How to Leverage Cellulose Acetate for Efficient Drug Delivery?
Cellulose Acetate in Drug Delivery: Background and Objectives
Cellulose acetate has emerged as a promising material in the field of drug delivery, offering unique properties that make it suitable for various pharmaceutical applications. The evolution of cellulose acetate in drug delivery systems can be traced back to the mid-20th century when researchers began exploring its potential as a controlled release matrix. Over the years, advancements in polymer science and drug delivery technologies have led to a deeper understanding of cellulose acetate's capabilities and limitations.
The primary objective of leveraging cellulose acetate for efficient drug delivery is to enhance the therapeutic efficacy of pharmaceutical compounds while minimizing side effects. This goal aligns with the broader trend in the pharmaceutical industry towards personalized medicine and targeted drug delivery. Cellulose acetate's biocompatibility, biodegradability, and versatile physicochemical properties make it an attractive candidate for developing novel drug delivery systems.
One of the key technological trends in this field is the development of cellulose acetate-based nanocarriers. These nanostructures offer improved drug solubility, controlled release profiles, and enhanced cellular uptake. Researchers are exploring various fabrication techniques, such as electrospinning and nanoprecipitation, to create cellulose acetate nanofibers and nanoparticles tailored for specific drug delivery applications.
Another significant trend is the modification of cellulose acetate to improve its functionality. Chemical modifications, such as grafting and crosslinking, are being investigated to enhance the material's mechanical properties, drug loading capacity, and release kinetics. These modifications aim to overcome some of the limitations associated with native cellulose acetate, such as its hydrophobicity and limited drug-polymer interactions.
The integration of cellulose acetate with other biomaterials and smart polymers is also gaining traction. Composite systems combining cellulose acetate with stimuli-responsive polymers or natural polysaccharides are being developed to achieve targeted and on-demand drug release. These advanced systems respond to external stimuli such as pH, temperature, or specific enzymes, allowing for precise control over drug release at the desired site of action.
As research in this field progresses, the focus is shifting towards developing cellulose acetate-based drug delivery systems for challenging therapeutic areas. These include the delivery of biologics, gene therapy agents, and poorly water-soluble drugs. The versatility of cellulose acetate allows for its application in various dosage forms, including oral tablets, transdermal patches, and implantable devices, expanding its potential impact across different therapeutic modalities.
Market Analysis for Cellulose Acetate-Based Drug Delivery Systems
The market for cellulose acetate-based drug delivery systems has shown significant growth potential in recent years, driven by the increasing demand for controlled-release medications and the growing emphasis on targeted drug delivery. Cellulose acetate, a biodegradable and biocompatible polymer, has emerged as a promising material for drug delivery applications due to its versatility and favorable properties.
The global market for cellulose acetate in pharmaceutical applications is expected to expand at a steady rate, with a particular focus on controlled-release formulations. This growth is attributed to the rising prevalence of chronic diseases, the need for improved patient compliance, and the push for more efficient drug delivery methods. The market is segmented based on drug type, formulation, and geographic region, with oral drug delivery systems currently dominating the market share.
North America holds the largest market share for cellulose acetate-based drug delivery systems, followed by Europe and Asia-Pacific. The United States, in particular, is a key market due to its advanced healthcare infrastructure and high investment in pharmaceutical research and development. However, emerging economies in Asia-Pacific, such as China and India, are expected to witness the fastest growth rates in the coming years, driven by increasing healthcare expenditure and a growing pharmaceutical industry.
Key market drivers include the aging population, which requires more sophisticated drug delivery systems for chronic conditions, and the increasing adoption of personalized medicine. Additionally, the shift towards value-based healthcare models is encouraging the development of more efficient and cost-effective drug delivery solutions, where cellulose acetate-based systems offer significant advantages.
The market faces challenges such as stringent regulatory requirements and the high cost of research and development for novel drug delivery systems. However, these barriers also serve as entry deterrents, potentially benefiting established players in the long run. Opportunities for market expansion lie in the development of innovative formulations that can enhance drug bioavailability and reduce side effects.
Collaborations between pharmaceutical companies and material science firms are becoming increasingly common, as they seek to leverage cellulose acetate's properties for advanced drug delivery applications. This trend is expected to drive further innovation and market growth in the coming years.
In conclusion, the market for cellulose acetate-based drug delivery systems shows promising growth prospects, driven by technological advancements, changing healthcare needs, and the material's inherent advantages. As research continues to unlock new applications and formulations, cellulose acetate is poised to play an increasingly important role in the future of drug delivery systems.
Current Challenges in Cellulose Acetate Drug Delivery
Despite the promising potential of cellulose acetate in drug delivery systems, several challenges currently hinder its widespread adoption and optimal utilization. One of the primary obstacles is the limited solubility of cellulose acetate in common solvents, which complicates the formulation process and restricts the range of drugs that can be effectively incorporated. This solubility issue often necessitates the use of harsh organic solvents, raising concerns about residual solvent toxicity and environmental impact.
Another significant challenge lies in controlling the release kinetics of drugs from cellulose acetate matrices. The inherent hydrophobicity of cellulose acetate can lead to inconsistent drug release profiles, particularly for hydrophilic drugs. This unpredictability in release rates can result in suboptimal therapeutic outcomes and potentially increase the risk of side effects due to burst release or inadequate drug concentrations at the target site.
The biodegradability of cellulose acetate presents a double-edged sword in drug delivery applications. While its slow degradation rate can be advantageous for sustained release formulations, it also poses challenges in achieving complete drug release and eliminating polymer residues from the body. This slow degradation may lead to accumulation of the polymer in tissues, raising concerns about long-term biocompatibility and potential inflammatory responses.
Surface modification of cellulose acetate to enhance its functionality in drug delivery systems remains a complex task. Achieving uniform and stable surface modifications without compromising the bulk properties of the material is challenging. This limitation restricts the ability to tailor cellulose acetate for specific drug delivery applications, such as targeted delivery or stimuli-responsive release mechanisms.
The scalability of cellulose acetate-based drug delivery systems also presents significant hurdles. Transitioning from laboratory-scale production to industrial-scale manufacturing while maintaining consistent quality and performance is a major challenge. Variations in cellulose acetate properties between batches can lead to inconsistencies in drug loading, release profiles, and overall efficacy of the delivery system.
Furthermore, regulatory considerations pose additional challenges in the development and commercialization of cellulose acetate drug delivery systems. The complex nature of these formulations, combined with the variability in cellulose acetate sources and processing methods, can complicate the regulatory approval process. Demonstrating consistent quality, safety, and efficacy across different batches and formulations remains a significant hurdle for pharmaceutical companies seeking to leverage cellulose acetate in their drug delivery platforms.
Existing Cellulose Acetate Drug Delivery Mechanisms
01 Improved cellulose acetate production methods
Various techniques have been developed to enhance the efficiency of cellulose acetate production. These methods focus on optimizing reaction conditions, improving catalyst systems, and refining purification processes to increase yield and quality of the final product.- Improved cellulose acetate production methods: Various techniques have been developed to enhance the efficiency of cellulose acetate production. These methods focus on optimizing reaction conditions, improving catalyst systems, and refining purification processes to increase yield and quality of the final product.
- Cellulose acetate applications in filtration: Cellulose acetate has shown high efficiency in filtration applications. Research has focused on developing cellulose acetate membranes with improved porosity, selectivity, and durability for use in water treatment, gas separation, and other filtration processes.
- Cellulose acetate in textile and fiber production: Advancements have been made in the use of cellulose acetate for textile and fiber production. These improvements include enhanced spinning techniques, modified fiber properties, and increased efficiency in the conversion of cellulose acetate into usable fibers for various applications.
- Biodegradability and environmental impact: Research has focused on improving the biodegradability of cellulose acetate and reducing its environmental impact. This includes developing more eco-friendly production processes, enhancing the material's ability to degrade in natural environments, and exploring recycling methods for cellulose acetate products.
- Cellulose acetate modifications for specific applications: Various modifications to cellulose acetate have been explored to enhance its efficiency in specific applications. These include chemical modifications to improve thermal stability, mechanical properties, and compatibility with other materials for use in packaging, coatings, and composite materials.
02 Cellulose acetate applications in filtration
Cellulose acetate has shown high efficiency in filtration applications. Research has focused on developing cellulose acetate membranes with improved porosity, selectivity, and durability for use in water treatment, gas separation, and other filtration processes.Expand Specific Solutions03 Cellulose acetate in textile and fiber production
Advancements have been made in using cellulose acetate for textile and fiber production. These improvements focus on enhancing the material's properties such as strength, elasticity, and moisture absorption, leading to more efficient and higher quality textile products.Expand Specific Solutions04 Biodegradability and environmental impact
Research has been conducted to improve the biodegradability of cellulose acetate and reduce its environmental impact. This includes developing more eco-friendly production processes and exploring ways to enhance the material's decomposition in natural environments.Expand Specific Solutions05 Cellulose acetate in drug delivery systems
Cellulose acetate has shown promise in drug delivery applications. Studies have focused on improving its efficiency as a carrier material for controlled release formulations, enhancing drug loading capacity, and optimizing release kinetics for various pharmaceutical compounds.Expand Specific Solutions
Key Players in Cellulose Acetate Drug Delivery Research
The field of cellulose acetate-based drug delivery is in a growth phase, with increasing market size and technological advancements. The global market for this technology is expanding due to its potential for improved drug efficacy and reduced side effects. Technologically, the field is progressing from basic research to more advanced applications, with companies like Cila Therapeutics, Intezyne Technologies, and BioNTech SE leading innovation. Academic institutions such as MIT, Tokyo Institute of Technology, and Fudan University are contributing significantly to research. The involvement of pharmaceutical giants like Regeneron Pharmaceuticals indicates the technology's growing maturity and commercial potential. However, challenges in scalability and regulatory approval remain, suggesting that the field is not yet fully mature but rapidly evolving.
BioNTech SE
Regeneron Pharmaceuticals, Inc.
Innovative Cellulose Acetate Formulations for Drug Release
- A carrier made of cellulose acetate with a specific ratio of hydroxyl groups substituted with acetyl groups (23-54%) and a total surface area of 157-627 cm² is used to selectively adsorb granulocytes from body fluids at a controlled linear velocity, improving both granulocyte removal and mononuclear cell recovery rates.
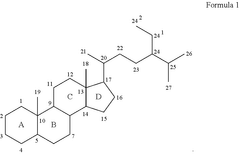
- A drug delivery system combining compounds with a cyclopenta[a]phenanthrene structure or derivatives with cellulose compounds, applied through a simple and cost-effective process, enhances the mechanical properties and efficiency of drug delivery, allowing for quick and effective absorption by body tissues.
Regulatory Considerations for Cellulose Acetate in Pharmaceuticals
The regulatory landscape for cellulose acetate in pharmaceuticals is complex and multifaceted, requiring careful consideration by drug developers and manufacturers. Cellulose acetate, as a versatile excipient in drug delivery systems, falls under the scrutiny of various regulatory bodies worldwide. In the United States, the Food and Drug Administration (FDA) oversees the use of cellulose acetate in pharmaceutical applications, with guidelines outlined in the Code of Federal Regulations (CFR) Title 21.
The European Medicines Agency (EMA) provides regulatory oversight in the European Union, where cellulose acetate must comply with the European Pharmacopoeia standards. These standards ensure the quality, safety, and efficacy of pharmaceutical products containing cellulose acetate. Similarly, regulatory agencies in other regions, such as Japan's Pharmaceuticals and Medical Devices Agency (PMDA), have their own specific requirements for cellulose acetate use in drug formulations.
One of the key regulatory considerations for cellulose acetate in pharmaceuticals is its classification as a generally recognized as safe (GRAS) substance by the FDA. This designation facilitates its use in various drug delivery systems, but manufacturers must still demonstrate the safety and efficacy of their specific formulations. Additionally, the purity and quality of cellulose acetate used in pharmaceutical applications are subject to stringent regulatory standards, including limits on residual solvents and impurities.
Regulatory bodies also focus on the manufacturing processes involving cellulose acetate. Good Manufacturing Practices (GMP) must be adhered to throughout the production and formulation stages. This includes maintaining proper documentation, implementing quality control measures, and ensuring traceability of materials used in the manufacturing process.
The use of cellulose acetate in controlled-release formulations requires particular attention from a regulatory perspective. Manufacturers must provide comprehensive data on the release kinetics, stability, and bioavailability of drugs formulated with cellulose acetate matrices. This often involves conducting extensive in vitro and in vivo studies to demonstrate the consistency and reliability of drug release profiles.
Environmental considerations are increasingly becoming a part of regulatory frameworks. As cellulose acetate is biodegradable, its environmental impact and disposal methods may be subject to regulatory scrutiny, especially in the context of sustainable pharmaceutical practices.
Lastly, regulatory bodies are continually updating their guidelines in response to new research and technological advancements. Drug developers and manufacturers must stay informed about these evolving regulations to ensure ongoing compliance and to leverage cellulose acetate effectively in their drug delivery systems.
Biocompatibility and Safety of Cellulose Acetate Drug Carriers
Cellulose acetate has emerged as a promising material for drug delivery systems due to its excellent biocompatibility and safety profile. As a naturally derived polymer, cellulose acetate exhibits low toxicity and minimal immunogenicity, making it an ideal candidate for pharmaceutical applications. The biocompatibility of cellulose acetate is attributed to its chemical structure, which closely resembles that of natural cellulose found in plant cell walls.
In vivo studies have demonstrated that cellulose acetate-based drug carriers are well-tolerated by the body, with minimal inflammatory responses observed in various tissues. This favorable biological interaction is crucial for maintaining the integrity of the drug delivery system and ensuring optimal therapeutic outcomes. Furthermore, the biodegradability of cellulose acetate contributes to its safety profile, as it can be broken down into non-toxic components and eliminated from the body through natural metabolic processes.
The safety of cellulose acetate drug carriers is further enhanced by their ability to protect encapsulated drugs from premature degradation or release. This controlled release mechanism not only improves the efficacy of the drug but also reduces the risk of systemic toxicity associated with sudden drug bursts. Additionally, the versatility of cellulose acetate allows for the incorporation of various functional groups, enabling the development of targeted drug delivery systems that can selectively accumulate in specific tissues or organs.
Extensive toxicological studies have been conducted to evaluate the long-term safety of cellulose acetate drug carriers. These investigations have consistently shown that cellulose acetate does not induce significant adverse effects on vital organs or cellular functions, even at high doses. The absence of genotoxicity and carcinogenicity further supports the use of cellulose acetate in drug delivery applications, particularly for chronic treatments that require prolonged exposure to the carrier material.
The biocompatibility of cellulose acetate extends to its interaction with blood components, making it suitable for intravenous administration. Studies have shown that cellulose acetate-based nanoparticles do not induce significant platelet aggregation or complement activation, reducing the risk of thrombosis or immune-mediated reactions. This hemocompatibility is crucial for developing safe and effective drug delivery systems for systemic applications.
In conclusion, the biocompatibility and safety of cellulose acetate drug carriers have been extensively validated through numerous preclinical and clinical studies. The combination of low toxicity, minimal immunogenicity, and controlled degradation makes cellulose acetate an excellent choice for developing advanced drug delivery systems. As research in this field continues to progress, it is expected that cellulose acetate will play an increasingly important role in the development of safe and effective pharmaceutical formulations.