Scale-Up Strategies For Microreactor Processes In Pharmaceutical Manufacturing
SEP 3, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Microreactor Technology Evolution and Objectives
Microreactor technology has evolved significantly over the past three decades, transitioning from laboratory curiosities to viable manufacturing tools in the pharmaceutical industry. The initial development phase in the 1990s focused primarily on proof-of-concept demonstrations, with researchers exploring the fundamental advantages of microscale processing such as enhanced heat and mass transfer. These early microreactors were typically fabricated from glass or silicon using techniques borrowed from the microelectronics industry.
By the early 2000s, the technology entered a more practical phase with the introduction of modular microreactor systems that could be configured for specific reactions. This period saw significant improvements in materials of construction, with stainless steel, hastelloy, and various polymers being employed to withstand harsh chemical environments. The integration of inline analytical techniques also began during this phase, enabling real-time monitoring of reaction parameters.
The 2010s marked a turning point for microreactor technology in pharmaceutical applications, with several major pharmaceutical companies establishing dedicated continuous manufacturing facilities incorporating microreactor technology. This period witnessed the development of more sophisticated flow chemistry platforms capable of handling multiphase reactions, solid-forming reactions, and highly exothermic processes that were previously challenging in continuous flow.
Current microreactor technology has evolved to address specific pharmaceutical manufacturing challenges, including the ability to handle solids, perform photochemical transformations, and execute electrochemical reactions. Modern systems feature advanced process control capabilities, improved safety features, and enhanced scalability options that facilitate the transition from laboratory to production scale.
The primary objectives of microreactor technology in pharmaceutical manufacturing include intensifying production processes to reduce equipment footprint, improving product quality through precise control of reaction parameters, enhancing safety profiles for hazardous chemistry, and enabling more sustainable manufacturing through reduced solvent usage and energy consumption. Additionally, microreactors aim to accelerate drug development timelines by providing a seamless path from discovery to commercial production.
Looking forward, the evolution of microreactor technology is expected to focus on addressing remaining challenges in scale-up strategies, particularly in developing predictive models that accurately translate microscale phenomena to production volumes. Objectives include creating standardized approaches for scale-up that minimize the need for extensive experimentation, developing more robust systems for handling crystallization and other solid-forming processes, and integrating advanced automation and artificial intelligence to optimize reaction conditions autonomously.
By the early 2000s, the technology entered a more practical phase with the introduction of modular microreactor systems that could be configured for specific reactions. This period saw significant improvements in materials of construction, with stainless steel, hastelloy, and various polymers being employed to withstand harsh chemical environments. The integration of inline analytical techniques also began during this phase, enabling real-time monitoring of reaction parameters.
The 2010s marked a turning point for microreactor technology in pharmaceutical applications, with several major pharmaceutical companies establishing dedicated continuous manufacturing facilities incorporating microreactor technology. This period witnessed the development of more sophisticated flow chemistry platforms capable of handling multiphase reactions, solid-forming reactions, and highly exothermic processes that were previously challenging in continuous flow.
Current microreactor technology has evolved to address specific pharmaceutical manufacturing challenges, including the ability to handle solids, perform photochemical transformations, and execute electrochemical reactions. Modern systems feature advanced process control capabilities, improved safety features, and enhanced scalability options that facilitate the transition from laboratory to production scale.
The primary objectives of microreactor technology in pharmaceutical manufacturing include intensifying production processes to reduce equipment footprint, improving product quality through precise control of reaction parameters, enhancing safety profiles for hazardous chemistry, and enabling more sustainable manufacturing through reduced solvent usage and energy consumption. Additionally, microreactors aim to accelerate drug development timelines by providing a seamless path from discovery to commercial production.
Looking forward, the evolution of microreactor technology is expected to focus on addressing remaining challenges in scale-up strategies, particularly in developing predictive models that accurately translate microscale phenomena to production volumes. Objectives include creating standardized approaches for scale-up that minimize the need for extensive experimentation, developing more robust systems for handling crystallization and other solid-forming processes, and integrating advanced automation and artificial intelligence to optimize reaction conditions autonomously.
Pharmaceutical Market Demand for Microreactor Scale-Up
The pharmaceutical industry is experiencing a significant shift towards continuous manufacturing processes, with microreactor technology emerging as a key enabler of this transformation. Market analysis indicates that the global pharmaceutical continuous manufacturing market is projected to reach $2.4 billion by 2027, growing at a CAGR of 13.8% from 2022. Within this broader trend, microreactor technology represents a rapidly expanding segment due to its potential to revolutionize pharmaceutical production methods.
The demand for microreactor scale-up solutions is primarily driven by increasing pressure on pharmaceutical companies to reduce time-to-market for new drugs while maintaining stringent quality standards. With patent cliffs threatening revenue streams, manufacturers are seeking more efficient production methods that can accelerate drug development cycles. Microreactor technology offers a promising solution by enabling faster process development and more efficient manufacturing.
Cost reduction represents another significant market driver. Traditional batch manufacturing requires substantial capital investment in large-scale equipment and facilities. In contrast, microreactor systems can potentially reduce manufacturing footprint by 40-60%, with corresponding reductions in capital expenditure. Additionally, the improved yield and reduced waste associated with microreactor processes directly address the industry's focus on sustainability and environmental responsibility.
Quality considerations further strengthen market demand for microreactor scale-up technologies. Regulatory bodies, including the FDA and EMA, have demonstrated increasing support for continuous manufacturing approaches due to their potential for improved process control and product consistency. The enhanced process analytical technology (PAT) integration capabilities of microreactor systems align perfectly with regulatory quality-by-design (QbD) initiatives.
Market segmentation reveals particularly strong demand in biologics manufacturing, where precise control of reaction conditions is critical for product quality. The biologics segment is expected to grow at 16.2% CAGR through 2027, outpacing small molecule applications. Geographically, North America currently leads market demand, accounting for approximately 42% of the global market, followed by Europe at 31% and Asia-Pacific at 22%, with the latter showing the fastest growth trajectory.
Customer needs analysis indicates that pharmaceutical manufacturers are specifically seeking scalable microreactor solutions that can bridge the gap between laboratory development and commercial production. The ideal systems must demonstrate reliable scale-up methodologies that maintain reaction performance across different production volumes while offering flexibility to accommodate diverse chemical processes. Integration capabilities with existing manufacturing infrastructure and compliance with regulatory requirements are additional critical factors driving purchasing decisions.
The demand for microreactor scale-up solutions is primarily driven by increasing pressure on pharmaceutical companies to reduce time-to-market for new drugs while maintaining stringent quality standards. With patent cliffs threatening revenue streams, manufacturers are seeking more efficient production methods that can accelerate drug development cycles. Microreactor technology offers a promising solution by enabling faster process development and more efficient manufacturing.
Cost reduction represents another significant market driver. Traditional batch manufacturing requires substantial capital investment in large-scale equipment and facilities. In contrast, microreactor systems can potentially reduce manufacturing footprint by 40-60%, with corresponding reductions in capital expenditure. Additionally, the improved yield and reduced waste associated with microreactor processes directly address the industry's focus on sustainability and environmental responsibility.
Quality considerations further strengthen market demand for microreactor scale-up technologies. Regulatory bodies, including the FDA and EMA, have demonstrated increasing support for continuous manufacturing approaches due to their potential for improved process control and product consistency. The enhanced process analytical technology (PAT) integration capabilities of microreactor systems align perfectly with regulatory quality-by-design (QbD) initiatives.
Market segmentation reveals particularly strong demand in biologics manufacturing, where precise control of reaction conditions is critical for product quality. The biologics segment is expected to grow at 16.2% CAGR through 2027, outpacing small molecule applications. Geographically, North America currently leads market demand, accounting for approximately 42% of the global market, followed by Europe at 31% and Asia-Pacific at 22%, with the latter showing the fastest growth trajectory.
Customer needs analysis indicates that pharmaceutical manufacturers are specifically seeking scalable microreactor solutions that can bridge the gap between laboratory development and commercial production. The ideal systems must demonstrate reliable scale-up methodologies that maintain reaction performance across different production volumes while offering flexibility to accommodate diverse chemical processes. Integration capabilities with existing manufacturing infrastructure and compliance with regulatory requirements are additional critical factors driving purchasing decisions.
Current Challenges in Microreactor Scale-Up Technologies
Despite significant advancements in microreactor technology for pharmaceutical manufacturing, several critical challenges persist in scaling up these processes from laboratory to industrial production. The fundamental issue lies in maintaining the inherent advantages of microreactors—excellent heat and mass transfer, precise residence time control, and enhanced safety profiles—while increasing throughput to commercially viable levels.
One primary challenge is the design of parallelization strategies. While numbering-up (adding identical microreactor units in parallel) seems conceptually straightforward, it introduces complex flow distribution problems. Ensuring uniform flow across multiple channels requires sophisticated manifold designs to prevent maldistribution, which can lead to inconsistent product quality and reduced yield. Current manifold technologies often struggle to maintain pressure equilibrium across numerous parallel channels.
Heat management presents another significant obstacle during scale-up. As production volumes increase, the surface-to-volume ratio—a key advantage of microreactors—inevitably decreases, compromising thermal control. This can lead to hotspot formation, runaway reactions, or insufficient cooling, particularly for highly exothermic pharmaceutical processes. Existing heat exchange technologies for larger-scale microreactor systems often fail to match the performance achieved at laboratory scale.
Material compatibility issues become more pronounced at scale. While laboratory prototypes can utilize exotic materials like silicon, glass, or specialized polymers, cost considerations for industrial implementation often necessitate more conventional materials that may have inferior chemical resistance or thermal properties. This creates a significant engineering challenge in maintaining process performance while using industrially viable materials.
Control system complexity increases exponentially with scale-up. Monitoring and controlling hundreds or thousands of parallel channels requires sophisticated sensor networks and control algorithms that can detect and respond to deviations in individual channels. Current sensor technologies often lack the resolution, response time, or reliability needed for comprehensive monitoring of large-scale microreactor arrays.
Fouling and clogging risks are amplified in scaled-up systems. Even minor precipitation or particulate formation that might be manageable in single laboratory channels can cascade into system-wide failures in industrial settings. Existing preventive and cleaning strategies developed for laboratory-scale operations often prove inadequate for continuous industrial production.
Regulatory and validation challenges further complicate scale-up efforts. Pharmaceutical manufacturers must demonstrate that product quality remains consistent throughout the scale-up process, requiring extensive validation studies and robust quality control systems that can accommodate the distributed nature of parallelized microreactor systems.
One primary challenge is the design of parallelization strategies. While numbering-up (adding identical microreactor units in parallel) seems conceptually straightforward, it introduces complex flow distribution problems. Ensuring uniform flow across multiple channels requires sophisticated manifold designs to prevent maldistribution, which can lead to inconsistent product quality and reduced yield. Current manifold technologies often struggle to maintain pressure equilibrium across numerous parallel channels.
Heat management presents another significant obstacle during scale-up. As production volumes increase, the surface-to-volume ratio—a key advantage of microreactors—inevitably decreases, compromising thermal control. This can lead to hotspot formation, runaway reactions, or insufficient cooling, particularly for highly exothermic pharmaceutical processes. Existing heat exchange technologies for larger-scale microreactor systems often fail to match the performance achieved at laboratory scale.
Material compatibility issues become more pronounced at scale. While laboratory prototypes can utilize exotic materials like silicon, glass, or specialized polymers, cost considerations for industrial implementation often necessitate more conventional materials that may have inferior chemical resistance or thermal properties. This creates a significant engineering challenge in maintaining process performance while using industrially viable materials.
Control system complexity increases exponentially with scale-up. Monitoring and controlling hundreds or thousands of parallel channels requires sophisticated sensor networks and control algorithms that can detect and respond to deviations in individual channels. Current sensor technologies often lack the resolution, response time, or reliability needed for comprehensive monitoring of large-scale microreactor arrays.
Fouling and clogging risks are amplified in scaled-up systems. Even minor precipitation or particulate formation that might be manageable in single laboratory channels can cascade into system-wide failures in industrial settings. Existing preventive and cleaning strategies developed for laboratory-scale operations often prove inadequate for continuous industrial production.
Regulatory and validation challenges further complicate scale-up efforts. Pharmaceutical manufacturers must demonstrate that product quality remains consistent throughout the scale-up process, requiring extensive validation studies and robust quality control systems that can accommodate the distributed nature of parallelized microreactor systems.
Current Scale-Up Methodologies for Pharmaceutical Microreactors
01 Numerical modeling and simulation for microreactor scale-up
Computational modeling and simulation techniques are essential for predicting microreactor performance during scale-up. These methods allow for the optimization of process parameters, flow dynamics, and reaction kinetics without extensive physical testing. Advanced simulation tools can model heat and mass transfer, fluid dynamics, and chemical reactions in microreactor systems, enabling engineers to identify potential issues before physical implementation and design more efficient scale-up strategies.- Numerical modeling and simulation for microreactor scale-up: Computational fluid dynamics (CFD) and numerical modeling techniques are essential tools for predicting microreactor performance during scale-up. These simulations help engineers understand flow patterns, mixing efficiency, heat transfer, and reaction kinetics at different scales. By creating accurate digital models, researchers can optimize reactor designs, identify potential bottlenecks, and validate scale-up strategies before physical implementation, significantly reducing development time and costs.
- Modular and parallel microreactor systems: Instead of traditional scale-up, microreactors can be scaled out through modular and parallel approaches. This strategy involves replicating identical microreactor units and operating them in parallel to increase production capacity while maintaining the advantageous characteristics of microscale processing. Modular designs allow for flexible production capacity, easier maintenance, and reduced risk, as individual units can be added or removed based on demand without redesigning the entire system.
- Process intensification and optimization techniques: Process intensification strategies focus on enhancing microreactor efficiency through optimized operating conditions, improved catalyst designs, and innovative mixing technologies. These techniques involve systematic parameter optimization, including residence time, temperature profiles, pressure conditions, and catalyst loading. Advanced control systems continuously monitor and adjust process variables to maintain optimal performance during scale-up, ensuring consistent product quality and yield across different production scales.
- Automated control systems and digital twins: Advanced control systems incorporating artificial intelligence and machine learning algorithms enable real-time monitoring and adjustment of microreactor processes during scale-up. Digital twin technology creates virtual replicas of physical microreactor systems that can predict performance, identify potential issues, and optimize operating conditions. These systems collect and analyze data from multiple sensors throughout the process, allowing for predictive maintenance and continuous improvement of scale-up strategies.
- Novel microreactor designs for enhanced scalability: Innovative microreactor designs specifically engineered for scalability incorporate features like improved heat exchange capabilities, enhanced mixing mechanisms, and specialized materials of construction. These designs often include adjustable geometries that can accommodate changing flow rates and reaction conditions during scale-up. Some advanced microreactors feature integrated sensing and monitoring capabilities that provide real-time feedback on reaction progress, enabling precise control and optimization throughout the scaling process.
02 Modular and parallel microreactor systems
Modular design approaches involve scaling up microreactor processes by numbering up identical reactor units rather than increasing the size of a single reactor. This strategy maintains the advantageous characteristics of microreactors while increasing production capacity. Parallel configuration of multiple microreactor modules allows for flexible production scaling, improved process control, and reduced risk during scale-up. The modular approach also facilitates maintenance and replacement of individual units without shutting down the entire production system.Expand Specific Solutions03 Process intensification and optimization techniques
Process intensification strategies focus on enhancing reaction efficiency and throughput in microreactor systems during scale-up. These techniques include optimizing reaction conditions, improving mixing efficiency, enhancing heat transfer, and implementing advanced catalyst designs. By intensifying the process, higher yields and selectivities can be achieved with smaller equipment footprints. Optimization algorithms and experimental design methodologies help identify the optimal operating parameters for scaled-up microreactor processes.Expand Specific Solutions04 Automated control systems for microreactor scale-up
Advanced control systems are crucial for maintaining precise process conditions during microreactor scale-up. These systems incorporate real-time monitoring, feedback control loops, and predictive algorithms to ensure consistent product quality across different scales. Automated control strategies can adjust process parameters in response to variations in feed composition, environmental conditions, or equipment performance. Integration of sensors, data analytics, and machine learning enhances the robustness of microreactor operations during scale-up.Expand Specific Solutions05 Continuous flow manufacturing and scale-up methodologies
Continuous flow manufacturing approaches provide a systematic framework for scaling up microreactor processes from laboratory to industrial production. These methodologies focus on maintaining consistent residence time distributions, mixing patterns, and heat transfer characteristics across different scales. Quality-by-design principles are applied to ensure product quality throughout the scale-up process. Risk assessment tools help identify critical process parameters and potential failure modes, enabling more reliable scale-up of microreactor technologies.Expand Specific Solutions
Leading Companies in Pharmaceutical Microreactor Manufacturing
The pharmaceutical microreactor process scale-up landscape is currently in a growth phase, with the market expanding as manufacturers seek more efficient, sustainable production methods. The global market for continuous flow chemistry in pharmaceuticals is estimated to be worth several billion dollars, with significant growth potential as technology adoption increases. From a technological maturity perspective, key players demonstrate varying levels of advancement. Massachusetts Institute of Technology, Dalian Institute of Chemical Physics, and East China University of Science & Technology lead academic research, while companies like Cytiva (Global Life Sciences Solutions), PBS Biotech, and Shanghai Huihe Huade Biotechnology are commercializing innovative solutions. Pharmaceutical giants and chemical corporations including FUJIFILM, Sinopec, and Hitachi are investing in microreactor technology to enhance manufacturing efficiency, indicating the technology's transition from experimental to practical implementation in industrial settings.
Massachusetts Institute of Technology
Technical Solution: MIT has developed advanced microreactor technologies for pharmaceutical manufacturing that focus on continuous flow processes. Their approach integrates microfluidic systems with real-time analytics for precise control of reaction parameters. MIT's platform employs modular microreactor designs that can be easily reconfigured for different pharmaceutical synthesis pathways, allowing for rapid scale-up from laboratory to production volumes. Their technology incorporates automated feedback control systems that maintain optimal reaction conditions throughout scale-up transitions, minimizing batch-to-batch variability[1]. MIT researchers have demonstrated successful scale-up of complex API syntheses by implementing numbering-up strategies, where multiple microreactor units operate in parallel rather than increasing individual reactor dimensions, preserving the advantageous heat and mass transfer characteristics of microscale operations[2]. Their systems also feature integrated purification modules that enable continuous downstream processing, further enhancing production efficiency.
Strengths: Superior heat and mass transfer efficiency allowing for safer handling of exothermic reactions; excellent process control leading to higher product quality consistency; reduced scale-up risks through numbering-up approach. Weaknesses: Higher initial capital investment compared to batch processes; potential challenges with solids handling in continuous flow systems; requires specialized expertise for implementation and operation.
Nirrin Bioprocess Analytics, Inc.
Technical Solution: Nirrin Bioprocess Analytics has developed innovative scale-up strategies for microreactor processes in pharmaceutical manufacturing centered around their proprietary spectroscopic monitoring technology. Their approach integrates advanced near-infrared (NIR) and Raman spectroscopy directly into microreactor systems, enabling real-time, non-destructive monitoring of chemical reactions and biological processes at the molecular level. This capability provides unprecedented insight into reaction kinetics and product formation during scale-up transitions[9]. Nirrin's platform incorporates chemometric modeling algorithms that correlate spectroscopic data with critical quality attributes, allowing for predictive scale-up based on molecular fingerprinting rather than traditional process parameters alone. Their technology features specialized probe designs that maintain consistent measurement geometry across different reactor scales, ensuring comparable spectral data from laboratory to production environments. Nirrin has implemented a systematic scale-up methodology that begins with comprehensive characterization of microreactor performance using their spectroscopic tools, followed by model-based prediction of larger-scale behavior, and verification through targeted pilot studies[10]. Their approach significantly reduces the empirical nature of traditional scale-up by providing direct visualization of molecular transformations throughout the process.
Strengths: Molecular-level process understanding enabling knowledge-based scale-up; early detection of scale-up issues through real-time monitoring; reduced material consumption during development phase. Weaknesses: Requires sophisticated data analysis capabilities and expertise in spectroscopy; initial calibration process can be time-consuming; potential challenges with highly turbid or heterogeneous systems.
Key Patents and Innovations in Microreactor Scale-Up
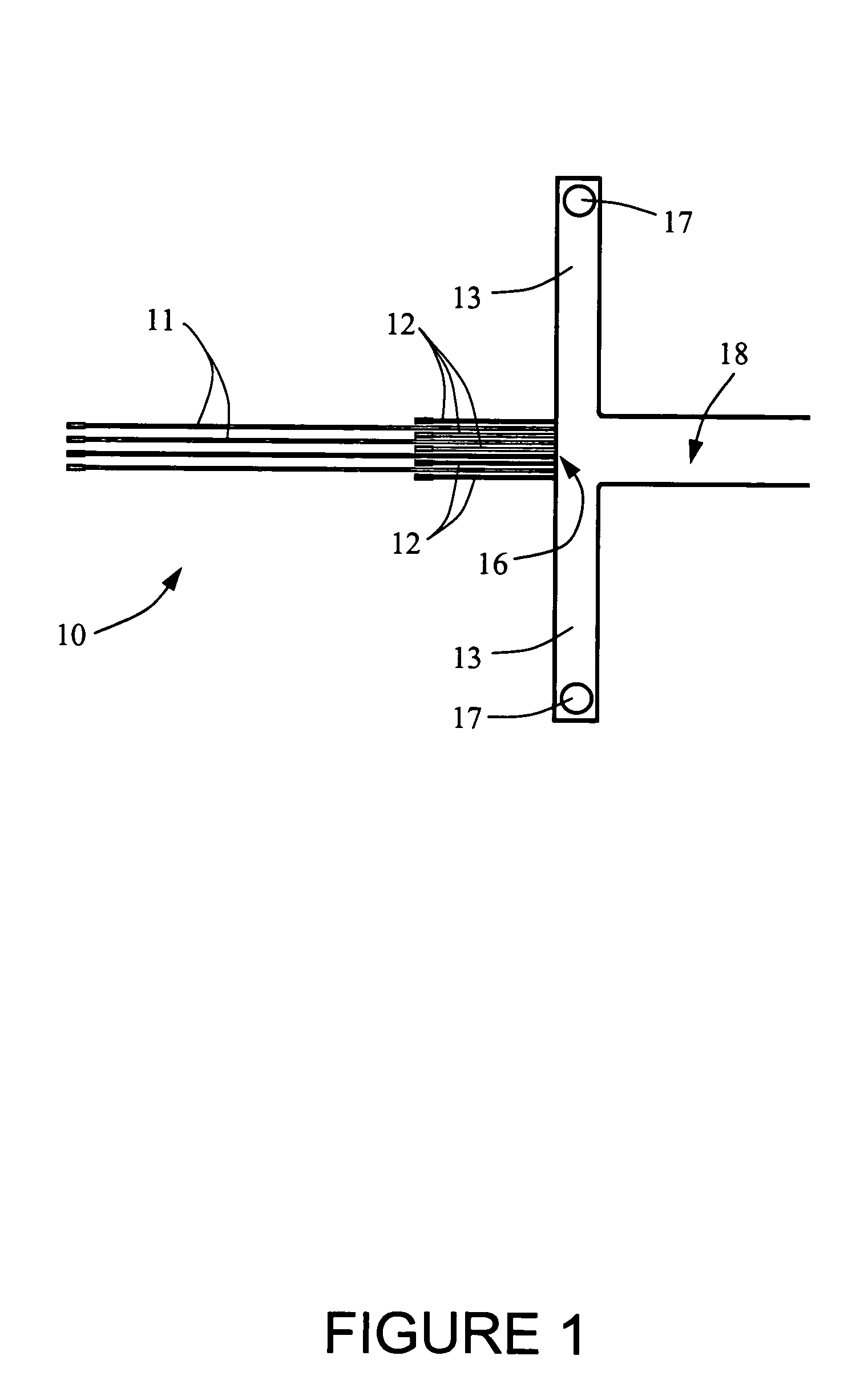
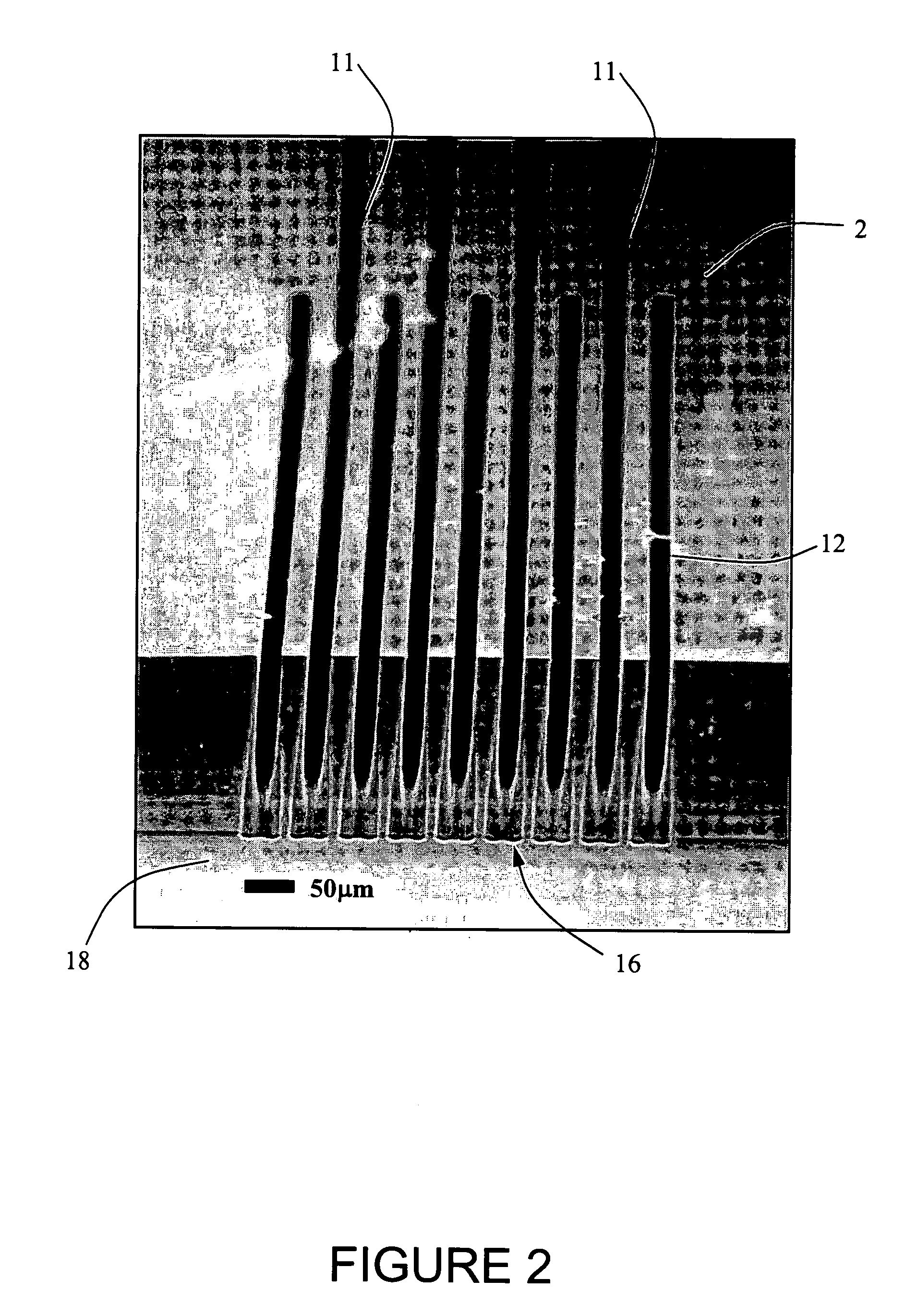
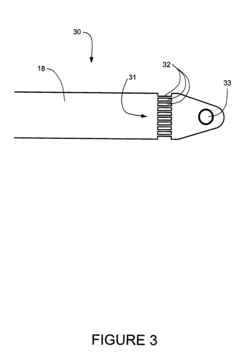
Modular numbering-up microreactor for increasing the production of pharmaceuticals
PatentActiveKR1020210040648A
Innovation
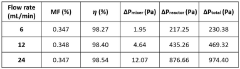
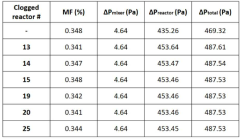
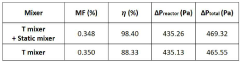
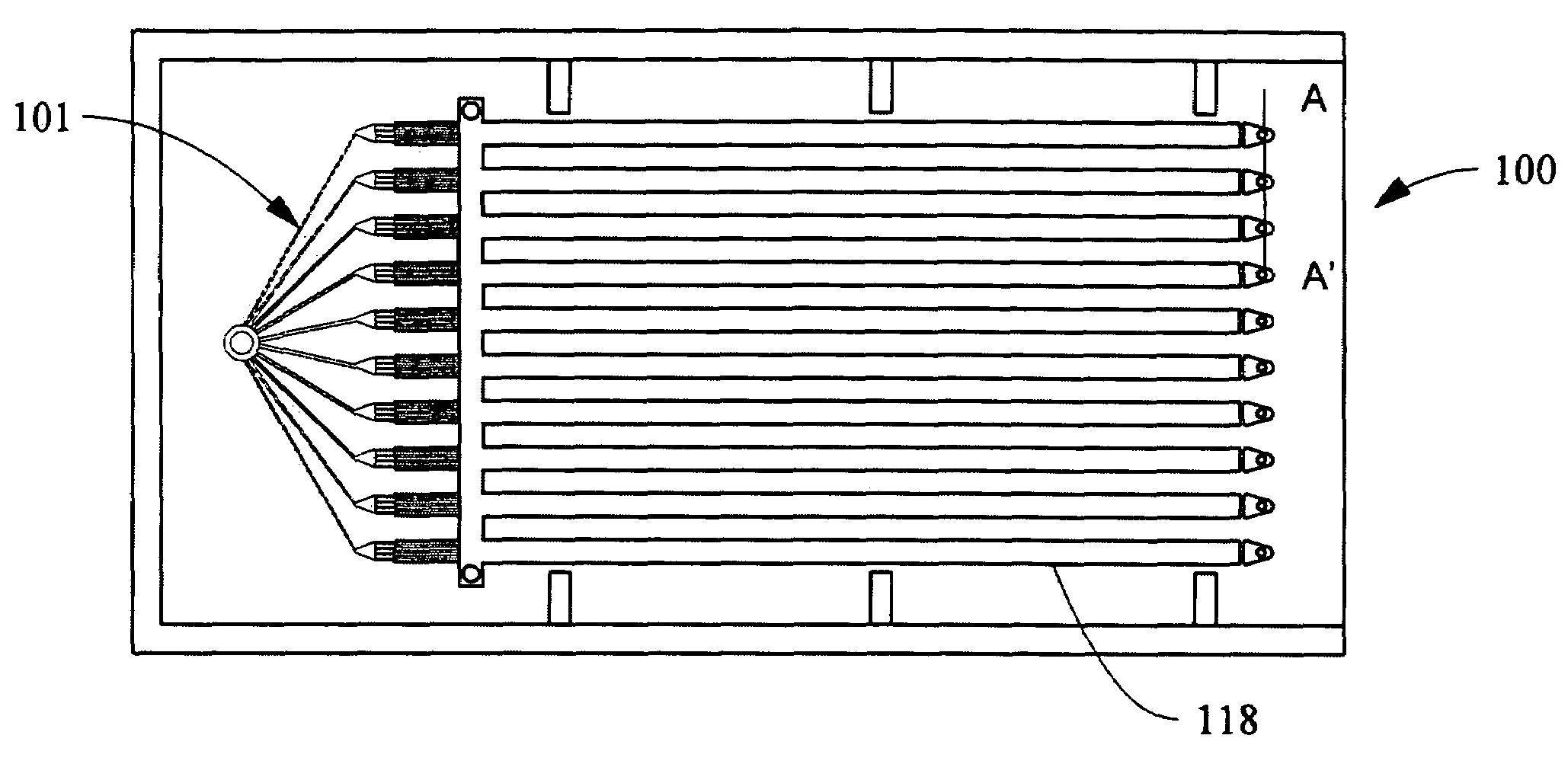
- A modular numbering-up microreactor assembly comprising a raw material injection and mixing module, flow distribution module, and integrated discharge module, utilizing a T-mixer and static mixer for uniform flow distribution and heterogeneous catalytic reaction modules, including copper capillaries, to enhance scalability and mixing efficiency.
Microfabricated chemical reactor
PatentInactiveUS6932951B1
Innovation
- A microfabricated chemical reactor with a plurality of laminae, featuring an inlet port, an outlet port, and a continuous channel with a microfluidic manifold and catalyst support structures, designed to enhance mixing and mass transfer while maintaining a low pressure drop, allowing for efficient heterogeneous catalytic reactions.
Economic Analysis of Microreactor Implementation
The economic analysis of microreactor implementation in pharmaceutical manufacturing reveals a complex cost-benefit landscape that differs significantly from traditional batch processing. Initial capital expenditure for microreactor systems typically ranges from $500,000 to $3 million, depending on scale and complexity, which may appear higher than conventional equipment on a per-unit basis. However, this investment must be evaluated against substantial operational savings and revenue enhancements that emerge over time.
Operational cost reductions represent a primary economic advantage, with studies indicating 30-45% lower energy consumption compared to batch reactors due to improved heat transfer efficiency and reduced heating/cooling requirements. Solvent usage typically decreases by 50-80%, significantly reducing both material costs and waste disposal expenses. Labor costs also diminish as microreactor systems enable higher levels of automation and continuous operation with minimal operator intervention.
Space utilization economics favor microreactors substantially, with production facilities requiring 40-70% less floor space than equivalent batch operations. This spatial efficiency translates to lower facility construction costs, reduced overhead, and greater production capacity per square foot—particularly valuable in high-cost manufacturing locations or when expanding within existing facilities.
The economic impact of quality improvements cannot be overlooked. Microreactors demonstrate 15-25% higher product yields and significantly reduced batch-to-batch variability. This consistency reduces costly quality testing, minimizes rejected batches, and decreases regulatory compliance expenses. Studies from major pharmaceutical manufacturers indicate that quality-related savings alone can offset microreactor implementation costs within 2-3 years of operation.
Time-to-market acceleration provides another economic advantage. Continuous processing enables faster scale-up from laboratory to production, potentially reducing development timelines by 30-50%. This acceleration can generate substantial additional revenue through earlier market entry, particularly for high-value pharmaceuticals where each day of patent-protected sales may represent millions in revenue.
Risk mitigation also carries economic value, with microreactors offering inherent safety advantages through smaller reaction volumes and improved process control. Insurance premiums typically decrease by 10-20% following microreactor implementation, while the likelihood of costly production interruptions due to quality or safety incidents decreases substantially.
Operational cost reductions represent a primary economic advantage, with studies indicating 30-45% lower energy consumption compared to batch reactors due to improved heat transfer efficiency and reduced heating/cooling requirements. Solvent usage typically decreases by 50-80%, significantly reducing both material costs and waste disposal expenses. Labor costs also diminish as microreactor systems enable higher levels of automation and continuous operation with minimal operator intervention.
Space utilization economics favor microreactors substantially, with production facilities requiring 40-70% less floor space than equivalent batch operations. This spatial efficiency translates to lower facility construction costs, reduced overhead, and greater production capacity per square foot—particularly valuable in high-cost manufacturing locations or when expanding within existing facilities.
The economic impact of quality improvements cannot be overlooked. Microreactors demonstrate 15-25% higher product yields and significantly reduced batch-to-batch variability. This consistency reduces costly quality testing, minimizes rejected batches, and decreases regulatory compliance expenses. Studies from major pharmaceutical manufacturers indicate that quality-related savings alone can offset microreactor implementation costs within 2-3 years of operation.
Time-to-market acceleration provides another economic advantage. Continuous processing enables faster scale-up from laboratory to production, potentially reducing development timelines by 30-50%. This acceleration can generate substantial additional revenue through earlier market entry, particularly for high-value pharmaceuticals where each day of patent-protected sales may represent millions in revenue.
Risk mitigation also carries economic value, with microreactors offering inherent safety advantages through smaller reaction volumes and improved process control. Insurance premiums typically decrease by 10-20% following microreactor implementation, while the likelihood of costly production interruptions due to quality or safety incidents decreases substantially.
Regulatory Compliance for Continuous Manufacturing
The regulatory landscape for continuous manufacturing in pharmaceutical production represents a critical consideration when implementing microreactor scale-up strategies. Regulatory bodies worldwide, including the FDA and EMA, have recognized the potential benefits of continuous manufacturing and have developed frameworks to support its implementation while ensuring product quality and patient safety.
The FDA's Emerging Technology Program specifically encourages pharmaceutical companies to adopt innovative manufacturing approaches, including continuous processing with microreactors. This program provides a pathway for early engagement between manufacturers and regulators, facilitating smoother regulatory approval processes for novel manufacturing technologies.
Quality by Design (QbD) principles form the cornerstone of regulatory compliance for continuous manufacturing systems. These principles emphasize thorough understanding of process parameters, material attributes, and their impact on product quality. For microreactor processes, this translates to comprehensive characterization of mixing efficiency, heat transfer capabilities, and reaction kinetics across different scales of operation.
Process Analytical Technology (PAT) implementation is essential for regulatory acceptance of continuous manufacturing processes. Real-time monitoring and control systems must demonstrate robust performance to ensure consistent product quality. Regulatory authorities expect manufacturers to establish appropriate control strategies with clearly defined critical process parameters (CPPs) and critical quality attributes (CQAs).
Validation requirements for continuous processes differ significantly from batch manufacturing. Regulators expect state-of-control demonstrations through process validation approaches that may include continuous process verification rather than traditional three-batch validation. This requires sophisticated statistical methods to analyze continuous data streams and establish process capability.
Change management presents unique challenges in the regulatory context of continuous manufacturing. When scaling up microreactor processes, manufacturers must establish clear protocols for assessing the impact of process modifications on product quality. Regulatory submissions should include comprehensive comparability studies demonstrating that scaled-up processes produce equivalent products.
International harmonization efforts, such as those through the International Council for Harmonisation (ICH), are gradually addressing regulatory inconsistencies across different regions. However, manufacturers must still navigate varying regional requirements when implementing continuous manufacturing technologies globally.
Data integrity and cybersecurity considerations have gained increasing regulatory attention for automated continuous manufacturing systems. Robust data management systems with appropriate audit trails and security measures are essential for regulatory compliance, particularly as microreactor processes often generate substantial volumes of process data requiring sophisticated analysis and storage solutions.
The FDA's Emerging Technology Program specifically encourages pharmaceutical companies to adopt innovative manufacturing approaches, including continuous processing with microreactors. This program provides a pathway for early engagement between manufacturers and regulators, facilitating smoother regulatory approval processes for novel manufacturing technologies.
Quality by Design (QbD) principles form the cornerstone of regulatory compliance for continuous manufacturing systems. These principles emphasize thorough understanding of process parameters, material attributes, and their impact on product quality. For microreactor processes, this translates to comprehensive characterization of mixing efficiency, heat transfer capabilities, and reaction kinetics across different scales of operation.
Process Analytical Technology (PAT) implementation is essential for regulatory acceptance of continuous manufacturing processes. Real-time monitoring and control systems must demonstrate robust performance to ensure consistent product quality. Regulatory authorities expect manufacturers to establish appropriate control strategies with clearly defined critical process parameters (CPPs) and critical quality attributes (CQAs).
Validation requirements for continuous processes differ significantly from batch manufacturing. Regulators expect state-of-control demonstrations through process validation approaches that may include continuous process verification rather than traditional three-batch validation. This requires sophisticated statistical methods to analyze continuous data streams and establish process capability.
Change management presents unique challenges in the regulatory context of continuous manufacturing. When scaling up microreactor processes, manufacturers must establish clear protocols for assessing the impact of process modifications on product quality. Regulatory submissions should include comprehensive comparability studies demonstrating that scaled-up processes produce equivalent products.
International harmonization efforts, such as those through the International Council for Harmonisation (ICH), are gradually addressing regulatory inconsistencies across different regions. However, manufacturers must still navigate varying regional requirements when implementing continuous manufacturing technologies globally.
Data integrity and cybersecurity considerations have gained increasing regulatory attention for automated continuous manufacturing systems. Robust data management systems with appropriate audit trails and security measures are essential for regulatory compliance, particularly as microreactor processes often generate substantial volumes of process data requiring sophisticated analysis and storage solutions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!