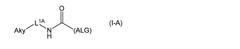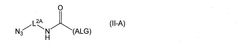How to Improve Biocompatibility of Cellulose Acetate Devices?
Cellulose Acetate Biocompatibility: Background and Objectives
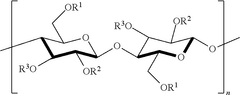
Cellulose acetate has been a widely used biomaterial in various medical applications for decades, owing to its unique properties such as biocompatibility, biodegradability, and ease of modification. The journey of cellulose acetate in biomedical applications began in the mid-20th century, with its initial use in hemodialysis membranes. Since then, its application has expanded to include drug delivery systems, tissue engineering scaffolds, and wound dressings.
The evolution of cellulose acetate technology has been driven by the increasing demand for more biocompatible and functional medical devices. As our understanding of biomaterial-tissue interactions has grown, so has the need to enhance the biocompatibility of cellulose acetate-based devices. This has led to a surge in research focused on surface modifications, composite formulations, and novel processing techniques to improve the material's performance in biological environments.
Recent technological advancements have opened up new possibilities for cellulose acetate in regenerative medicine and personalized healthcare. The integration of nanotechnology and smart materials with cellulose acetate has paved the way for responsive and adaptive medical devices. These developments align with the broader trends in biomaterials science, which emphasize biomimetic approaches and sustainable, eco-friendly solutions.
The primary objective of improving the biocompatibility of cellulose acetate devices is to enhance their interaction with biological systems, minimizing adverse reactions and maximizing therapeutic efficacy. This involves addressing several key challenges, including reducing protein adsorption, improving cell adhesion and proliferation, and controlling the material's degradation rate in vivo.
Researchers aim to develop cellulose acetate-based devices that can seamlessly integrate with host tissues, promote healing, and potentially even stimulate tissue regeneration. Another crucial goal is to tailor the material's properties to specific medical applications, allowing for customized solutions in areas such as drug delivery, tissue engineering, and implantable devices.
As we look towards the future, the focus is on creating multifunctional cellulose acetate devices that can adapt to dynamic biological environments. This includes developing smart materials that can respond to physiological cues, incorporating bioactive molecules for enhanced therapeutic effects, and exploring new manufacturing techniques like 3D printing for personalized medical devices.
The pursuit of improved biocompatibility in cellulose acetate devices is not only driven by medical needs but also by regulatory requirements and the push for more sustainable healthcare solutions. As such, this field of research sits at the intersection of materials science, bioengineering, and medical technology, promising significant advancements in patient care and treatment outcomes.
Market Analysis for Biocompatible Cellulose Acetate Devices
The market for biocompatible cellulose acetate devices is experiencing significant growth, driven by increasing demand in various medical and healthcare applications. Cellulose acetate, a versatile biopolymer, has gained attention due to its potential for improved biocompatibility in medical devices and implants.
The global market for biocompatible materials is projected to expand rapidly in the coming years, with cellulose acetate devices playing a crucial role in this growth. Key factors contributing to market expansion include the rising prevalence of chronic diseases, an aging population, and advancements in medical technology.
In the healthcare sector, cellulose acetate devices find applications in wound dressings, drug delivery systems, and tissue engineering scaffolds. The wound care segment, in particular, shows promising growth potential due to the increasing incidence of chronic wounds and diabetic ulcers. Cellulose acetate-based wound dressings offer advantages such as moisture retention, bacterial barrier properties, and enhanced healing rates.
The pharmaceutical industry represents another significant market for biocompatible cellulose acetate devices. Drug delivery systems utilizing cellulose acetate membranes or matrices are gaining traction due to their controlled release properties and biocompatibility. This trend is expected to continue as the demand for targeted and sustained drug delivery solutions grows.
Geographically, North America and Europe currently dominate the market for biocompatible cellulose acetate devices, owing to well-established healthcare infrastructure and high adoption rates of advanced medical technologies. However, the Asia-Pacific region is anticipated to witness the fastest growth in the coming years, driven by improving healthcare access, rising disposable incomes, and increasing awareness of advanced medical treatments.
Despite the positive market outlook, challenges remain in the widespread adoption of biocompatible cellulose acetate devices. These include the need for extensive clinical trials to demonstrate long-term safety and efficacy, regulatory hurdles, and competition from alternative biomaterials. Addressing these challenges through continued research and development efforts will be crucial for realizing the full market potential of biocompatible cellulose acetate devices.
In conclusion, the market for biocompatible cellulose acetate devices shows promising growth prospects across various medical applications. As research advances and new applications emerge, the demand for these devices is expected to increase, presenting opportunities for innovation and market expansion in the coming years.
Current Challenges in Cellulose Acetate Biocompatibility
Despite the widespread use of cellulose acetate in various biomedical applications, several challenges persist in improving its biocompatibility. One of the primary issues is the material's hydrophobic nature, which can lead to protein adsorption and subsequent foreign body responses. This protein layer formation often results in reduced device functionality and increased risk of inflammation or rejection.
Another significant challenge is the potential release of acetyl groups during degradation, which can alter the local pH and potentially cause tissue irritation or damage. This degradation process can also lead to changes in the mechanical properties of the device over time, potentially compromising its intended function and longevity in the biological environment.
The surface topography of cellulose acetate devices presents an additional hurdle. Micro-scale irregularities can provide sites for bacterial adhesion and biofilm formation, increasing the risk of device-associated infections. Modifying the surface without altering the bulk properties of the material remains a complex task.
Cellulose acetate's limited cell adhesion properties pose challenges for applications requiring tissue integration. This characteristic can lead to poor wound healing around implanted devices and reduced overall biocompatibility. Enhancing cell attachment and proliferation on cellulose acetate surfaces without compromising other beneficial properties is an ongoing area of research.
The material's relatively low mechanical strength in physiological conditions is another concern, particularly for load-bearing applications. Improving the mechanical properties while maintaining biocompatibility and biodegradability presents a significant engineering challenge.
Furthermore, the controlled release of therapeutic agents from cellulose acetate matrices remains difficult to optimize. Achieving sustained and predictable drug release profiles without compromising the structural integrity of the device is crucial for many biomedical applications.
Lastly, the immune response to cellulose acetate devices, while generally mild, can still lead to complications in some patients. Developing strategies to modulate or minimize this response without resorting to systemic immunosuppression is a key area of focus in improving the overall biocompatibility of cellulose acetate-based medical devices.
Existing Biocompatibility Enhancement Methods
01 Biocompatible cellulose acetate materials for medical devices
Cellulose acetate materials are being developed and modified to enhance their biocompatibility for use in medical devices. These materials are engineered to minimize adverse reactions when in contact with biological tissues, making them suitable for implants, drug delivery systems, and other biomedical applications.- Biocompatible cellulose acetate materials for medical devices: Development of cellulose acetate materials with enhanced biocompatibility for use in medical devices. These materials are designed to minimize adverse reactions when in contact with biological tissues, making them suitable for implants, drug delivery systems, and other biomedical applications.
- Surface modification of cellulose acetate for improved biocompatibility: Techniques for modifying the surface of cellulose acetate devices to enhance their biocompatibility. This may include coating with biocompatible polymers, plasma treatment, or chemical modifications to improve cell adhesion, reduce protein adsorption, and minimize inflammatory responses.
- Cellulose acetate composites for tissue engineering: Development of biocompatible cellulose acetate composites for tissue engineering applications. These composites may incorporate other materials or bioactive agents to promote cell growth, differentiation, and tissue regeneration while maintaining biocompatibility.
- Biodegradable cellulose acetate devices: Creation of biodegradable cellulose acetate devices that maintain biocompatibility throughout their degradation process. These devices are designed to be gradually absorbed by the body without causing adverse reactions, making them suitable for temporary implants or drug delivery systems.
- Biocompatibility testing methods for cellulose acetate devices: Development and standardization of biocompatibility testing methods specifically tailored for cellulose acetate devices. These methods assess the material's interaction with biological systems, including cytotoxicity, inflammatory responses, and long-term biocompatibility, to ensure the safety and efficacy of cellulose acetate-based medical devices.
02 Surface modification techniques for cellulose acetate devices
Various surface modification techniques are employed to improve the biocompatibility of cellulose acetate devices. These methods include plasma treatment, chemical grafting, and coating with biocompatible polymers to enhance cell adhesion, reduce protein adsorption, and minimize inflammatory responses.Expand Specific Solutions03 Cellulose acetate composites for tissue engineering
Cellulose acetate is being combined with other biocompatible materials to create composite scaffolds for tissue engineering applications. These composites aim to mimic the extracellular matrix, promote cell growth and differentiation, and provide mechanical support for tissue regeneration.Expand Specific Solutions04 Biodegradable cellulose acetate formulations
Research is focused on developing biodegradable cellulose acetate formulations that can be safely absorbed by the body over time. These formulations are designed to maintain their structural integrity during the healing process and then gradually break down into non-toxic components.Expand Specific Solutions05 Biocompatibility testing methods for cellulose acetate devices
Standardized biocompatibility testing methods are being established to evaluate the safety and efficacy of cellulose acetate devices. These tests assess cytotoxicity, genotoxicity, immunogenicity, and long-term tissue responses to ensure the devices meet regulatory requirements for medical use.Expand Specific Solutions
Key Players in Biocompatible Cellulose Acetate Industry
The biocompatibility improvement of cellulose acetate devices is in a growth phase, with increasing market demand driven by medical and pharmaceutical applications. The global market for biocompatible materials is expanding, with cellulose acetate gaining traction due to its versatility. Technologically, the field is moderately mature, with ongoing research focused on enhancing biocompatibility. Companies like Daicel Corp., Eastman Chemical Co., and Celanese International Corp. are leading innovators, while academic institutions such as South China University of Technology and Kyushu University contribute significant research. Collaboration between industry leaders and research institutions is accelerating progress in this field, with a focus on surface modifications and composite formulations to improve biocompatibility.
Daicel Corp.
Eastman Chemical Co.
Innovative Approaches to Cellulose Acetate Modification
- A biocompatible device featuring a composite membrane with a semipermeable layer containing a cellulose derivative laminated on a thermoplastic nonwoven fabric, providing improved strength and immunoisolation, and a nonwoven fabric layer that enhances cell and tissue encapsulation within a hydrogel, preventing immune cell invasion and maintaining tissue function.
- A melt-processable cellulose acetate composition comprising cellulose acetate, fatty acid, and an optional processing aid, which improves processability and article properties, allowing for the production of biodegradable and compostable products with enhanced performance.
Regulatory Framework for Biocompatible Medical Devices
The regulatory framework for biocompatible medical devices plays a crucial role in ensuring the safety and efficacy of cellulose acetate devices. In the United States, the Food and Drug Administration (FDA) oversees the regulation of medical devices, including those made from cellulose acetate. The FDA's regulatory process is based on a risk-based classification system, with Class I devices being low-risk and Class III devices being high-risk.
For cellulose acetate devices, the classification depends on their intended use and level of risk. Many cellulose acetate devices fall under Class II, requiring a 510(k) premarket notification. This process involves demonstrating that the device is substantially equivalent to a legally marketed predicate device in terms of safety and effectiveness.
The FDA's guidance document on biocompatibility testing for medical devices outlines the requirements for evaluating the biocompatibility of materials used in medical devices. This includes a series of tests such as cytotoxicity, sensitization, irritation, and systemic toxicity. For cellulose acetate devices, manufacturers must provide data demonstrating the material's biocompatibility in accordance with these guidelines.
In the European Union, the Medical Device Regulation (MDR) governs the approval and marketing of medical devices. The MDR places a strong emphasis on clinical evidence and post-market surveillance. Cellulose acetate devices must comply with the General Safety and Performance Requirements outlined in the MDR, which includes demonstrating biocompatibility.
The International Organization for Standardization (ISO) has developed several standards relevant to biocompatibility testing, such as ISO 10993. This series of standards provides a framework for the biological evaluation of medical devices and is widely recognized by regulatory bodies worldwide.
Manufacturers of cellulose acetate devices must also consider specific regulations related to the device's intended use. For example, devices intended for long-term implantation may require more extensive biocompatibility testing compared to those intended for short-term use.
As the field of biomaterials advances, regulatory frameworks are evolving to keep pace with new technologies. This includes the development of guidance documents for novel materials and manufacturing processes, which may impact the regulatory pathway for innovative cellulose acetate devices.
Environmental Impact of Biocompatible Cellulose Acetate
The environmental impact of biocompatible cellulose acetate devices is a crucial consideration in their development and application. As these devices gain prominence in various fields, particularly in medical and consumer products, their interaction with the environment throughout their lifecycle becomes increasingly significant.
Cellulose acetate, derived from natural cellulose, offers several environmental advantages over synthetic polymers. Its biodegradability is a key factor in reducing long-term environmental pollution. When properly disposed of, cellulose acetate devices can decompose naturally, minimizing their persistence in ecosystems. This characteristic is particularly valuable in addressing the growing concern of plastic pollution in marine and terrestrial environments.
The production process of biocompatible cellulose acetate also tends to have a lower environmental footprint compared to many synthetic alternatives. The raw material, cellulose, is renewable and abundant, often sourced from sustainably managed forests or agricultural byproducts. This reduces the reliance on fossil fuel-based resources, contributing to a more sustainable manufacturing cycle.
However, the environmental impact is not entirely benign. The acetylation process, which converts cellulose to cellulose acetate, involves the use of acetic anhydride and other chemicals. Proper management of these chemicals and their byproducts is essential to prevent environmental contamination. Additionally, the energy consumption in the manufacturing process can contribute to carbon emissions, although this impact can be mitigated through the use of renewable energy sources.
The end-of-life management of cellulose acetate devices is another critical aspect of their environmental impact. While biodegradable, the rate of decomposition can vary significantly depending on environmental conditions. In controlled composting environments, these devices can break down relatively quickly. However, in landfills or natural settings, the process may be considerably slower, potentially leading to accumulation if not properly managed.
The biocompatibility of cellulose acetate devices also influences their environmental impact. Improved biocompatibility often means reduced toxicity and better integration with biological systems. This can lead to fewer adverse effects on organisms that may come into contact with these devices in the environment, whether during use or after disposal.
In the context of medical applications, biocompatible cellulose acetate devices offer the potential for reduced medical waste. Their ability to be safely absorbed or broken down by the body in certain applications can decrease the volume of waste generated from medical procedures and treatments. This aspect is particularly relevant in addressing the growing concern over medical waste management and its environmental implications.
As research continues to improve the biocompatibility of cellulose acetate devices, there is an opportunity to further enhance their environmental profile. Innovations in material science and manufacturing processes may lead to even more sustainable production methods and improved end-of-life characteristics. This ongoing development underscores the potential for cellulose acetate to play a significant role in the transition towards more environmentally friendly materials in various industries.