Flow Chemistry For Enzymatic And Biocatalytic Transformations
SEP 3, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Biocatalysis Flow Chemistry Background and Objectives
Flow chemistry for enzymatic and biocatalytic transformations represents a convergence of continuous flow processing technology with the power of biological catalysts. This field has evolved significantly over the past two decades, transitioning from traditional batch processes to more efficient continuous flow systems. The integration of enzymes and whole-cell biocatalysts into flow reactors marks a paradigm shift in chemical synthesis methodology, offering unprecedented control over reaction parameters while harnessing the inherent selectivity of biological systems.
Historically, biocatalytic processes were predominantly conducted in batch reactors, which limited scalability and often resulted in enzyme deactivation due to prolonged exposure to reaction conditions. The emergence of flow chemistry in the early 2000s provided a technological platform to address these limitations. By 2010, pioneering research groups began exploring the immobilization of enzymes in microreactors, establishing the foundational principles for enzymatic flow chemistry.
The technological evolution has been driven by increasing demands for sustainable manufacturing processes in pharmaceutical, fine chemical, and agrochemical industries. Environmental regulations and the push toward greener chemistry have accelerated interest in biocatalytic approaches that operate under mild conditions with reduced waste generation. Flow chemistry offers an ideal complement to these sustainability goals by minimizing solvent usage and improving energy efficiency.
Current technological objectives in this field focus on several key areas: enhancing enzyme stability in continuous flow environments, developing robust immobilization techniques for extended catalyst lifetime, improving reactor designs for optimal mass transfer, and establishing scalable processes that maintain the selectivity advantages of biocatalysis. Additionally, there is significant interest in multi-enzymatic cascade reactions in flow, which mimic natural metabolic pathways to perform complex transformations in a single process.
The integration of digital technologies represents another important objective, with real-time monitoring and process analytical technology (PAT) becoming essential for quality control and process optimization. Machine learning approaches are increasingly being applied to predict enzyme performance and optimize reaction conditions in flow systems.
Looking forward, the field aims to develop "plug-and-play" biocatalytic flow modules that can be readily integrated into existing manufacturing infrastructure. This would democratize access to biocatalytic technology across various industrial sectors. Another ambitious goal is the creation of self-sustaining biocatalytic flow systems with in-situ cofactor regeneration and enzyme production capabilities, potentially revolutionizing how complex molecules are synthesized in the chemical industry.
Historically, biocatalytic processes were predominantly conducted in batch reactors, which limited scalability and often resulted in enzyme deactivation due to prolonged exposure to reaction conditions. The emergence of flow chemistry in the early 2000s provided a technological platform to address these limitations. By 2010, pioneering research groups began exploring the immobilization of enzymes in microreactors, establishing the foundational principles for enzymatic flow chemistry.
The technological evolution has been driven by increasing demands for sustainable manufacturing processes in pharmaceutical, fine chemical, and agrochemical industries. Environmental regulations and the push toward greener chemistry have accelerated interest in biocatalytic approaches that operate under mild conditions with reduced waste generation. Flow chemistry offers an ideal complement to these sustainability goals by minimizing solvent usage and improving energy efficiency.
Current technological objectives in this field focus on several key areas: enhancing enzyme stability in continuous flow environments, developing robust immobilization techniques for extended catalyst lifetime, improving reactor designs for optimal mass transfer, and establishing scalable processes that maintain the selectivity advantages of biocatalysis. Additionally, there is significant interest in multi-enzymatic cascade reactions in flow, which mimic natural metabolic pathways to perform complex transformations in a single process.
The integration of digital technologies represents another important objective, with real-time monitoring and process analytical technology (PAT) becoming essential for quality control and process optimization. Machine learning approaches are increasingly being applied to predict enzyme performance and optimize reaction conditions in flow systems.
Looking forward, the field aims to develop "plug-and-play" biocatalytic flow modules that can be readily integrated into existing manufacturing infrastructure. This would democratize access to biocatalytic technology across various industrial sectors. Another ambitious goal is the creation of self-sustaining biocatalytic flow systems with in-situ cofactor regeneration and enzyme production capabilities, potentially revolutionizing how complex molecules are synthesized in the chemical industry.
Market Analysis for Enzymatic Flow Processes
The global market for enzymatic flow processes is experiencing robust growth, driven by increasing demand for sustainable manufacturing solutions across pharmaceutical, fine chemical, and food industries. Current market valuation stands at approximately 1.2 billion USD with a compound annual growth rate of 8.7% projected through 2028. This growth trajectory reflects the industrial shift toward greener chemistry and more efficient production methods.
Pharmaceutical applications represent the largest market segment, accounting for nearly 45% of the total market share. This dominance stems from the industry's stringent requirements for high-purity products and the significant advantages flow processes offer in terms of reaction control and product consistency. The fine chemicals sector follows at 30%, while food and beverage applications constitute about 15% of the market.
Regionally, North America leads with 38% market share, followed closely by Europe at 35%. The European market demonstrates particularly strong growth due to stringent environmental regulations and substantial investments in sustainable manufacturing technologies. Asia-Pacific represents the fastest-growing region with a 12.3% growth rate, driven by rapid industrialization in China and India coupled with increasing adoption of advanced biocatalytic processes.
Customer demand analysis reveals three primary market drivers: cost efficiency, sustainability credentials, and product quality enhancement. End-users increasingly value the reduced enzyme consumption and higher space-time yields offered by flow processes, which can decrease production costs by up to 25% compared to batch operations. Additionally, the reduced solvent usage and energy requirements align with corporate sustainability goals and regulatory pressures.
Market barriers include high initial capital investment requirements, technical complexity in system design, and challenges in enzyme immobilization technology. The average implementation cost for a mid-scale enzymatic flow system ranges between 500,000 to 2 million USD, creating adoption hurdles particularly for small and medium enterprises.
Future market expansion is expected in emerging applications such as biofuel production, waste valorization, and personalized medicine manufacturing. The integration of enzymatic flow processes with other technologies like artificial intelligence for process optimization and continuous downstream processing represents significant growth opportunities. Market forecasts suggest particular acceleration in hybrid chemo-enzymatic flow processes, which are projected to grow at 14.2% annually as industries seek to combine the selectivity of biocatalysis with the robustness of chemical catalysis.
Pharmaceutical applications represent the largest market segment, accounting for nearly 45% of the total market share. This dominance stems from the industry's stringent requirements for high-purity products and the significant advantages flow processes offer in terms of reaction control and product consistency. The fine chemicals sector follows at 30%, while food and beverage applications constitute about 15% of the market.
Regionally, North America leads with 38% market share, followed closely by Europe at 35%. The European market demonstrates particularly strong growth due to stringent environmental regulations and substantial investments in sustainable manufacturing technologies. Asia-Pacific represents the fastest-growing region with a 12.3% growth rate, driven by rapid industrialization in China and India coupled with increasing adoption of advanced biocatalytic processes.
Customer demand analysis reveals three primary market drivers: cost efficiency, sustainability credentials, and product quality enhancement. End-users increasingly value the reduced enzyme consumption and higher space-time yields offered by flow processes, which can decrease production costs by up to 25% compared to batch operations. Additionally, the reduced solvent usage and energy requirements align with corporate sustainability goals and regulatory pressures.
Market barriers include high initial capital investment requirements, technical complexity in system design, and challenges in enzyme immobilization technology. The average implementation cost for a mid-scale enzymatic flow system ranges between 500,000 to 2 million USD, creating adoption hurdles particularly for small and medium enterprises.
Future market expansion is expected in emerging applications such as biofuel production, waste valorization, and personalized medicine manufacturing. The integration of enzymatic flow processes with other technologies like artificial intelligence for process optimization and continuous downstream processing represents significant growth opportunities. Market forecasts suggest particular acceleration in hybrid chemo-enzymatic flow processes, which are projected to grow at 14.2% annually as industries seek to combine the selectivity of biocatalysis with the robustness of chemical catalysis.
Current Challenges in Flow Biocatalysis
Despite the significant advancements in flow biocatalysis, several critical challenges continue to impede its widespread industrial adoption. One of the primary obstacles remains enzyme stability under continuous flow conditions. Many enzymes experience accelerated deactivation when exposed to mechanical stress from pumping systems, interfaces in multiphase systems, and prolonged contact with synthetic materials used in flow reactors. This stability issue is particularly pronounced for complex multi-enzymatic cascades where each enzyme may require different optimal conditions.
Immobilization techniques, while offering solutions for enzyme retention, introduce their own complications. Current immobilization methods often result in partial loss of catalytic activity, altered selectivity, and diffusional limitations that reduce reaction efficiency. The trade-off between stability gained through immobilization and activity loss remains a significant engineering challenge that requires case-by-case optimization.
Scale-up represents another formidable barrier in flow biocatalysis. The transition from laboratory-scale microreactors to industrial production volumes frequently encounters issues with flow distribution, heat transfer limitations, and pressure drop considerations. These parameters can dramatically affect reaction performance and are difficult to predict using small-scale models, necessitating extensive empirical testing during scale-up.
Oxygen supply presents a unique challenge for oxidative biocatalytic reactions in flow systems. The low solubility of oxygen in aqueous media combined with the high oxygen demand of many enzymatic oxidations creates a fundamental limitation. Current gas-liquid contactors often struggle to provide sufficient oxygen transfer rates without introducing damaging interfaces or causing enzyme denaturation at gas-liquid boundaries.
Economic considerations further complicate implementation. The high cost of enzyme production, particularly for non-commodity enzymes, coupled with the capital investment required for specialized flow equipment, creates significant barriers to entry. This economic pressure is intensified by the relatively low space-time yields achieved in many biocatalytic processes compared to traditional chemical catalysis.
Regulatory and quality control challenges also persist, especially for pharmaceutical applications. Continuous monitoring and real-time analytics for biocatalytic processes lag behind those available for chemical processes, making quality assurance more difficult in continuous flow biocatalysis. The lack of standardized protocols for validation of such processes further complicates regulatory approval pathways.
Immobilization techniques, while offering solutions for enzyme retention, introduce their own complications. Current immobilization methods often result in partial loss of catalytic activity, altered selectivity, and diffusional limitations that reduce reaction efficiency. The trade-off between stability gained through immobilization and activity loss remains a significant engineering challenge that requires case-by-case optimization.
Scale-up represents another formidable barrier in flow biocatalysis. The transition from laboratory-scale microreactors to industrial production volumes frequently encounters issues with flow distribution, heat transfer limitations, and pressure drop considerations. These parameters can dramatically affect reaction performance and are difficult to predict using small-scale models, necessitating extensive empirical testing during scale-up.
Oxygen supply presents a unique challenge for oxidative biocatalytic reactions in flow systems. The low solubility of oxygen in aqueous media combined with the high oxygen demand of many enzymatic oxidations creates a fundamental limitation. Current gas-liquid contactors often struggle to provide sufficient oxygen transfer rates without introducing damaging interfaces or causing enzyme denaturation at gas-liquid boundaries.
Economic considerations further complicate implementation. The high cost of enzyme production, particularly for non-commodity enzymes, coupled with the capital investment required for specialized flow equipment, creates significant barriers to entry. This economic pressure is intensified by the relatively low space-time yields achieved in many biocatalytic processes compared to traditional chemical catalysis.
Regulatory and quality control challenges also persist, especially for pharmaceutical applications. Continuous monitoring and real-time analytics for biocatalytic processes lag behind those available for chemical processes, making quality assurance more difficult in continuous flow biocatalysis. The lack of standardized protocols for validation of such processes further complicates regulatory approval pathways.
Current Methodologies for Enzyme Immobilization in Flow
01 Microfluidic systems for flow chemistry
Microfluidic systems provide precise control over reaction conditions in flow chemistry applications. These systems utilize small channels to manipulate fluids at the microscale, enabling efficient mixing, heat transfer, and reaction control. The technology allows for continuous processing with improved yield and selectivity compared to batch reactions, while requiring smaller reagent volumes and generating less waste.- Microfluidic systems for flow chemistry: Microfluidic systems enable precise control over chemical reactions in flow chemistry. These systems utilize small channels and chambers to manipulate small volumes of fluids, allowing for better mixing, heat transfer, and reaction control. The miniaturized nature of these systems enhances reaction efficiency, reduces reagent consumption, and enables rapid screening of reaction conditions. These advantages make microfluidic platforms particularly valuable for applications requiring precise control over reaction parameters.
- Continuous flow reactors and equipment: Continuous flow reactors are essential components in flow chemistry, allowing for uninterrupted chemical processing. These reactors come in various designs including tubular reactors, packed bed reactors, and plate reactors, each optimized for specific reaction types. The equipment often features integrated heating/cooling systems, pressure regulators, and mixing elements to maintain optimal reaction conditions. These systems enable scaling of reactions from laboratory to production scale while maintaining consistent product quality and reaction efficiency.
- Process control and automation in flow chemistry: Advanced process control and automation systems are critical for optimizing flow chemistry operations. These systems incorporate sensors, controllers, and software that monitor and adjust reaction parameters in real-time. Automated flow chemistry platforms can perform sequential operations, adjust flow rates, control temperatures, and respond to analytical feedback. This automation reduces human error, increases reproducibility, and enables continuous operation with minimal supervision, making complex multi-step syntheses more practical and efficient.
- Novel applications of flow chemistry: Flow chemistry techniques are being applied to diverse fields beyond traditional chemical synthesis. These applications include pharmaceutical manufacturing, nanomaterial synthesis, biocatalysis, and polymerization reactions. The controlled environment of flow systems allows for safer handling of hazardous reagents, improved selectivity in reactions, and more efficient multiphase processes. Flow chemistry also enables reactions under extreme conditions that would be challenging in batch processes, opening new pathways for chemical transformations and material production.
- Integration of analytical techniques with flow chemistry: The integration of real-time analytical techniques with flow chemistry systems enables continuous monitoring and optimization of reactions. Techniques such as spectroscopy, chromatography, and mass spectrometry can be coupled directly to flow reactors to provide immediate feedback on reaction progress and product quality. This integration facilitates rapid reaction optimization, quality control, and process understanding. Advanced systems can use analytical data to automatically adjust reaction parameters, creating self-optimizing chemical processes that maximize yield and selectivity.
02 Flow reactors and equipment design
Specialized reactors and equipment are essential for effective flow chemistry processes. These designs include continuous flow reactors, tubular reactors, and modular systems that can be configured for various reaction types. Advanced reactor designs incorporate features for enhanced mixing, controlled residence time, and improved heat exchange, allowing for safer handling of exothermic reactions and hazardous intermediates.Expand Specific Solutions03 Process control and automation in flow chemistry
Automation and process control systems are critical for maintaining consistent conditions in flow chemistry operations. These systems monitor and adjust parameters such as flow rates, temperature, pressure, and residence time to ensure reproducible results. Advanced control strategies incorporate real-time analytics, feedback loops, and predictive algorithms to optimize reaction conditions and respond to process variations.Expand Specific Solutions04 Pharmaceutical and biological applications
Flow chemistry offers significant advantages for pharmaceutical and biological applications, including drug synthesis, formulation, and testing. The continuous processing approach enables precise control over reaction conditions, leading to improved product quality and consistency. Flow systems also facilitate rapid screening of reaction conditions, accelerated drug development, and the implementation of quality-by-design principles in pharmaceutical manufacturing.Expand Specific Solutions05 Novel catalytic methods in flow chemistry
Innovative catalytic approaches have been developed specifically for flow chemistry applications. These include heterogeneous catalysts immobilized in packed beds or wall-coated channels, supported enzyme systems, and photocatalytic processes optimized for continuous flow. The flow environment allows for more efficient catalyst utilization, simplified catalyst recovery, and the ability to perform multi-step catalytic transformations in sequence without intermediate purification steps.Expand Specific Solutions
Leading Organizations in Biocatalytic Flow Chemistry
Flow chemistry for enzymatic and biocatalytic transformations is currently in a growth phase, with the market expanding rapidly due to increasing demand for sustainable manufacturing processes. The global market size is estimated to reach $2-3 billion by 2025, driven by pharmaceutical and fine chemical applications. Technologically, the field is advancing from early-stage development toward commercial maturity, with companies like DuPont de Nemours, Bristol Myers Squibb, and FUJIFILM leading industrial implementation. Academic institutions including Beijing University of Chemical Technology and Karlsruher Institut für Technologie are contributing significant research advances, while specialized firms such as UOP LLC and Cognis IP Management are developing proprietary biocatalytic systems. The convergence of continuous flow technology with enzymatic processes represents a transformative approach for green chemistry applications.
DuPont de Nemours, Inc.
Technical Solution: DuPont has developed integrated continuous flow biocatalysis platforms that combine enzyme immobilization technology with microfluidic reactors. Their approach utilizes specialized enzyme carriers with controlled porosity that allow for high enzyme loading while maintaining excellent mass transfer characteristics. The system incorporates precise temperature and pH control mechanisms to maintain optimal enzyme activity throughout the reaction process. DuPont's platform enables multi-step enzymatic cascades by compartmentalizing different enzymes in sequential reactor modules, allowing for the synthesis of complex molecules without intermediate isolation steps. Their technology also features in-line monitoring capabilities using spectroscopic methods to provide real-time reaction progress data and automated feedback control systems to adjust process parameters accordingly.
Strengths: Superior enzyme stability through proprietary immobilization techniques, resulting in extended catalyst lifetime and improved process economics. High volumetric productivity compared to batch processes. Weaknesses: Higher initial capital investment required compared to traditional batch systems. Some complex enzyme systems may face challenges with immobilization without activity loss.
Bristol Myers Squibb Co.
Technical Solution: Bristol Myers Squibb has pioneered a modular flow chemistry platform specifically designed for biocatalytic transformations in pharmaceutical manufacturing. Their system employs packed-bed reactors containing immobilized enzymes on specialized polymer supports that maintain enzyme activity while providing excellent mechanical stability under continuous flow conditions. The platform incorporates sophisticated process analytical technology (PAT) tools for real-time monitoring of reaction parameters and product quality. BMS has developed proprietary membrane separation units integrated directly into their flow systems, allowing for continuous product isolation and enzyme recycling. Their technology enables precise control of residence time distribution, critical for optimizing yield and selectivity in enzymatic reactions. The company has successfully implemented this platform for the commercial production of several API intermediates, demonstrating significant improvements in process efficiency and sustainability compared to traditional batch methods.
Strengths: Highly adaptable system that can be rapidly reconfigured for different enzymatic transformations, reducing development timelines. Excellent process control leading to consistent product quality. Weaknesses: System complexity requires specialized expertise for operation and troubleshooting. Higher enzyme loadings sometimes needed to compensate for activity loss during immobilization.
Key Innovations in Continuous Biocatalytic Transformations
Method of enzymatic biotransformation of petroleum hydrocarbon
PatentWO2019213754A1
Innovation
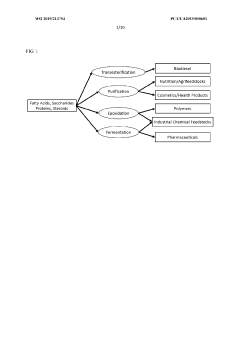
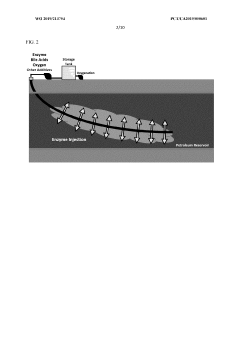
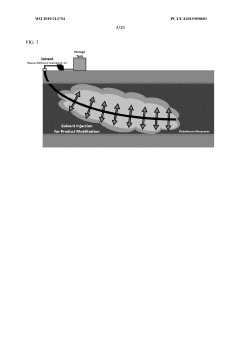
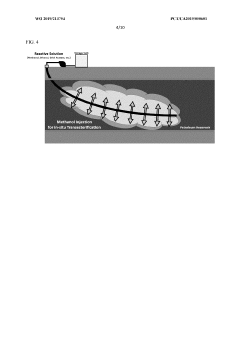
- A method involving the injection of hydrolytic enzymes such as lipases, cellulases, and oxygen into petroleum reservoirs to biodegrade hydrocarbons into bioconversion products, which are then processed into value-added products like fatty acids, saccharides, and steroids, followed by further conversion using reactive agents and solvents to produce biodiesel, biodegradable plastics, and pharmaceuticals.
Enzymatic process using immobilized microbial cells
PatentInactiveUSRE29136E1
Innovation
- Flocculation of microbial cells using polyelectrolytes and other agents to create aggregates that can be used directly in batch or continuous processes, eliminating the need for enzyme separation and purification, and enhancing structural integrity through freezing or dehydration to maintain enzyme activity.
Sustainability Impact of Enzymatic Flow Processes
The integration of enzymatic and biocatalytic processes into flow chemistry frameworks represents a significant advancement in sustainable manufacturing practices. Flow-based enzymatic processes demonstrate substantial environmental benefits compared to traditional batch reactions, primarily through reduced resource consumption. Studies indicate that continuous flow enzymatic reactions can achieve up to 40-60% reduction in solvent usage and 30-50% decrease in energy requirements, directly contributing to smaller carbon footprints in chemical manufacturing operations.
Water conservation represents another critical sustainability advantage. Flow enzymatic systems typically require 25-45% less water than comparable batch processes, addressing growing concerns about industrial water consumption. This efficiency stems from improved heat and mass transfer characteristics inherent to microfluidic and continuous flow systems, allowing for more precise reaction control with minimal resource input.
The waste reduction profile of enzymatic flow processes further enhances their sustainability credentials. These systems generate approximately 35-55% less waste compared to conventional methods, primarily due to higher conversion rates, improved selectivity, and reduced side-product formation. The controlled reaction environment in flow systems enables enzymes to operate at their optimal efficiency, maximizing atom economy and minimizing downstream purification requirements.
From a life cycle assessment perspective, enzymatic flow processes demonstrate favorable environmental profiles across multiple impact categories. Research indicates reductions of 20-40% in global warming potential, 30-50% in ecotoxicity measures, and 25-45% in resource depletion indicators when compared to traditional chemical synthesis routes. These improvements become particularly significant when scaled to industrial production volumes.
The sustainability benefits extend to operational aspects as well. Flow enzymatic processes typically require smaller physical footprints for equivalent production capacity, reducing land use requirements by 40-60%. Their modular nature facilitates distributed manufacturing models that can decrease transportation-related emissions by enabling production closer to points of consumption.
Looking forward, the integration of renewable energy sources with enzymatic flow processes presents opportunities for further sustainability enhancements. Pilot projects combining solar or wind power with continuous flow biocatalysis have demonstrated the potential for near-carbon-neutral manufacturing of pharmaceuticals and fine chemicals, establishing a pathway toward truly sustainable chemical production systems that align with circular economy principles and global climate objectives.
Water conservation represents another critical sustainability advantage. Flow enzymatic systems typically require 25-45% less water than comparable batch processes, addressing growing concerns about industrial water consumption. This efficiency stems from improved heat and mass transfer characteristics inherent to microfluidic and continuous flow systems, allowing for more precise reaction control with minimal resource input.
The waste reduction profile of enzymatic flow processes further enhances their sustainability credentials. These systems generate approximately 35-55% less waste compared to conventional methods, primarily due to higher conversion rates, improved selectivity, and reduced side-product formation. The controlled reaction environment in flow systems enables enzymes to operate at their optimal efficiency, maximizing atom economy and minimizing downstream purification requirements.
From a life cycle assessment perspective, enzymatic flow processes demonstrate favorable environmental profiles across multiple impact categories. Research indicates reductions of 20-40% in global warming potential, 30-50% in ecotoxicity measures, and 25-45% in resource depletion indicators when compared to traditional chemical synthesis routes. These improvements become particularly significant when scaled to industrial production volumes.
The sustainability benefits extend to operational aspects as well. Flow enzymatic processes typically require smaller physical footprints for equivalent production capacity, reducing land use requirements by 40-60%. Their modular nature facilitates distributed manufacturing models that can decrease transportation-related emissions by enabling production closer to points of consumption.
Looking forward, the integration of renewable energy sources with enzymatic flow processes presents opportunities for further sustainability enhancements. Pilot projects combining solar or wind power with continuous flow biocatalysis have demonstrated the potential for near-carbon-neutral manufacturing of pharmaceuticals and fine chemicals, establishing a pathway toward truly sustainable chemical production systems that align with circular economy principles and global climate objectives.
Scalability and Industrial Implementation Considerations
Scaling up flow chemistry processes for enzymatic and biocatalytic transformations from laboratory to industrial scale presents unique challenges that require careful consideration. The transition demands not only technical adaptations but also economic viability assessments. Industrial implementation necessitates robust process design that can maintain consistent enzyme activity and stability under continuous operation conditions.
Equipment selection becomes critical at industrial scale, with specialized reactors needed to accommodate the particular requirements of biocatalysts. Packed-bed reactors with immobilized enzymes offer advantages for long-term operations, while membrane reactors provide solutions for enzyme retention and product separation. The material of construction must be biocompatible and resistant to the often mild but potentially corrosive conditions of biocatalytic processes.
Process parameters require significant optimization during scale-up. Residence time distribution, mixing efficiency, and mass transfer characteristics differ substantially between laboratory microreactors and industrial-scale flow systems. These differences can significantly impact enzyme performance and reaction outcomes. Temperature control systems must be more sophisticated at larger scales to manage the heat generated by exothermic biocatalytic reactions while maintaining optimal enzyme activity.
Economic considerations drive many scale-up decisions. The cost of enzymes represents a substantial portion of operational expenses, making enzyme immobilization and recycling strategies essential for cost-effective production. Recovery systems that allow multiple reuses of biocatalysts can dramatically improve process economics. Additionally, downstream processing must be integrated with the flow system to minimize intermediate handling and maximize overall efficiency.
Regulatory compliance adds another layer of complexity to industrial implementation. Pharmaceutical and food applications face stringent requirements regarding process validation, reproducibility, and product purity. Flow chemistry offers advantages in meeting these requirements through precise control and continuous monitoring, but validation protocols must be established specifically for enzymatic flow processes.
Sustainability metrics increasingly influence industrial adoption decisions. Flow-based enzymatic processes typically demonstrate improved environmental performance compared to traditional batch methods, with reduced solvent usage, energy consumption, and waste generation. Quantifying these benefits through life cycle assessment becomes important for justifying technology transitions and meeting corporate sustainability goals.
Equipment selection becomes critical at industrial scale, with specialized reactors needed to accommodate the particular requirements of biocatalysts. Packed-bed reactors with immobilized enzymes offer advantages for long-term operations, while membrane reactors provide solutions for enzyme retention and product separation. The material of construction must be biocompatible and resistant to the often mild but potentially corrosive conditions of biocatalytic processes.
Process parameters require significant optimization during scale-up. Residence time distribution, mixing efficiency, and mass transfer characteristics differ substantially between laboratory microreactors and industrial-scale flow systems. These differences can significantly impact enzyme performance and reaction outcomes. Temperature control systems must be more sophisticated at larger scales to manage the heat generated by exothermic biocatalytic reactions while maintaining optimal enzyme activity.
Economic considerations drive many scale-up decisions. The cost of enzymes represents a substantial portion of operational expenses, making enzyme immobilization and recycling strategies essential for cost-effective production. Recovery systems that allow multiple reuses of biocatalysts can dramatically improve process economics. Additionally, downstream processing must be integrated with the flow system to minimize intermediate handling and maximize overall efficiency.
Regulatory compliance adds another layer of complexity to industrial implementation. Pharmaceutical and food applications face stringent requirements regarding process validation, reproducibility, and product purity. Flow chemistry offers advantages in meeting these requirements through precise control and continuous monitoring, but validation protocols must be established specifically for enzymatic flow processes.
Sustainability metrics increasingly influence industrial adoption decisions. Flow-based enzymatic processes typically demonstrate improved environmental performance compared to traditional batch methods, with reduced solvent usage, energy consumption, and waste generation. Quantifying these benefits through life cycle assessment becomes important for justifying technology transitions and meeting corporate sustainability goals.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!