Flow Chemistry For Late-Stage Functionalization In Drug Discovery
SEP 3, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Flow Chemistry Background and Objectives
Flow chemistry represents a paradigm shift in synthetic chemistry, evolving from traditional batch processes to continuous flow systems. This methodology, which emerged in the early 2000s, has gained significant momentum in pharmaceutical research due to its precision, efficiency, and scalability. The continuous nature of flow chemistry allows for precise control over reaction parameters such as temperature, pressure, and mixing, which is particularly valuable for complex transformations required in drug discovery.
The evolution of flow chemistry has been marked by technological advancements in microreactors, pumping systems, and inline analytical tools. Initially limited to simple transformations, modern flow systems now accommodate multistep syntheses, heterogeneous catalysis, and photochemical reactions—capabilities that have revolutionized late-stage functionalization approaches in medicinal chemistry.
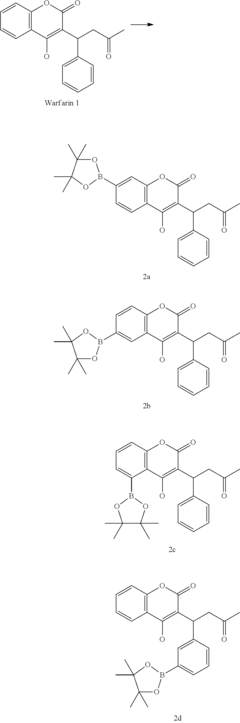
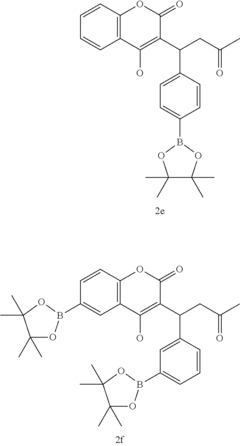
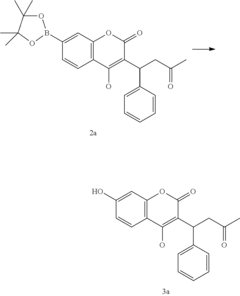
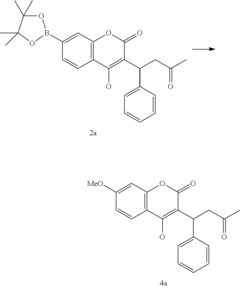
Late-stage functionalization (LSF) refers to the selective modification of complex molecules at specific positions without disturbing existing functional groups. This approach has become increasingly important in drug discovery as it enables rapid diversification of lead compounds, accelerating the exploration of structure-activity relationships and potentially reducing development timelines.
The integration of flow chemistry with LSF addresses several critical challenges in pharmaceutical development. Traditional batch methods often struggle with reactions requiring precise control over hazardous reagents, extreme conditions, or short-lived intermediates—all common in LSF processes. Flow systems mitigate these challenges through enhanced heat transfer, improved mixing, and the ability to safely handle unstable intermediates.
The primary objectives of flow chemistry for LSF in drug discovery include: enhancing reaction efficiency and selectivity; enabling previously challenging or impossible transformations; reducing reaction times from hours to minutes; minimizing waste generation through optimized reagent usage; and facilitating seamless scale-up from discovery to production without significant process redesign.
Recent technological trends indicate movement toward fully automated flow platforms that integrate reaction optimization, purification, and analysis. Machine learning algorithms are increasingly being applied to predict optimal flow conditions, while modular flow systems offer flexibility for diverse chemical transformations required in modern drug discovery campaigns.
The convergence of flow chemistry with emerging technologies such as photochemistry, electrochemistry, and biocatalysis presents particularly promising avenues for expanding the LSF toolkit. These hybrid approaches aim to access novel chemical space with greater efficiency and sustainability than conventional methods, potentially unlocking new therapeutic opportunities through previously inaccessible molecular modifications.
The evolution of flow chemistry has been marked by technological advancements in microreactors, pumping systems, and inline analytical tools. Initially limited to simple transformations, modern flow systems now accommodate multistep syntheses, heterogeneous catalysis, and photochemical reactions—capabilities that have revolutionized late-stage functionalization approaches in medicinal chemistry.
Late-stage functionalization (LSF) refers to the selective modification of complex molecules at specific positions without disturbing existing functional groups. This approach has become increasingly important in drug discovery as it enables rapid diversification of lead compounds, accelerating the exploration of structure-activity relationships and potentially reducing development timelines.
The integration of flow chemistry with LSF addresses several critical challenges in pharmaceutical development. Traditional batch methods often struggle with reactions requiring precise control over hazardous reagents, extreme conditions, or short-lived intermediates—all common in LSF processes. Flow systems mitigate these challenges through enhanced heat transfer, improved mixing, and the ability to safely handle unstable intermediates.
The primary objectives of flow chemistry for LSF in drug discovery include: enhancing reaction efficiency and selectivity; enabling previously challenging or impossible transformations; reducing reaction times from hours to minutes; minimizing waste generation through optimized reagent usage; and facilitating seamless scale-up from discovery to production without significant process redesign.
Recent technological trends indicate movement toward fully automated flow platforms that integrate reaction optimization, purification, and analysis. Machine learning algorithms are increasingly being applied to predict optimal flow conditions, while modular flow systems offer flexibility for diverse chemical transformations required in modern drug discovery campaigns.
The convergence of flow chemistry with emerging technologies such as photochemistry, electrochemistry, and biocatalysis presents particularly promising avenues for expanding the LSF toolkit. These hybrid approaches aim to access novel chemical space with greater efficiency and sustainability than conventional methods, potentially unlocking new therapeutic opportunities through previously inaccessible molecular modifications.
Market Demand Analysis for Flow Chemistry in Pharmaceuticals
The pharmaceutical industry is witnessing a significant shift towards more efficient and sustainable drug discovery processes, with flow chemistry emerging as a transformative technology. The global market for flow chemistry in pharmaceutical applications was valued at approximately $1.2 billion in 2022 and is projected to grow at a CAGR of 10.5% through 2030, driven primarily by increasing demand for more efficient drug development methodologies.
Late-stage functionalization (LSF) represents a particularly promising application area within flow chemistry, addressing the pharmaceutical industry's need to reduce development timelines and costs. Traditional batch synthesis methods often require 7-10 years and investments exceeding $2.6 billion to bring a new drug to market, creating substantial demand for technologies that can streamline this process.
Market research indicates that over 65% of pharmaceutical companies are actively exploring flow chemistry technologies, with late-stage functionalization capabilities being a priority investment area. This trend is particularly pronounced among the top 20 pharmaceutical companies, where adoption rates have increased by approximately 30% over the past five years.
The demand for flow chemistry in LSF is being driven by several key market factors. First, regulatory pressures to reduce environmental impact have intensified, with flow chemistry offering up to 90% reduction in solvent usage and waste generation compared to traditional methods. Second, patent cliffs facing major pharmaceutical companies necessitate more efficient methods to develop and optimize drug candidates, with flow chemistry enabling rapid synthesis and testing of multiple derivatives.
Regional analysis reveals varying adoption rates, with North America leading the market (38% share), followed by Europe (32%) and Asia-Pacific (25%). However, the Asia-Pacific region is experiencing the fastest growth rate at 12.8% annually, driven by expanding pharmaceutical manufacturing capabilities in China and India.
Contract research organizations (CROs) and contract development and manufacturing organizations (CDMOs) represent a rapidly growing market segment, with 72% reporting increased client requests for flow chemistry capabilities, particularly for late-stage functionalization applications. This trend reflects the broader pharmaceutical industry's move toward outsourcing specialized chemistry services.
Market forecasts suggest that flow chemistry technologies specifically designed for late-stage functionalization could capture 18% of the total pharmaceutical process technology market by 2028, representing a significant opportunity for technology providers and pharmaceutical companies alike. The convergence of automation, artificial intelligence, and flow chemistry is expected to further accelerate market growth, with integrated platforms that combine these technologies commanding premium pricing and showing the highest demand growth rates.
Late-stage functionalization (LSF) represents a particularly promising application area within flow chemistry, addressing the pharmaceutical industry's need to reduce development timelines and costs. Traditional batch synthesis methods often require 7-10 years and investments exceeding $2.6 billion to bring a new drug to market, creating substantial demand for technologies that can streamline this process.
Market research indicates that over 65% of pharmaceutical companies are actively exploring flow chemistry technologies, with late-stage functionalization capabilities being a priority investment area. This trend is particularly pronounced among the top 20 pharmaceutical companies, where adoption rates have increased by approximately 30% over the past five years.
The demand for flow chemistry in LSF is being driven by several key market factors. First, regulatory pressures to reduce environmental impact have intensified, with flow chemistry offering up to 90% reduction in solvent usage and waste generation compared to traditional methods. Second, patent cliffs facing major pharmaceutical companies necessitate more efficient methods to develop and optimize drug candidates, with flow chemistry enabling rapid synthesis and testing of multiple derivatives.
Regional analysis reveals varying adoption rates, with North America leading the market (38% share), followed by Europe (32%) and Asia-Pacific (25%). However, the Asia-Pacific region is experiencing the fastest growth rate at 12.8% annually, driven by expanding pharmaceutical manufacturing capabilities in China and India.
Contract research organizations (CROs) and contract development and manufacturing organizations (CDMOs) represent a rapidly growing market segment, with 72% reporting increased client requests for flow chemistry capabilities, particularly for late-stage functionalization applications. This trend reflects the broader pharmaceutical industry's move toward outsourcing specialized chemistry services.
Market forecasts suggest that flow chemistry technologies specifically designed for late-stage functionalization could capture 18% of the total pharmaceutical process technology market by 2028, representing a significant opportunity for technology providers and pharmaceutical companies alike. The convergence of automation, artificial intelligence, and flow chemistry is expected to further accelerate market growth, with integrated platforms that combine these technologies commanding premium pricing and showing the highest demand growth rates.
Current State and Challenges in Late-Stage Functionalization
Late-stage functionalization (LSF) has emerged as a powerful approach in drug discovery, allowing chemists to modify complex molecules at previously unreactive sites. Currently, flow chemistry represents a significant advancement in implementing LSF strategies, offering enhanced control over reaction parameters compared to traditional batch processes. The integration of flow technology with LSF has demonstrated remarkable success in C-H activation, heteroatom incorporation, and selective oxidation reactions.
Despite these advances, several technical challenges persist in the field. Reaction selectivity remains a primary concern, as achieving site-specific modifications on complex drug scaffolds containing multiple similar functional groups continues to be difficult. Many LSF protocols still require harsh conditions or specialized catalysts that may not be compatible with sensitive functional groups present in drug candidates, limiting their broad applicability.
The scalability of flow-based LSF processes presents another significant hurdle. While flow chemistry inherently offers advantages for scale-up compared to batch methods, transitioning from laboratory-scale to production-scale for LSF reactions often encounters engineering challenges related to mixing efficiency, heat transfer, and residence time distribution. These factors can significantly impact reaction performance and reproducibility at larger scales.
Catalyst development represents a critical area requiring further innovation. Current catalytic systems for LSF often suffer from limited substrate scope, poor functional group tolerance, or dependence on precious metals. The development of more robust, sustainable catalysts that can operate efficiently under flow conditions remains an active research focus.
Equipment standardization and accessibility pose additional barriers to widespread adoption. Specialized flow reactors and microfluidic devices required for certain LSF transformations are not universally available, and the expertise needed to operate these systems effectively is not yet commonplace in medicinal chemistry laboratories.
Globally, research in flow-enabled LSF is concentrated primarily in academic institutions and pharmaceutical companies across North America, Europe, and increasingly in Asia. The United States, United Kingdom, Germany, Japan, and China lead in patent filings and publications related to this technology. This geographical distribution reflects both the technical infrastructure requirements and the strategic importance of advanced synthetic methodologies in regions with strong pharmaceutical research presence.
Regulatory considerations also present challenges, as novel synthetic routes using flow-based LSF may require additional validation and documentation compared to established processes. The pharmaceutical industry's inherently conservative approach to manufacturing processes can slow the adoption of these innovative methodologies despite their potential advantages.
Despite these advances, several technical challenges persist in the field. Reaction selectivity remains a primary concern, as achieving site-specific modifications on complex drug scaffolds containing multiple similar functional groups continues to be difficult. Many LSF protocols still require harsh conditions or specialized catalysts that may not be compatible with sensitive functional groups present in drug candidates, limiting their broad applicability.
The scalability of flow-based LSF processes presents another significant hurdle. While flow chemistry inherently offers advantages for scale-up compared to batch methods, transitioning from laboratory-scale to production-scale for LSF reactions often encounters engineering challenges related to mixing efficiency, heat transfer, and residence time distribution. These factors can significantly impact reaction performance and reproducibility at larger scales.
Catalyst development represents a critical area requiring further innovation. Current catalytic systems for LSF often suffer from limited substrate scope, poor functional group tolerance, or dependence on precious metals. The development of more robust, sustainable catalysts that can operate efficiently under flow conditions remains an active research focus.
Equipment standardization and accessibility pose additional barriers to widespread adoption. Specialized flow reactors and microfluidic devices required for certain LSF transformations are not universally available, and the expertise needed to operate these systems effectively is not yet commonplace in medicinal chemistry laboratories.
Globally, research in flow-enabled LSF is concentrated primarily in academic institutions and pharmaceutical companies across North America, Europe, and increasingly in Asia. The United States, United Kingdom, Germany, Japan, and China lead in patent filings and publications related to this technology. This geographical distribution reflects both the technical infrastructure requirements and the strategic importance of advanced synthetic methodologies in regions with strong pharmaceutical research presence.
Regulatory considerations also present challenges, as novel synthetic routes using flow-based LSF may require additional validation and documentation compared to established processes. The pharmaceutical industry's inherently conservative approach to manufacturing processes can slow the adoption of these innovative methodologies despite their potential advantages.
Current Flow Chemistry Solutions for Late-Stage Functionalization
01 Continuous flow reactors for late-stage functionalization
Continuous flow reactors provide advantages for late-stage functionalization of complex molecules by enabling precise control of reaction parameters. These systems allow for better heat and mass transfer, controlled residence times, and improved safety profiles when handling hazardous reagents. The technology enables efficient functionalization of complex pharmaceutical intermediates and other high-value compounds under milder conditions than traditional batch processes.- Continuous flow reactors for late-stage functionalization: Continuous flow reactors provide advantages for late-stage functionalization of complex molecules by enabling precise control of reaction parameters. These systems allow for better heat and mass transfer, controlled residence times, and improved safety profiles when handling hazardous reagents. Flow chemistry platforms can be designed specifically for late-stage modifications of pharmaceuticals and other high-value compounds, increasing efficiency and reproducibility compared to batch processes.
- Microfluidic systems for targeted chemical modifications: Microfluidic devices offer unique capabilities for performing selective late-stage functionalization reactions. These systems utilize small reaction channels to enhance mixing efficiency, control reaction kinetics, and enable rapid screening of reaction conditions. The miniaturized format allows for precise manipulation of sensitive intermediates and efficient use of expensive catalysts or reagents, making them particularly valuable for complex transformations on elaborate molecular scaffolds.
- Automated flow chemistry platforms for pharmaceutical applications: Automated flow chemistry systems have been developed specifically for pharmaceutical applications, including late-stage functionalization of drug candidates. These platforms integrate reaction execution, in-line analysis, and feedback control to optimize reaction conditions in real-time. The automation enables high-throughput experimentation, accelerating the discovery and development of novel synthetic routes for introducing functional groups into complex molecular structures at late stages of synthesis.
- Photochemical and electrochemical flow processes: Flow chemistry enables efficient implementation of photochemical and electrochemical methods for late-stage functionalization. These approaches allow for selective C-H activation and other challenging transformations under mild conditions. The controlled exposure to light or electrical potential in flow systems overcomes limitations of batch processes, such as limited light penetration or electrode surface area, resulting in improved yields and selectivities for complex molecule modifications.
- Scale-up strategies for flow-based late-stage functionalization: Scaling up late-stage functionalization reactions in flow chemistry presents unique challenges and opportunities. Various approaches have been developed to increase throughput while maintaining the advantages of flow processing, including numbering-up (parallel reactors), extending residence time, and designing modular systems. These strategies enable the translation of laboratory-scale late-stage functionalization methods to production-scale processes while preserving reaction efficiency and selectivity.
02 Microfluidic systems for selective chemical transformations
Microfluidic platforms offer unique capabilities for late-stage functionalization by providing precise control over mixing, residence time, and reaction conditions at microscale. These systems enable selective chemical transformations on complex substrates with enhanced efficiency and selectivity. The small channel dimensions facilitate rapid heat transfer and mixing, allowing for more controlled reactions and improved yields for sensitive late-stage modifications.Expand Specific Solutions03 Automated flow chemistry platforms for high-throughput functionalization
Automated flow chemistry platforms integrate reaction, purification, and analysis steps to enable high-throughput late-stage functionalization of diverse compound libraries. These systems incorporate inline analytics, feedback control mechanisms, and machine learning algorithms to optimize reaction conditions in real-time. The technology accelerates the discovery and development of novel compounds by enabling rapid diversification of complex scaffolds through automated late-stage modifications.Expand Specific Solutions04 Photochemical and electrochemical flow processes
Flow chemistry enables efficient photochemical and electrochemical late-stage functionalization by providing uniform irradiation or electrical current distribution. These processes allow for selective C-H activation and other challenging transformations under mild conditions. The controlled exposure of reactants to light or electrical potential in flow systems overcomes limitations of traditional batch methods, enabling novel transformations on complex molecules with improved selectivity and yield.Expand Specific Solutions05 Scale-up strategies for flow-based late-stage functionalization
Scale-up strategies for flow-based late-stage functionalization involve parallel processing, numbering-up of microreactors, and continuous operation protocols. These approaches maintain the advantages of flow chemistry while increasing throughput for industrial applications. The technology enables consistent translation of laboratory-scale late-stage functionalization methods to production scale with preserved selectivity and yield, addressing challenges in manufacturing complex functionalized compounds.Expand Specific Solutions
Key Industry Players and Competitive Landscape
Flow chemistry for late-stage functionalization in drug discovery is evolving rapidly, currently in a growth phase characterized by increasing adoption across pharmaceutical research. The market is expanding significantly, driven by demands for more efficient, sustainable drug development processes. Technologically, the field shows varying maturity levels among key players. Companies like Merck Sharp & Dohme and BASF are leading with advanced continuous flow systems, while Sunshine Lake Pharma and Chengdu Tiantaishan Pharmaceutical are developing specialized applications for targeted drug modifications. Academic institutions including ETH Zurich and University of Edinburgh contribute fundamental research, creating a competitive landscape where industry-academia partnerships accelerate innovation in microreactor technology and process intensification methodologies.
Merck Sharp & Dohme Corp.
Technical Solution: Merck has developed an integrated flow chemistry platform specifically for late-stage functionalization in drug discovery. Their approach combines microfluidic reactors with inline analytics for real-time reaction monitoring. The system employs photochemical and electrochemical modules that enable selective C-H activation under mild conditions, particularly valuable for complex pharmaceutical intermediates. Merck's platform incorporates automated high-throughput experimentation capabilities, allowing rapid optimization of reaction parameters across multiple substrates simultaneously. Their technology integrates machine learning algorithms to predict optimal conditions for new substrates based on molecular descriptors and reaction outcomes from previous experiments. This system has demonstrated particular success with heterocyclic scaffolds common in drug development, achieving functionalization at positions traditionally considered unreactive using conventional batch methods.
Strengths: Superior scalability from discovery to manufacturing scales without significant process redesign; enhanced safety profile when handling hazardous reagents; improved reproducibility through precise control of reaction parameters. Weaknesses: Higher initial capital investment compared to batch systems; requires specialized expertise in flow chemistry; some complex transformations still challenging to translate from batch to flow.
Chengdu Tiantaishan Pharmaceutical Co., Ltd.
Technical Solution: Chengdu Tiantaishan Pharmaceutical has developed a comprehensive flow chemistry platform called "FlowDrug" specifically optimized for late-stage functionalization of traditional Chinese medicine (TCM) derivatives and modern small molecule drugs. Their system employs specialized corrosion-resistant microreactors capable of handling aggressive reagents commonly used in C-H activation chemistry. The platform incorporates proprietary mixing technology that enables precise control of reaction parameters even with multiphasic systems. Tiantaishan's approach integrates electrochemical modules that facilitate selective oxidation reactions without requiring stoichiometric oxidants, significantly improving atom economy. Their technology has demonstrated particular success with selective hydroxylation and amination of complex natural product scaffolds. The FlowDrug system includes inline purification capabilities through continuous extraction and crystallization modules, enabling seamless integration of multiple reaction steps. Tiantaishan has implemented this technology across their R&D pipeline, reporting significant reductions in development timelines for drug candidates.
Strengths: Specialized expertise with complex natural product scaffolds; excellent scalability from discovery to manufacturing; reduced environmental impact through decreased solvent usage and improved atom economy. Weaknesses: Limited published validation outside their proprietary compounds; significant expertise required for optimal utilization; challenges with handling highly viscous natural extracts.
Core Innovations in Flow Reactors and Catalysis
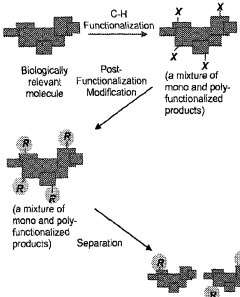
Compound diversification using late stage functionalization
PatentInactiveUS20150274755A1
Innovation
- The method involves functionalizing compounds using C—H functionalization chemistry to form mixtures of functionalized compounds, followed by separation and post-functionalization modification to generate a library of modified compounds, allowing for testing of physical and biological properties at various stages, thereby introducing limited chemical diversity and optimizing lead drug candidates.
Compound diversification using late stage functionalization
PatentWO2014052174A1
Innovation
- The method involves functionalizing compounds using C-H functionalization chemistry to form mixtures of functionalized compounds, followed by separation and post-functionalization modification to generate a library of modified compounds, allowing for testing of physical and biological properties at various stages, thereby introducing limited chemical diversity and optimizing lead drug candidates.
Regulatory Considerations for Flow Chemistry Implementation
The implementation of flow chemistry in pharmaceutical manufacturing environments necessitates careful navigation of complex regulatory frameworks. Regulatory bodies such as the FDA, EMA, and ICH have established guidelines that directly impact the adoption of continuous flow processes for late-stage functionalization in drug discovery and development.
Current regulatory frameworks primarily address batch processing methodologies, creating challenges for pharmaceutical companies transitioning to flow chemistry approaches. The FDA's Process Analytical Technology (PAT) initiative and Quality by Design (QbD) principles provide foundational guidance, though specific provisions for continuous manufacturing require interpretation and adaptation.
Key regulatory considerations include validation protocols for continuous processes, which differ significantly from traditional batch validation. Flow chemistry systems must demonstrate consistent product quality through real-time monitoring capabilities and robust control strategies. Regulatory agencies expect comprehensive documentation of process parameters, critical quality attributes, and design space definitions that account for the dynamic nature of flow processes.
Risk assessment frameworks must be adapted for flow chemistry implementation, particularly addressing concerns related to process stability, contamination control, and system reliability during extended operation periods. The establishment of appropriate in-process controls and sampling strategies presents unique challenges in continuous processing environments.
Regulatory submissions for flow chemistry processes require enhanced data packages demonstrating process understanding and control. This includes detailed characterization of residence time distributions, mixing efficiency, heat transfer dynamics, and scale-dependent parameters. Companies must develop strategies to address potential scale-up challenges while maintaining regulatory compliance.
International harmonization efforts are gradually evolving to accommodate continuous manufacturing technologies. The ICH Q13 guideline development specifically addresses continuous manufacturing of drug substances and drug products, providing a framework for global implementation strategies. Companies engaged in early adoption of flow chemistry for late-stage functionalization should actively monitor these evolving regulatory landscapes.
Engagement with regulatory authorities through early consultation programs such as the FDA's Emerging Technology Program can facilitate smoother implementation pathways. These programs offer opportunities for collaborative discussions regarding novel technological approaches before formal submission processes, potentially reducing regulatory uncertainties and accelerating approval timelines.
Compliance with Good Manufacturing Practice (GMP) requirements presents unique considerations for flow chemistry systems, particularly regarding equipment qualification, cleaning validation, and batch definition concepts. Establishing clear strategies for batch traceability, material reconciliation, and deviation management within continuous processing environments remains critical for regulatory acceptance.
Current regulatory frameworks primarily address batch processing methodologies, creating challenges for pharmaceutical companies transitioning to flow chemistry approaches. The FDA's Process Analytical Technology (PAT) initiative and Quality by Design (QbD) principles provide foundational guidance, though specific provisions for continuous manufacturing require interpretation and adaptation.
Key regulatory considerations include validation protocols for continuous processes, which differ significantly from traditional batch validation. Flow chemistry systems must demonstrate consistent product quality through real-time monitoring capabilities and robust control strategies. Regulatory agencies expect comprehensive documentation of process parameters, critical quality attributes, and design space definitions that account for the dynamic nature of flow processes.
Risk assessment frameworks must be adapted for flow chemistry implementation, particularly addressing concerns related to process stability, contamination control, and system reliability during extended operation periods. The establishment of appropriate in-process controls and sampling strategies presents unique challenges in continuous processing environments.
Regulatory submissions for flow chemistry processes require enhanced data packages demonstrating process understanding and control. This includes detailed characterization of residence time distributions, mixing efficiency, heat transfer dynamics, and scale-dependent parameters. Companies must develop strategies to address potential scale-up challenges while maintaining regulatory compliance.
International harmonization efforts are gradually evolving to accommodate continuous manufacturing technologies. The ICH Q13 guideline development specifically addresses continuous manufacturing of drug substances and drug products, providing a framework for global implementation strategies. Companies engaged in early adoption of flow chemistry for late-stage functionalization should actively monitor these evolving regulatory landscapes.
Engagement with regulatory authorities through early consultation programs such as the FDA's Emerging Technology Program can facilitate smoother implementation pathways. These programs offer opportunities for collaborative discussions regarding novel technological approaches before formal submission processes, potentially reducing regulatory uncertainties and accelerating approval timelines.
Compliance with Good Manufacturing Practice (GMP) requirements presents unique considerations for flow chemistry systems, particularly regarding equipment qualification, cleaning validation, and batch definition concepts. Establishing clear strategies for batch traceability, material reconciliation, and deviation management within continuous processing environments remains critical for regulatory acceptance.
Scalability and Manufacturing Integration Strategies
The scalability of flow chemistry processes represents a critical consideration for pharmaceutical companies seeking to translate late-stage functionalization techniques from discovery to manufacturing. Current integration strategies focus on modular flow systems that can be reconfigured for different reaction types while maintaining consistent performance across scales. These systems typically incorporate in-line monitoring capabilities through spectroscopic methods (UV-Vis, IR, Raman) to ensure reaction quality and consistency during scale-up operations.
Manufacturing integration of flow chemistry requires careful consideration of several key factors. Process intensification techniques enable higher throughput in smaller equipment footprints, with microreactor technology allowing for improved heat and mass transfer compared to traditional batch processes. This results in more efficient reactions with reduced solvent usage and waste generation, aligning with green chemistry principles increasingly prioritized in pharmaceutical manufacturing.
Continuous manufacturing approaches present significant advantages for late-stage functionalization, particularly for high-value, low-volume drug candidates. The ability to produce smaller batches on demand reduces inventory costs and minimizes product degradation concerns. Additionally, the controlled reaction environment of flow systems enables more precise handling of sensitive intermediates and potentially hazardous reagents, enhancing safety profiles across manufacturing operations.
Regulatory considerations remain paramount when implementing flow chemistry at scale. The FDA's Emerging Technology Team has established frameworks for evaluating continuous manufacturing processes, with several approved drugs now manufactured using flow techniques. Documentation of process analytical technology (PAT) implementation and control strategies is essential for regulatory approval, requiring pharmaceutical companies to develop robust validation protocols specific to flow chemistry operations.
Economic analysis indicates that while initial capital investment for flow chemistry equipment may exceed traditional batch reactors, the long-term operational benefits often justify these costs. Reduced cycle times, improved yields, and decreased waste treatment expenses contribute to favorable return on investment calculations. Companies implementing late-stage functionalization via flow chemistry report 15-30% reductions in overall manufacturing costs for complex API synthesis, with particularly significant savings in reactions requiring cryogenic conditions or involving unstable intermediates.
Manufacturing integration of flow chemistry requires careful consideration of several key factors. Process intensification techniques enable higher throughput in smaller equipment footprints, with microreactor technology allowing for improved heat and mass transfer compared to traditional batch processes. This results in more efficient reactions with reduced solvent usage and waste generation, aligning with green chemistry principles increasingly prioritized in pharmaceutical manufacturing.
Continuous manufacturing approaches present significant advantages for late-stage functionalization, particularly for high-value, low-volume drug candidates. The ability to produce smaller batches on demand reduces inventory costs and minimizes product degradation concerns. Additionally, the controlled reaction environment of flow systems enables more precise handling of sensitive intermediates and potentially hazardous reagents, enhancing safety profiles across manufacturing operations.
Regulatory considerations remain paramount when implementing flow chemistry at scale. The FDA's Emerging Technology Team has established frameworks for evaluating continuous manufacturing processes, with several approved drugs now manufactured using flow techniques. Documentation of process analytical technology (PAT) implementation and control strategies is essential for regulatory approval, requiring pharmaceutical companies to develop robust validation protocols specific to flow chemistry operations.
Economic analysis indicates that while initial capital investment for flow chemistry equipment may exceed traditional batch reactors, the long-term operational benefits often justify these costs. Reduced cycle times, improved yields, and decreased waste treatment expenses contribute to favorable return on investment calculations. Companies implementing late-stage functionalization via flow chemistry report 15-30% reductions in overall manufacturing costs for complex API synthesis, with particularly significant savings in reactions requiring cryogenic conditions or involving unstable intermediates.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!