Flow Chemistry In Regenerative Pharma: Trends And Adoption Barriers
SEP 3, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Flow Chemistry Evolution and Objectives
Flow chemistry represents a paradigm shift in pharmaceutical manufacturing, evolving from traditional batch processing to continuous production methods. This evolution began in the early 2000s when researchers recognized the potential advantages of miniaturized reaction environments for precise control over reaction parameters. The initial applications focused primarily on simple organic transformations, but by 2010, more complex multi-step syntheses were being demonstrated in academic laboratories.
The technological progression accelerated between 2010-2015 with the development of integrated flow systems incorporating in-line analytics and automated control mechanisms. This period marked a critical transition from laboratory curiosity to industrial implementation, with several pharmaceutical companies establishing dedicated flow chemistry divisions. The evolution was driven by increasing regulatory pressure for quality-by-design approaches and the need for more efficient manufacturing processes.
In the context of regenerative pharmaceuticals, flow chemistry has followed a distinct evolutionary path. Initial applications emerged around 2012-2014, focusing on the synthesis of specialized biomaterials and scaffold components. The field gained significant momentum after 2015 when researchers demonstrated the advantages of continuous processing for maintaining the integrity of sensitive biological molecules and achieving more consistent product quality.
The primary objectives of flow chemistry in regenerative pharmaceuticals encompass several dimensions. First, enhancing reproducibility and scalability of complex biological molecule synthesis, which is crucial for clinical translation of regenerative therapies. Second, enabling precise control over reaction conditions to optimize the biological activity of synthesized compounds. Third, reducing production costs through more efficient use of expensive reagents and catalysts, thereby addressing the economic barriers to widespread adoption of regenerative therapies.
Another key objective is regulatory compliance facilitation. Flow chemistry's inherent advantages in process monitoring and control align well with regulatory requirements for consistent product quality and traceability. This is particularly important in regenerative pharmaceuticals where product consistency directly impacts therapeutic efficacy and safety.
Looking forward, the field aims to develop fully integrated continuous manufacturing platforms that can seamlessly connect synthesis, purification, and formulation steps. The ultimate goal is to establish end-to-end manufacturing solutions that can produce complex regenerative pharmaceutical products with unprecedented consistency, while simultaneously reducing production costs and environmental impact.
The technological progression accelerated between 2010-2015 with the development of integrated flow systems incorporating in-line analytics and automated control mechanisms. This period marked a critical transition from laboratory curiosity to industrial implementation, with several pharmaceutical companies establishing dedicated flow chemistry divisions. The evolution was driven by increasing regulatory pressure for quality-by-design approaches and the need for more efficient manufacturing processes.
In the context of regenerative pharmaceuticals, flow chemistry has followed a distinct evolutionary path. Initial applications emerged around 2012-2014, focusing on the synthesis of specialized biomaterials and scaffold components. The field gained significant momentum after 2015 when researchers demonstrated the advantages of continuous processing for maintaining the integrity of sensitive biological molecules and achieving more consistent product quality.
The primary objectives of flow chemistry in regenerative pharmaceuticals encompass several dimensions. First, enhancing reproducibility and scalability of complex biological molecule synthesis, which is crucial for clinical translation of regenerative therapies. Second, enabling precise control over reaction conditions to optimize the biological activity of synthesized compounds. Third, reducing production costs through more efficient use of expensive reagents and catalysts, thereby addressing the economic barriers to widespread adoption of regenerative therapies.
Another key objective is regulatory compliance facilitation. Flow chemistry's inherent advantages in process monitoring and control align well with regulatory requirements for consistent product quality and traceability. This is particularly important in regenerative pharmaceuticals where product consistency directly impacts therapeutic efficacy and safety.
Looking forward, the field aims to develop fully integrated continuous manufacturing platforms that can seamlessly connect synthesis, purification, and formulation steps. The ultimate goal is to establish end-to-end manufacturing solutions that can produce complex regenerative pharmaceutical products with unprecedented consistency, while simultaneously reducing production costs and environmental impact.
Regenerative Pharma Market Analysis
The regenerative pharmaceuticals market has experienced significant growth over the past decade, with a global market value reaching $28.5 billion in 2023. This sector is projected to grow at a compound annual growth rate (CAGR) of 23.4% through 2030, driven primarily by increasing prevalence of chronic diseases, aging populations, and advancements in cell and gene therapy technologies. North America currently dominates the market with approximately 45% share, followed by Europe at 30% and Asia-Pacific at 20%.
The market can be segmented into several key categories: cell therapy, gene therapy, tissue engineering, and small molecule drugs. Cell therapy represents the largest segment, accounting for 38% of the market, with CAR-T cell therapies emerging as particularly lucrative products commanding premium pricing structures. Gene therapy follows at 32%, with recent regulatory approvals accelerating market penetration.
Demand drivers for regenerative pharmaceuticals include the rising incidence of degenerative diseases, increasing healthcare expenditure, and growing acceptance of personalized medicine approaches. The oncology application segment currently leads with 42% market share, followed by cardiovascular diseases (18%), neurodegenerative disorders (15%), and orthopedic applications (12%).
Reimbursement landscapes significantly impact market dynamics, with favorable policies in developed markets accelerating adoption. However, emerging economies face challenges with limited coverage for these high-cost therapies, creating market access barriers despite growing disease burdens in these regions.
The competitive landscape features both established pharmaceutical giants and specialized biotech companies. Key players include Novartis, Gilead Sciences (Kite Pharma), Bristol Myers Squibb, Takeda, and emerging players like Bluebird Bio and Spark Therapeutics. Strategic partnerships between pharmaceutical companies and academic institutions have become increasingly common to accelerate innovation pipelines.
Investment trends reveal substantial venture capital interest, with $12.8 billion invested in regenerative medicine companies in 2022 alone. This represents a 15% increase from the previous year, indicating strong investor confidence despite broader economic uncertainties.
Customer adoption patterns show varying rates across different healthcare systems, with academic medical centers and specialized treatment facilities leading implementation. Patient awareness and acceptance continue to grow, though concerns about long-term efficacy, safety profiles, and treatment costs remain significant barriers to wider adoption.
The market can be segmented into several key categories: cell therapy, gene therapy, tissue engineering, and small molecule drugs. Cell therapy represents the largest segment, accounting for 38% of the market, with CAR-T cell therapies emerging as particularly lucrative products commanding premium pricing structures. Gene therapy follows at 32%, with recent regulatory approvals accelerating market penetration.
Demand drivers for regenerative pharmaceuticals include the rising incidence of degenerative diseases, increasing healthcare expenditure, and growing acceptance of personalized medicine approaches. The oncology application segment currently leads with 42% market share, followed by cardiovascular diseases (18%), neurodegenerative disorders (15%), and orthopedic applications (12%).
Reimbursement landscapes significantly impact market dynamics, with favorable policies in developed markets accelerating adoption. However, emerging economies face challenges with limited coverage for these high-cost therapies, creating market access barriers despite growing disease burdens in these regions.
The competitive landscape features both established pharmaceutical giants and specialized biotech companies. Key players include Novartis, Gilead Sciences (Kite Pharma), Bristol Myers Squibb, Takeda, and emerging players like Bluebird Bio and Spark Therapeutics. Strategic partnerships between pharmaceutical companies and academic institutions have become increasingly common to accelerate innovation pipelines.
Investment trends reveal substantial venture capital interest, with $12.8 billion invested in regenerative medicine companies in 2022 alone. This represents a 15% increase from the previous year, indicating strong investor confidence despite broader economic uncertainties.
Customer adoption patterns show varying rates across different healthcare systems, with academic medical centers and specialized treatment facilities leading implementation. Patient awareness and acceptance continue to grow, though concerns about long-term efficacy, safety profiles, and treatment costs remain significant barriers to wider adoption.
Flow Chemistry Technical Challenges
Flow chemistry in regenerative pharmaceuticals faces several significant technical challenges that currently impede its widespread adoption. The transition from traditional batch processing to continuous flow systems requires overcoming complex engineering hurdles related to reaction control, scalability, and process integration.
One primary challenge is maintaining precise control over reaction parameters in continuous flow environments. Unlike batch processes where conditions remain relatively stable, flow chemistry demands real-time monitoring and adjustment of temperature, pressure, concentration, and residence time. This becomes particularly critical when dealing with sensitive biological materials common in regenerative pharmaceuticals, such as stem cells, growth factors, and specialized proteins, which can degrade or lose functionality with even minor parameter variations.
Scalability presents another substantial obstacle. While laboratory-scale flow chemistry demonstrations have shown promising results, scaling these processes to commercial production levels introduces complications in maintaining uniform flow dynamics, preventing channel clogging, and ensuring consistent product quality. The non-linear scaling relationships between reaction parameters at different production volumes often necessitate complete process redesign rather than simple dimensional scaling.
Material compatibility issues also plague flow chemistry implementation in regenerative pharma. The microreactors, pumps, valves, and sensors must withstand not only the chemical reagents but also maintain biocompatibility with cellular and biological components. Finding materials that meet these dual requirements while also providing necessary optical clarity for monitoring or specific surface properties for reaction control remains challenging.
Integration with downstream processing represents a significant technical hurdle. Flow chemistry produces continuous output that must seamlessly connect with subsequent purification, formulation, and quality control steps. This integration often requires developing novel interfaces between continuous and batch processes or completely reimagining the entire production chain as a continuous system.
Regulatory compliance adds another layer of complexity. Current good manufacturing practice (cGMP) guidelines were largely developed around batch processing paradigms, making validation of continuous flow processes more challenging. Demonstrating process control, batch definition, and product consistency requires new approaches to quality assurance and regulatory documentation.
Equipment standardization remains underdeveloped in the flow chemistry space. Unlike batch reactors with established designs and operational parameters, flow chemistry equipment varies significantly between manufacturers, making knowledge transfer and process replication difficult across different facilities or scaling stages.
One primary challenge is maintaining precise control over reaction parameters in continuous flow environments. Unlike batch processes where conditions remain relatively stable, flow chemistry demands real-time monitoring and adjustment of temperature, pressure, concentration, and residence time. This becomes particularly critical when dealing with sensitive biological materials common in regenerative pharmaceuticals, such as stem cells, growth factors, and specialized proteins, which can degrade or lose functionality with even minor parameter variations.
Scalability presents another substantial obstacle. While laboratory-scale flow chemistry demonstrations have shown promising results, scaling these processes to commercial production levels introduces complications in maintaining uniform flow dynamics, preventing channel clogging, and ensuring consistent product quality. The non-linear scaling relationships between reaction parameters at different production volumes often necessitate complete process redesign rather than simple dimensional scaling.
Material compatibility issues also plague flow chemistry implementation in regenerative pharma. The microreactors, pumps, valves, and sensors must withstand not only the chemical reagents but also maintain biocompatibility with cellular and biological components. Finding materials that meet these dual requirements while also providing necessary optical clarity for monitoring or specific surface properties for reaction control remains challenging.
Integration with downstream processing represents a significant technical hurdle. Flow chemistry produces continuous output that must seamlessly connect with subsequent purification, formulation, and quality control steps. This integration often requires developing novel interfaces between continuous and batch processes or completely reimagining the entire production chain as a continuous system.
Regulatory compliance adds another layer of complexity. Current good manufacturing practice (cGMP) guidelines were largely developed around batch processing paradigms, making validation of continuous flow processes more challenging. Demonstrating process control, batch definition, and product consistency requires new approaches to quality assurance and regulatory documentation.
Equipment standardization remains underdeveloped in the flow chemistry space. Unlike batch reactors with established designs and operational parameters, flow chemistry equipment varies significantly between manufacturers, making knowledge transfer and process replication difficult across different facilities or scaling stages.
Current Flow Chemistry Solutions
01 Microfluidic systems for flow chemistry
Microfluidic systems provide precise control over reaction conditions in flow chemistry applications. These systems utilize small channels and chambers to manipulate small volumes of fluids, enabling efficient mixing, heat transfer, and reaction control. The miniaturized nature of these devices allows for improved reaction kinetics, reduced reagent consumption, and enhanced safety for hazardous reactions. These systems are particularly valuable for applications requiring precise temperature control and rapid mixing of reagents.- Microfluidic systems for flow chemistry: Microfluidic systems enable precise control of chemical reactions in continuous flow processes. These systems utilize small channels and chambers to manipulate small volumes of fluids, allowing for enhanced mixing, heat transfer, and reaction control. The miniaturized nature of these devices provides advantages such as reduced reagent consumption, improved safety, and the ability to perform reactions under more controlled conditions than traditional batch processes.
- Flow reactors and reaction apparatus design: Specialized reactor designs for flow chemistry applications focus on optimizing reaction parameters such as mixing efficiency, residence time, and temperature control. These reactors may incorporate features like static mixers, heat exchangers, and modular components that can be reconfigured for different reaction types. Advanced reactor designs enable more efficient chemical transformations, improved scalability, and enhanced process safety compared to conventional batch reactors.
- Continuous flow synthesis methods: Continuous flow synthesis methods involve performing chemical reactions in a flowing stream rather than in a batch vessel. This approach offers advantages including consistent product quality, improved reaction control, reduced reaction times, and safer handling of hazardous intermediates. These methods are particularly valuable for reactions that require precise temperature control, involve unstable intermediates, or benefit from immediate product isolation to prevent degradation.
- Process monitoring and control systems: Advanced monitoring and control systems are essential for optimizing flow chemistry processes. These systems incorporate sensors for real-time measurement of parameters such as temperature, pressure, flow rate, and concentration. Integration with automated control systems allows for precise adjustment of reaction conditions, ensuring consistent product quality and process efficiency. These technologies enable continuous process verification and facilitate the implementation of quality-by-design principles in chemical manufacturing.
- Applications of flow chemistry in specific industries: Flow chemistry techniques have been adapted for specialized applications across various industries including pharmaceuticals, materials science, and biotechnology. These applications leverage the advantages of continuous flow processes to address specific challenges such as synthesizing complex molecules, producing nanomaterials with controlled properties, or performing enzymatic reactions under precisely controlled conditions. Industry-specific implementations often incorporate customized reactor designs and process parameters optimized for particular reaction classes.
02 Continuous flow reactors and processing equipment
Continuous flow reactors represent a core technology in flow chemistry, allowing for uninterrupted chemical processing as opposed to batch methods. These reactors feature specialized designs that facilitate continuous movement of reagents through reaction zones with controlled residence times. The equipment often includes pumps, mixers, heat exchangers, and monitoring systems that work together to maintain consistent reaction conditions. This approach offers advantages in scalability, process consistency, and the ability to handle reactions that would be challenging or dangerous in batch processes.Expand Specific Solutions03 Flow chemistry applications in pharmaceutical and biological processes
Flow chemistry has revolutionized pharmaceutical and biological processing by enabling more efficient synthesis of active pharmaceutical ingredients and biomolecules. The controlled environment of flow systems allows for precise reaction control, leading to higher purity products and reduced formation of unwanted byproducts. These systems are particularly valuable for multi-step syntheses, hazardous reactions, and processes requiring sterile conditions. The technology facilitates rapid optimization of reaction conditions and easier scale-up from laboratory to production scale.Expand Specific Solutions04 Automated control systems for flow chemistry
Advanced control systems are essential components of modern flow chemistry setups, providing automated monitoring and adjustment of reaction parameters. These systems incorporate sensors for real-time measurement of temperature, pressure, flow rate, and concentration, coupled with feedback control mechanisms to maintain optimal conditions. Integration with software platforms enables data collection, process visualization, and implementation of machine learning algorithms for process optimization. Automation reduces human error, increases reproducibility, and allows for continuous operation with minimal supervision.Expand Specific Solutions05 Novel reactor designs and materials for flow chemistry
Innovative reactor designs and materials are expanding the capabilities of flow chemistry systems. These include reactors made from specialized materials that offer enhanced chemical resistance, improved heat transfer, or catalytic properties. Some designs incorporate features like static mixers, structured packing, or membrane separators to improve mixing efficiency and reaction selectivity. Modular reactor designs allow for flexible configuration of reaction pathways and easy maintenance. These advancements enable the application of flow chemistry to a wider range of reaction types and operating conditions.Expand Specific Solutions
Key Industry Players and Competitors
Flow chemistry in regenerative pharmaceuticals is currently in the early growth phase, with a rapidly expanding market expected to reach significant scale as adoption increases. The technology demonstrates moderate maturity, with companies like F. Hoffmann-La Roche, Merck & Co., Novartis, and Bristol Myers Squibb leading implementation in continuous manufacturing processes. Despite proven benefits in process efficiency and product quality, adoption faces barriers including high initial investment costs, regulatory uncertainties, and technical expertise requirements. Smaller players like SoluBest and academic institutions such as KU Leuven are advancing innovative applications, while established pharmaceutical manufacturers are gradually integrating flow chemistry into regenerative medicine production platforms to enhance scalability and reproducibility of cell and gene therapies.
F. Hoffmann-La Roche Ltd.
Technical Solution: Roche has developed a sophisticated flow chemistry platform called "Continuous Regenerative Synthesis" (CRS) specifically designed for the production of advanced regenerative pharmaceuticals. Their system integrates microfluidic technology with precision control systems to enable the synthesis of complex biological molecules with exceptional reproducibility. Roche's approach incorporates specialized flow reactors with enhanced heat and mass transfer capabilities, allowing for precise control of reaction parameters critical for regenerative medicine applications. Their platform features in-line analytical technologies that provide real-time monitoring of critical quality attributes, enabling immediate process adjustments to maintain product quality. Roche has also implemented advanced automation systems that reduce human intervention and associated variability. Their flow chemistry technology includes novel photochemical reactors that enable previously challenging transformations relevant to regenerative pharmaceutical synthesis, such as controlled protein modification and peptide coupling reactions under continuous flow conditions.
Strengths: Superior product consistency through precise process control; significant reduction in manufacturing time from weeks to days; enhanced safety profile for handling potent compounds; improved scalability from laboratory to commercial production. Weaknesses: Regulatory challenges associated with novel manufacturing approaches; high initial capital investment requirements; need for specialized expertise in both flow chemistry and regenerative medicine fields.
Genentech, Inc.
Technical Solution: Genentech has developed an innovative flow chemistry platform called "Continuous Bioprocess Integration" (CBI) specifically tailored for regenerative pharmaceutical applications. Their technology seamlessly integrates continuous flow synthesis with downstream processing, creating an end-to-end manufacturing solution for complex biological therapeutics. Genentech's system features specialized microreactors with enhanced mixing capabilities that enable precise control over critical reaction parameters, particularly important for temperature-sensitive biomolecules used in regenerative medicine. Their platform incorporates advanced process analytical technology (PAT) that provides real-time monitoring of product quality attributes, allowing for immediate process adjustments. Genentech has pioneered the use of membrane-based separation techniques integrated directly into their flow chemistry systems, enabling continuous purification of regenerative pharmaceutical compounds. Additionally, their technology includes novel biocatalytic flow reactors that facilitate enzymatic transformations under continuous conditions, significantly enhancing the sustainability profile of their manufacturing processes.
Strengths: Exceptional product quality consistency through integrated quality control; reduced manufacturing footprint and capital requirements; significantly improved process efficiency with yields typically 15-25% higher than batch processes; enhanced safety profile for handling sensitive biological materials. Weaknesses: Complex technology transfer to contract manufacturing organizations; regulatory hurdles for novel continuous manufacturing approaches; specialized training requirements for operational personnel.
Critical Patents and Technical Literature
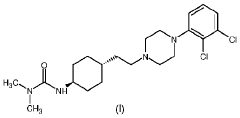
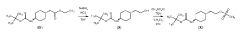
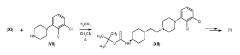
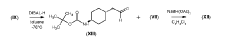
Process for consecutive continuous-flow reductions in the synthesis of medicinally relevant piperazine derivatives using a tubular reactor with alternating diameter
PatentWO2022190047A1
Innovation
- A consecutive continuous-flow process using an alternating diameter flow reactor for selective ester reduction followed by reductive amination with a 5% Pt/C catalyst in a toluene:methanol solvent mixture, optimizing reaction conditions for higher conversions and selectivity.
Regulatory Compliance Framework
The regulatory landscape for flow chemistry in regenerative pharmaceutical manufacturing presents a complex framework that manufacturers must navigate to ensure compliance. Current regulations from major authorities like the FDA, EMA, and ICH were not specifically designed with continuous manufacturing processes in mind, creating significant challenges for implementation. These agencies have traditionally focused on batch processing validation, requiring adaptation of existing guidelines to accommodate the unique aspects of flow chemistry.
Quality by Design (QbD) principles have become increasingly important in the regulatory approach to flow chemistry. Regulatory bodies now emphasize process understanding, risk assessment, and establishing design spaces that define critical process parameters. This shift allows for more flexible manufacturing while maintaining strict quality standards, though implementation remains challenging for many organizations.
Process Analytical Technology (PAT) integration forms a critical component of the regulatory framework, enabling real-time monitoring and control of manufacturing processes. Regulators increasingly expect pharmaceutical companies to implement PAT tools for continuous verification of product quality, representing both a compliance requirement and a technical hurdle for adoption.
International harmonization efforts are gradually addressing regulatory inconsistencies across different regions. The ICH Q13 guideline development specifically targeting continuous manufacturing represents a significant step toward standardized approaches. However, regional differences in interpretation and implementation timelines continue to create compliance challenges for global pharmaceutical operations.
Validation protocols for flow chemistry systems require substantial adaptation from traditional batch processing approaches. Regulatory expectations include demonstrating process robustness across extended operating periods, establishing appropriate sampling strategies, and validating cleaning procedures for continuous operations. These requirements often necessitate innovative approaches to validation that may lack clear regulatory precedent.
Change management within the regulatory framework presents particular challenges for flow chemistry adoption. Manufacturers must establish clear protocols for implementing process modifications, equipment changes, and scale adjustments while maintaining compliance. The dynamic nature of flow processes often conflicts with traditional regulatory approaches to manufacturing changes.
Emerging regulatory considerations include specific guidelines for cell and gene therapy applications using flow chemistry. These advanced therapeutic products face additional regulatory scrutiny regarding process consistency, sterility assurance, and product characterization. The intersection of regenerative medicine and continuous manufacturing creates a particularly complex regulatory environment requiring specialized expertise.
Quality by Design (QbD) principles have become increasingly important in the regulatory approach to flow chemistry. Regulatory bodies now emphasize process understanding, risk assessment, and establishing design spaces that define critical process parameters. This shift allows for more flexible manufacturing while maintaining strict quality standards, though implementation remains challenging for many organizations.
Process Analytical Technology (PAT) integration forms a critical component of the regulatory framework, enabling real-time monitoring and control of manufacturing processes. Regulators increasingly expect pharmaceutical companies to implement PAT tools for continuous verification of product quality, representing both a compliance requirement and a technical hurdle for adoption.
International harmonization efforts are gradually addressing regulatory inconsistencies across different regions. The ICH Q13 guideline development specifically targeting continuous manufacturing represents a significant step toward standardized approaches. However, regional differences in interpretation and implementation timelines continue to create compliance challenges for global pharmaceutical operations.
Validation protocols for flow chemistry systems require substantial adaptation from traditional batch processing approaches. Regulatory expectations include demonstrating process robustness across extended operating periods, establishing appropriate sampling strategies, and validating cleaning procedures for continuous operations. These requirements often necessitate innovative approaches to validation that may lack clear regulatory precedent.
Change management within the regulatory framework presents particular challenges for flow chemistry adoption. Manufacturers must establish clear protocols for implementing process modifications, equipment changes, and scale adjustments while maintaining compliance. The dynamic nature of flow processes often conflicts with traditional regulatory approaches to manufacturing changes.
Emerging regulatory considerations include specific guidelines for cell and gene therapy applications using flow chemistry. These advanced therapeutic products face additional regulatory scrutiny regarding process consistency, sterility assurance, and product characterization. The intersection of regenerative medicine and continuous manufacturing creates a particularly complex regulatory environment requiring specialized expertise.
Scale-up and Manufacturing Considerations
Scaling up flow chemistry processes from laboratory to commercial production represents a critical transition point in the adoption of this technology for regenerative pharmaceutical manufacturing. The inherent advantages of flow systems—precise control over reaction parameters, improved heat and mass transfer, and enhanced safety profiles—become even more pronounced at larger scales. However, this transition introduces unique engineering challenges that must be addressed systematically.
Material compatibility becomes increasingly important at production scale, as extended contact with reagents can lead to degradation of system components. Selection of appropriate construction materials that maintain integrity while avoiding contamination is essential for GMP compliance and product quality. Additionally, the design of larger flow reactors must carefully consider fluid dynamics to maintain the mixing efficiency and residence time distribution that were optimized at lab scale.
Process control systems require significant enhancement when scaling up flow chemistry operations. Real-time monitoring capabilities must be expanded to accommodate increased throughput while maintaining sensitivity. Implementation of advanced PAT (Process Analytical Technology) tools becomes necessary to ensure consistent product quality across longer production runs. These systems must be validated according to regulatory standards, adding complexity to the scale-up process.
Heat management presents another significant challenge, as the surface-to-volume ratio decreases with increasing reactor dimensions. Engineering solutions such as segmented flow designs, microstructured heat exchangers, and advanced cooling systems are often required to maintain the thermal control that makes flow chemistry advantageous in the first place. Failure to address these thermal considerations can lead to hotspots, reduced yield, or even safety hazards.
Economic considerations heavily influence scale-up decisions in regenerative pharmaceutical manufacturing. The capital investment required for commercial-scale flow chemistry equipment is substantial, necessitating thorough cost-benefit analysis. While continuous processing typically offers reduced operating costs and improved space utilization compared to batch methods, the initial investment can be prohibitive for smaller organizations without clear ROI projections.
Regulatory compliance adds another layer of complexity to scale-up efforts. Validation of continuous manufacturing processes requires different approaches than traditional batch validation. Companies must develop robust strategies for demonstrating process understanding, establishing control strategies, and defining appropriate sampling methodologies. Regulatory agencies have shown increasing acceptance of continuous manufacturing approaches, but the pathway to approval still contains uncertainties that companies must navigate carefully.
Material compatibility becomes increasingly important at production scale, as extended contact with reagents can lead to degradation of system components. Selection of appropriate construction materials that maintain integrity while avoiding contamination is essential for GMP compliance and product quality. Additionally, the design of larger flow reactors must carefully consider fluid dynamics to maintain the mixing efficiency and residence time distribution that were optimized at lab scale.
Process control systems require significant enhancement when scaling up flow chemistry operations. Real-time monitoring capabilities must be expanded to accommodate increased throughput while maintaining sensitivity. Implementation of advanced PAT (Process Analytical Technology) tools becomes necessary to ensure consistent product quality across longer production runs. These systems must be validated according to regulatory standards, adding complexity to the scale-up process.
Heat management presents another significant challenge, as the surface-to-volume ratio decreases with increasing reactor dimensions. Engineering solutions such as segmented flow designs, microstructured heat exchangers, and advanced cooling systems are often required to maintain the thermal control that makes flow chemistry advantageous in the first place. Failure to address these thermal considerations can lead to hotspots, reduced yield, or even safety hazards.
Economic considerations heavily influence scale-up decisions in regenerative pharmaceutical manufacturing. The capital investment required for commercial-scale flow chemistry equipment is substantial, necessitating thorough cost-benefit analysis. While continuous processing typically offers reduced operating costs and improved space utilization compared to batch methods, the initial investment can be prohibitive for smaller organizations without clear ROI projections.
Regulatory compliance adds another layer of complexity to scale-up efforts. Validation of continuous manufacturing processes requires different approaches than traditional batch validation. Companies must develop robust strategies for demonstrating process understanding, establishing control strategies, and defining appropriate sampling methodologies. Regulatory agencies have shown increasing acceptance of continuous manufacturing approaches, but the pathway to approval still contains uncertainties that companies must navigate carefully.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!