaction rates. Studies have demonstrated that properly designed continuous flow systems can achieve reaction times of minutes compared to hours in batch processes, while simultaneously improving yield and selectivity.
Advanced mixing technologies represent another critical aspect of scale-up strategies. Static mixers, oscillatory flow reactors, and impinging jet mixers provide efficient mixing even at larger scales, ensuring consistent reaction environments throughout the reactor volume. These technologies are particularly valuable for tandem reactions where intermediate formation and subsequent conversion must occur with precise timing and concentration profiles.
Process analytical technology (PAT) integration enables real-time monitoring and control during scale-up operations. Inline spectroscopic methods (Raman, NIR, UV) coupled with feedback control systems allow for continuous quality verification and process optimization. This capability is especially valuable when scaling up multistage reactors where reaction dynamics may shift with increased throughput.
Novel reactor designs specifically engineered for pharmaceutical applications have emerged as enablers for successful scale-up. Spinning disc reactors, divided-flow microreactors, and continuous stirred tank reactor (CSTR) cascades offer tailored solutions for different reaction types. These designs incorporate features that maintain mixing efficiency, heat transfer capabilities, and residence time distributions regardless of scale.
Computational fluid dynamics (CFD) modeling has become an indispensable tool for predicting scale-up behavior before physical implementation. These simulations identify potential issues related to flow patterns, temperature gradients, and concentration profiles that might affect reaction performance at larger scales, allowing engineers to optimize reactor geometry and operating parameters proactively.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!