Cellulose Acetate Advancements in Drug Delivery Systems
Cellulose Acetate in Drug Delivery: Background and Objectives
Cellulose acetate has emerged as a promising material in the field of drug delivery systems, offering unique properties that enhance the efficacy and control of pharmaceutical formulations. The evolution of cellulose acetate in drug delivery can be traced back to the mid-20th century when researchers began exploring its potential as a polymer matrix for controlled release applications. Over the decades, advancements in material science and pharmaceutical technology have propelled cellulose acetate to the forefront of innovative drug delivery solutions.
The primary objective of utilizing cellulose acetate in drug delivery systems is to achieve precise control over the release kinetics of active pharmaceutical ingredients (APIs). This control allows for optimized therapeutic outcomes, reduced side effects, and improved patient compliance. Cellulose acetate's versatility in forming various structures, such as membranes, fibers, and microspheres, has enabled its application in a wide range of drug delivery platforms, including transdermal patches, oral controlled-release tablets, and implantable devices.
One of the key drivers behind the growing interest in cellulose acetate is its biocompatibility and biodegradability. As the pharmaceutical industry increasingly focuses on sustainable and environmentally friendly materials, cellulose acetate's natural origin and potential for biodegradation align well with these objectives. Furthermore, its ability to be chemically modified allows for tailoring its properties to suit specific drug delivery requirements, enhancing its versatility in pharmaceutical formulations.
The technological evolution of cellulose acetate in drug delivery systems has been marked by several significant milestones. Early research focused on understanding the fundamental properties of cellulose acetate and its interaction with various drugs. This led to the development of simple matrix systems for sustained drug release. As the field progressed, more sophisticated formulations emerged, incorporating cellulose acetate in multi-layered tablets, osmotic pump systems, and nanofiber-based delivery platforms.
Recent advancements have seen the integration of cellulose acetate with other polymers and nanomaterials, creating hybrid systems with enhanced functionalities. These developments have opened up new possibilities for targeted drug delivery, stimuli-responsive release mechanisms, and improved bioavailability of poorly soluble drugs. The ongoing research in this area aims to further expand the capabilities of cellulose acetate-based drug delivery systems, addressing challenges such as site-specific targeting, controlled release of multiple drugs, and overcoming biological barriers.
As we look towards the future, the objectives for cellulose acetate in drug delivery systems are multifaceted. Researchers are striving to develop more sophisticated and intelligent delivery systems that can respond to physiological cues and provide personalized therapeutic interventions. Additionally, there is a growing emphasis on scaling up production processes to ensure the commercial viability of cellulose acetate-based drug delivery technologies. The ultimate goal is to translate these advancements into clinically effective and commercially successful pharmaceutical products that can significantly improve patient outcomes and quality of life.
Market Analysis for CA-based Drug Delivery Systems
The market for cellulose acetate (CA)-based drug delivery systems has shown significant growth potential in recent years, driven by the increasing demand for controlled-release medications and the growing prevalence of chronic diseases. The global market for CA-based drug delivery systems is expected to expand at a steady pace, with a compound annual growth rate (CAGR) projected to be in the high single digits over the next five years.
One of the key factors contributing to this market growth is the versatility of cellulose acetate in drug delivery applications. CA offers excellent biocompatibility, biodegradability, and the ability to control drug release rates, making it an attractive material for pharmaceutical companies developing innovative drug formulations. The market has seen a particular surge in demand for CA-based oral drug delivery systems, which account for the largest share of the overall market.
The pharmaceutical industry's focus on patient-centric drug delivery solutions has also bolstered the adoption of CA-based systems. These systems can improve patient compliance by reducing dosing frequency and minimizing side effects through controlled release mechanisms. This trend aligns well with the growing emphasis on personalized medicine and targeted drug delivery approaches.
Geographically, North America currently holds the largest market share for CA-based drug delivery systems, followed by Europe. This dominance can be attributed to the presence of major pharmaceutical companies, advanced healthcare infrastructure, and favorable regulatory environments in these regions. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by increasing healthcare expenditure, rising awareness about advanced drug delivery technologies, and the growing pharmaceutical manufacturing sector in countries like China and India.
The market landscape for CA-based drug delivery systems is characterized by a mix of established pharmaceutical companies and innovative startups. Major players are investing heavily in research and development to enhance the performance of CA-based systems and expand their application areas. Collaborations between material science companies and drug manufacturers are becoming increasingly common, fostering innovation in this space.
Despite the positive outlook, the market faces certain challenges. The high cost of developing and manufacturing advanced drug delivery systems, along with stringent regulatory requirements, can pose barriers to market entry for smaller companies. Additionally, competition from alternative drug delivery technologies, such as liposomes and nanoparticles, may impact the growth trajectory of CA-based systems in specific therapeutic areas.
Looking ahead, the market for CA-based drug delivery systems is poised for continued expansion, driven by technological advancements, increasing research activities, and the growing need for efficient drug delivery solutions. The development of novel CA derivatives with enhanced properties and the exploration of new application areas, such as transdermal and ocular drug delivery, are expected to open up new opportunities for market growth in the coming years.
Current Challenges in CA Drug Delivery Applications
Despite the promising potential of cellulose acetate (CA) in drug delivery systems, several challenges persist in its practical applications. One of the primary obstacles is the limited control over drug release rates. CA-based systems often exhibit an initial burst release followed by a slower, sustained release phase. This inconsistent release profile can lead to suboptimal therapeutic outcomes, particularly for drugs requiring precise dosing over extended periods.
Another significant challenge is the hydrophobic nature of CA, which can hinder the encapsulation and release of hydrophilic drugs. This limitation restricts the range of pharmaceuticals that can be effectively delivered using CA-based systems. Researchers are actively exploring various modification techniques to improve the hydrophilicity of CA, but achieving a balance between enhanced hydrophilicity and maintaining the structural integrity of the polymer remains a complex task.
The biodegradability of CA in physiological conditions is also a concern. While CA is generally considered biodegradable, its degradation rate in the human body can be slow and unpredictable. This characteristic may lead to the accumulation of polymer residues in tissues, potentially causing adverse effects or complicating long-term drug delivery strategies.
Furthermore, the mechanical properties of CA-based drug delivery systems pose challenges in certain applications. CA films and membranes can be brittle and lack the flexibility required for some delivery routes, such as transdermal patches or implantable devices. Improving the mechanical strength and elasticity of CA-based materials without compromising their drug release properties is an ongoing area of research.
The scalability of CA-based drug delivery systems presents another hurdle. While laboratory-scale production may yield promising results, translating these processes to industrial-scale manufacturing while maintaining consistent quality and performance can be challenging. Issues such as batch-to-batch variability and the need for specialized equipment can impede the widespread adoption of CA-based drug delivery technologies.
Lastly, regulatory considerations pose significant challenges in the development and commercialization of CA-based drug delivery systems. As a relatively new material in this application, extensive safety and efficacy data are required to gain regulatory approval. The complex nature of these systems, often involving multiple components and novel formulation techniques, can lead to prolonged and costly regulatory processes.
Existing CA Formulations for Drug Delivery
01 Cellulose acetate production methods
Various methods for producing cellulose acetate are described, including improvements in acetylation processes, solvent systems, and reaction conditions. These methods aim to enhance the efficiency and quality of cellulose acetate production for different applications.- Cellulose acetate production methods: Various methods for producing cellulose acetate are described, including improvements in acetylation processes, solvent systems, and reaction conditions. These methods aim to enhance the efficiency and quality of cellulose acetate production for different applications.
- Cellulose acetate fiber applications: Cellulose acetate fibers are utilized in diverse applications such as textiles, filters, and membranes. The patents discuss modifications to improve fiber properties, processing techniques, and the development of novel cellulose acetate-based materials for specific uses.
- Cellulose acetate film and coating technologies: Innovations in cellulose acetate film and coating technologies are presented, including methods to enhance film properties, improve coating processes, and develop new formulations for various industrial and consumer applications.
- Cellulose acetate modification and blending: Techniques for modifying cellulose acetate and blending it with other materials are explored to create composites with enhanced properties. These modifications aim to improve characteristics such as biodegradability, mechanical strength, and thermal stability.
- Cellulose acetate in pharmaceutical and biomedical applications: The use of cellulose acetate in pharmaceutical and biomedical fields is discussed, including drug delivery systems, controlled release formulations, and biocompatible materials for medical devices. Patents describe various formulations and processing methods to optimize cellulose acetate for these applications.
02 Cellulose acetate fiber applications
Cellulose acetate fibers are utilized in diverse applications, including textiles, filters, and composite materials. The properties of these fibers can be tailored for specific uses through modifications in the production process or post-treatment methods.Expand Specific Solutions03 Cellulose acetate film and membrane technology
Advancements in cellulose acetate film and membrane technology are presented, focusing on improved formulations and manufacturing techniques. These developments enhance the performance of cellulose acetate in applications such as separation processes and packaging materials.Expand Specific Solutions04 Cellulose acetate modification and blending
Techniques for modifying cellulose acetate and blending it with other materials are explored to enhance its properties. These modifications can improve characteristics such as biodegradability, mechanical strength, and thermal stability for various applications.Expand Specific Solutions05 Cellulose acetate in sustainable and biodegradable products
The use of cellulose acetate in developing sustainable and biodegradable products is discussed. This includes applications in packaging, disposable items, and other environmentally friendly alternatives to conventional plastics.Expand Specific Solutions
Key Players in CA-based Drug Delivery Research
The field of cellulose acetate advancements in drug delivery systems is in a growth phase, with increasing market size and technological maturity. The global drug delivery market is expanding rapidly, driven by the demand for innovative and efficient delivery methods. Companies like Allergan, Inc., Novartis AG, and Eastman Chemical Co. are at the forefront of research and development in this area. The technology is progressing from basic research to practical applications, with academic institutions such as Massachusetts Institute of Technology and Tsinghua University contributing significantly to its advancement. Collaborations between industry leaders and research institutions are accelerating the development of novel cellulose acetate-based drug delivery systems, promising improved therapeutic outcomes and patient compliance.
Eastman Chemical Co.
Novartis AG
Innovative CA Modifications for Enhanced Drug Release
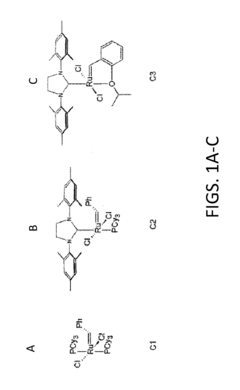
- The method involves cross-metathesis of polysaccharides with olefin-terminated side chains using the Hoveyda-Grubbs' 2nd generation catalyst, employing an excess of acrylic acid to avoid self-metathesis and oligomerization, and incorporating free radical scavengers to maintain solubility and stability, allowing for the synthesis of cellulose w-carboxyesters and other polysaccharide derivatives under mild conditions.
- The development of novel drug delivery systems utilizing nanoparticle-based carriers, liposomal formulations, micellar systems, hydrogel-based systems, and stimuli-responsive materials to enhance drug solubility, stability, targeting, and release kinetics, enabling precise and sustained delivery of therapeutic agents.
Regulatory Considerations for CA-based Pharmaceuticals
The regulatory landscape for cellulose acetate (CA)-based pharmaceuticals is complex and evolving, requiring careful consideration by drug developers and manufacturers. The U.S. Food and Drug Administration (FDA) plays a crucial role in overseeing the approval process for CA-based drug delivery systems. These systems must meet stringent safety and efficacy standards before they can be marketed.
One of the primary regulatory considerations is the classification of CA-based drug delivery systems. Depending on their specific characteristics and intended use, these systems may be categorized as drugs, medical devices, or combination products. This classification significantly impacts the regulatory pathway and requirements for approval.
For CA-based pharmaceuticals classified as drugs, manufacturers must follow the New Drug Application (NDA) process. This involves extensive preclinical and clinical trials to demonstrate safety and efficacy. The FDA's Center for Drug Evaluation and Research (CDER) evaluates these applications, focusing on the drug's formulation, manufacturing process, and quality control measures.
In cases where CA-based systems are considered medical devices, the regulatory pathway may involve premarket notification (510(k)) or premarket approval (PMA), depending on the device's risk classification. The FDA's Center for Devices and Radiological Health (CDRH) oversees this process, evaluating factors such as biocompatibility, mechanical properties, and performance characteristics.
Combination products, which incorporate both drug and device components, require a more complex regulatory approach. The FDA's Office of Combination Products (OCP) determines the primary mode of action and assigns the lead center for review. This process often involves collaboration between CDER and CDRH to ensure comprehensive evaluation of the product's safety and effectiveness.
Environmental considerations also play a role in the regulatory landscape for CA-based pharmaceuticals. The FDA may require environmental assessments to evaluate the potential impact of these products on the environment, particularly concerning biodegradability and disposal methods.
International regulatory bodies, such as the European Medicines Agency (EMA), have their own requirements for CA-based pharmaceuticals. Manufacturers seeking global market access must navigate these diverse regulatory frameworks, often necessitating harmonized development strategies to meet various regional standards.
As the field of CA-based drug delivery systems continues to advance, regulatory agencies are adapting their approaches to keep pace with technological innovations. This may include the development of new guidance documents, the establishment of specialized review pathways, or the implementation of accelerated approval processes for certain types of CA-based pharmaceuticals that address unmet medical needs.
Biocompatibility and Biodegradability of CA in Drug Delivery
Cellulose acetate (CA) has emerged as a promising material in drug delivery systems due to its exceptional biocompatibility and biodegradability. These properties are crucial for ensuring the safety and efficacy of drug delivery vehicles while minimizing potential adverse effects on the human body and the environment.
The biocompatibility of CA is attributed to its natural origin and chemical structure. As a derivative of cellulose, CA shares many of the beneficial properties of its parent compound, including low toxicity and high compatibility with biological systems. Numerous studies have demonstrated that CA-based drug delivery systems exhibit minimal inflammatory responses and do not elicit significant immune reactions when introduced into the body.
In vitro and in vivo studies have consistently shown that CA-based materials are well-tolerated by various cell types and tissues. This compatibility extends to both short-term and long-term exposure, making CA suitable for a wide range of drug delivery applications, from rapid-release formulations to sustained-release implants.
The biodegradability of CA is another key advantage in drug delivery applications. CA undergoes hydrolysis in physiological conditions, breaking down into non-toxic components that can be easily metabolized or excreted by the body. This property is particularly valuable for controlled-release systems, where the gradual degradation of the CA matrix can be harnessed to modulate drug release rates.
The rate of CA biodegradation can be fine-tuned by adjusting its degree of substitution and molecular weight, allowing for precise control over the drug release profile. This versatility enables the development of tailored drug delivery systems for various therapeutic needs, from rapid-acting formulations to long-term sustained release.
Furthermore, the biodegradability of CA addresses environmental concerns associated with pharmaceutical waste. As CA-based drug delivery systems break down into harmless byproducts, they minimize the accumulation of persistent materials in the environment, aligning with the growing emphasis on sustainable healthcare solutions.
Recent advancements in CA modification techniques have further enhanced its biocompatibility and biodegradability profiles. Surface modifications, such as grafting with bioactive molecules or incorporating nanoparticles, have been shown to improve cell adhesion, tissue integration, and controlled degradation rates.
The combination of biocompatibility and biodegradability makes CA an ideal candidate for advanced drug delivery applications, including targeted delivery to specific tissues or organs. By leveraging these properties, researchers have developed CA-based nanocarriers, microparticles, and scaffolds that can effectively encapsulate and deliver a wide range of therapeutic agents, from small molecule drugs to proteins and nucleic acids.