Chemical Mechanisms in Nickel Phosphorus Coating Formation
OCT 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Nickel Phosphorus Coating Technology Background and Objectives
Nickel phosphorus (Ni-P) coating technology has evolved significantly since its inception in the mid-20th century. Initially developed as a corrosion-resistant alternative to chromium plating, Ni-P coatings have progressively expanded their application scope across multiple industries. The technology's evolution has been marked by continuous improvements in deposition techniques, bath formulations, and process control parameters, leading to enhanced coating properties and performance characteristics.
The chemical mechanisms underlying Ni-P coating formation represent a complex interplay of electrochemical reactions, primarily involving the reduction of nickel ions and hypophosphite. This process, known as electroless nickel plating, operates through an autocatalytic reaction mechanism that enables uniform deposition on complex geometries without requiring an external current source. Understanding these mechanisms is crucial for optimizing coating properties and expanding application possibilities.
Current technological trends in Ni-P coating development focus on achieving precise control over phosphorus content, which significantly influences coating hardness, wear resistance, corrosion protection, and magnetic properties. The industry is witnessing a shift toward environmentally friendly formulations that eliminate hazardous chemicals while maintaining or improving coating performance. Additionally, there is growing interest in nano-structured Ni-P coatings and composite variants incorporating particles such as silicon carbide or diamond for specialized applications.
The primary technical objectives in this field include developing a comprehensive understanding of the nucleation and growth mechanisms during deposition, establishing precise correlations between process parameters and resulting microstructures, and formulating predictive models for coating behavior under various service conditions. Researchers aim to achieve greater control over phosphorus distribution within the coating matrix and enhance the stability of bath formulations to extend operational lifespans.
From an industrial perspective, there is significant interest in reducing process energy requirements, minimizing waste generation, and developing more efficient recovery methods for spent plating solutions. The technology roadmap also emphasizes the need for standardized testing protocols to accurately evaluate coating performance across different application environments and establish reliable quality control parameters.
As environmental regulations become increasingly stringent worldwide, a key objective is to develop completely lead-free and cadmium-free Ni-P coating processes that maintain the exceptional performance characteristics of traditional formulations. This environmental imperative is driving innovation in catalyst systems and stabilizer chemistries, opening new avenues for technological advancement in this mature but still evolving field.
The chemical mechanisms underlying Ni-P coating formation represent a complex interplay of electrochemical reactions, primarily involving the reduction of nickel ions and hypophosphite. This process, known as electroless nickel plating, operates through an autocatalytic reaction mechanism that enables uniform deposition on complex geometries without requiring an external current source. Understanding these mechanisms is crucial for optimizing coating properties and expanding application possibilities.
Current technological trends in Ni-P coating development focus on achieving precise control over phosphorus content, which significantly influences coating hardness, wear resistance, corrosion protection, and magnetic properties. The industry is witnessing a shift toward environmentally friendly formulations that eliminate hazardous chemicals while maintaining or improving coating performance. Additionally, there is growing interest in nano-structured Ni-P coatings and composite variants incorporating particles such as silicon carbide or diamond for specialized applications.
The primary technical objectives in this field include developing a comprehensive understanding of the nucleation and growth mechanisms during deposition, establishing precise correlations between process parameters and resulting microstructures, and formulating predictive models for coating behavior under various service conditions. Researchers aim to achieve greater control over phosphorus distribution within the coating matrix and enhance the stability of bath formulations to extend operational lifespans.
From an industrial perspective, there is significant interest in reducing process energy requirements, minimizing waste generation, and developing more efficient recovery methods for spent plating solutions. The technology roadmap also emphasizes the need for standardized testing protocols to accurately evaluate coating performance across different application environments and establish reliable quality control parameters.
As environmental regulations become increasingly stringent worldwide, a key objective is to develop completely lead-free and cadmium-free Ni-P coating processes that maintain the exceptional performance characteristics of traditional formulations. This environmental imperative is driving innovation in catalyst systems and stabilizer chemistries, opening new avenues for technological advancement in this mature but still evolving field.
Industrial Applications and Market Demand Analysis
Nickel Phosphorus (Ni-P) coatings have established themselves as critical surface treatment solutions across multiple industries due to their exceptional combination of properties. The global market for electroless nickel plating, which includes Ni-P coatings, was valued at approximately 2.1 billion USD in 2022 and is projected to grow at a compound annual growth rate of 3.8% through 2030.
The automotive industry represents one of the largest application sectors for Ni-P coatings, where they are extensively used for components requiring high wear resistance and corrosion protection. These include fuel system components, brake pistons, and transmission parts. The transition toward electric vehicles has further expanded demand, as these coatings provide electromagnetic shielding for sensitive electronic components.
In the electronics industry, Ni-P coatings serve as essential elements in printed circuit board manufacturing, providing solderable surfaces and corrosion resistance. The miniaturization trend in electronics has intensified requirements for uniform, defect-free coatings, driving innovation in Ni-P deposition techniques. The semiconductor industry specifically demands high-phosphorus Ni-P coatings for their non-magnetic properties and excellent diffusion barrier characteristics.
The aerospace sector utilizes Ni-P coatings for critical components exposed to extreme conditions. The coatings' ability to maintain structural integrity under high temperatures and corrosive environments makes them ideal for turbine components and hydraulic systems. Market analysis indicates growing demand in this sector, with an estimated annual growth rate of 4.2%.
Oil and gas industries rely on Ni-P coatings for downhole tools, valves, and pumping equipment operating in highly corrosive environments. The coatings' resistance to hydrogen sulfide and carbon dioxide corrosion extends equipment lifespan significantly, providing substantial cost savings in maintenance and replacement.
The chemical processing industry represents another significant market, where Ni-P coatings protect equipment from chemical attack. Reactors, heat exchangers, and storage tanks benefit from the coatings' chemical stability and non-catalytic surface properties.
Market trends indicate growing demand for environmentally friendly Ni-P coating processes that reduce or eliminate hazardous chemicals. Regulatory pressures, particularly in Europe and North America, are driving research into green chemistry approaches for Ni-P deposition. Additionally, there is increasing interest in functionalized Ni-P coatings with enhanced properties such as hydrophobicity, antimicrobial activity, or self-healing capabilities.
The automotive industry represents one of the largest application sectors for Ni-P coatings, where they are extensively used for components requiring high wear resistance and corrosion protection. These include fuel system components, brake pistons, and transmission parts. The transition toward electric vehicles has further expanded demand, as these coatings provide electromagnetic shielding for sensitive electronic components.
In the electronics industry, Ni-P coatings serve as essential elements in printed circuit board manufacturing, providing solderable surfaces and corrosion resistance. The miniaturization trend in electronics has intensified requirements for uniform, defect-free coatings, driving innovation in Ni-P deposition techniques. The semiconductor industry specifically demands high-phosphorus Ni-P coatings for their non-magnetic properties and excellent diffusion barrier characteristics.
The aerospace sector utilizes Ni-P coatings for critical components exposed to extreme conditions. The coatings' ability to maintain structural integrity under high temperatures and corrosive environments makes them ideal for turbine components and hydraulic systems. Market analysis indicates growing demand in this sector, with an estimated annual growth rate of 4.2%.
Oil and gas industries rely on Ni-P coatings for downhole tools, valves, and pumping equipment operating in highly corrosive environments. The coatings' resistance to hydrogen sulfide and carbon dioxide corrosion extends equipment lifespan significantly, providing substantial cost savings in maintenance and replacement.
The chemical processing industry represents another significant market, where Ni-P coatings protect equipment from chemical attack. Reactors, heat exchangers, and storage tanks benefit from the coatings' chemical stability and non-catalytic surface properties.
Market trends indicate growing demand for environmentally friendly Ni-P coating processes that reduce or eliminate hazardous chemicals. Regulatory pressures, particularly in Europe and North America, are driving research into green chemistry approaches for Ni-P deposition. Additionally, there is increasing interest in functionalized Ni-P coatings with enhanced properties such as hydrophobicity, antimicrobial activity, or self-healing capabilities.
Current State and Technical Challenges in Ni-P Coating
The global landscape of Nickel-Phosphorus (Ni-P) coating technology has witnessed significant advancements in recent years, with applications spanning across automotive, aerospace, electronics, and chemical industries. Currently, electroless Ni-P coating dominates commercial applications due to its uniform deposition capability on complex geometries and non-conductive substrates. The technology has evolved from basic single-layer coatings to sophisticated multi-layer and composite systems incorporating particles like SiC, Al2O3, and PTFE to enhance specific properties.
Despite widespread industrial adoption, several technical challenges persist in Ni-P coating formation. Bath stability remains a critical issue, with spontaneous decomposition occurring due to factors including pH fluctuation, contamination, and temperature variations. This instability not only reduces bath life but also compromises coating quality and increases operational costs. Current stabilizer systems show limitations in maintaining long-term bath performance, particularly in high-phosphorus formulations.
Coating adhesion presents another significant challenge, especially on difficult substrates like aluminum alloys and certain steels. The conventional activation processes often fail to create sufficient nucleation sites, resulting in poor adhesion and premature coating failure. The industry still lacks standardized pre-treatment protocols that work consistently across diverse substrate materials.
Phosphorus content control represents a persistent technical hurdle. While low (1-5%), medium (5-9%), and high (>9%) phosphorus coatings offer distinct property profiles, precise control within these ranges remains difficult. Current technologies struggle to maintain consistent phosphorus distribution throughout the coating thickness, leading to property variations that affect performance reliability.
Environmental and health concerns constitute growing challenges for Ni-P coating technology. Traditional formulations rely on chemicals like sodium hypophosphite (reducing agent), lead compounds (stabilizers), and cadmium (brighteners) that face increasing regulatory restrictions. The industry is under pressure to develop more environmentally friendly alternatives while maintaining coating performance and cost-effectiveness.
Scale-up and process control challenges become evident in industrial implementation. Laboratory-optimized formulations often perform inconsistently at production scale due to differences in agitation patterns, temperature gradients, and loading factors. Real-time monitoring technologies for bath composition remain inadequate, forcing manufacturers to rely on periodic sampling that may miss critical process deviations.
Emerging applications in microelectronics and medical devices demand increasingly stringent performance requirements that current Ni-P coating technologies struggle to meet consistently. These include ultra-thin coatings (<1 μm) with uniform phosphorus distribution, defect-free surfaces at nanoscale, and biocompatibility for medical applications.
Despite widespread industrial adoption, several technical challenges persist in Ni-P coating formation. Bath stability remains a critical issue, with spontaneous decomposition occurring due to factors including pH fluctuation, contamination, and temperature variations. This instability not only reduces bath life but also compromises coating quality and increases operational costs. Current stabilizer systems show limitations in maintaining long-term bath performance, particularly in high-phosphorus formulations.
Coating adhesion presents another significant challenge, especially on difficult substrates like aluminum alloys and certain steels. The conventional activation processes often fail to create sufficient nucleation sites, resulting in poor adhesion and premature coating failure. The industry still lacks standardized pre-treatment protocols that work consistently across diverse substrate materials.
Phosphorus content control represents a persistent technical hurdle. While low (1-5%), medium (5-9%), and high (>9%) phosphorus coatings offer distinct property profiles, precise control within these ranges remains difficult. Current technologies struggle to maintain consistent phosphorus distribution throughout the coating thickness, leading to property variations that affect performance reliability.
Environmental and health concerns constitute growing challenges for Ni-P coating technology. Traditional formulations rely on chemicals like sodium hypophosphite (reducing agent), lead compounds (stabilizers), and cadmium (brighteners) that face increasing regulatory restrictions. The industry is under pressure to develop more environmentally friendly alternatives while maintaining coating performance and cost-effectiveness.
Scale-up and process control challenges become evident in industrial implementation. Laboratory-optimized formulations often perform inconsistently at production scale due to differences in agitation patterns, temperature gradients, and loading factors. Real-time monitoring technologies for bath composition remain inadequate, forcing manufacturers to rely on periodic sampling that may miss critical process deviations.
Emerging applications in microelectronics and medical devices demand increasingly stringent performance requirements that current Ni-P coating technologies struggle to meet consistently. These include ultra-thin coatings (<1 μm) with uniform phosphorus distribution, defect-free surfaces at nanoscale, and biocompatibility for medical applications.
Current Chemical Reaction Mechanisms and Process Solutions
01 Electroless nickel-phosphorus coating composition
Electroless nickel-phosphorus coating compositions typically contain nickel salts, reducing agents (such as sodium hypophosphite), complexing agents, stabilizers, and pH adjusters. The phosphorus content in these coatings can be controlled by adjusting bath parameters such as pH, temperature, and concentration of reducing agents. Different phosphorus contents (low, medium, and high) result in coatings with varying properties including hardness, corrosion resistance, and magnetic characteristics.- Electroless nickel phosphorus coating composition: Electroless nickel phosphorus coating compositions typically include nickel salts, reducing agents (such as sodium hypophosphite), complexing agents, stabilizers, and pH adjusters. The phosphorus content in these coatings can be controlled by adjusting bath parameters such as pH, temperature, and concentration of reducing agents. Different phosphorus contents (low, medium, and high) result in coatings with varying properties including hardness, corrosion resistance, and magnetic characteristics.
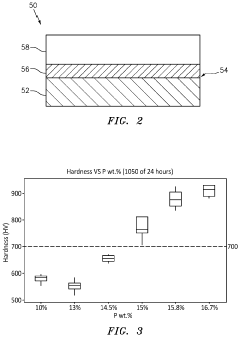
- Heat treatment of nickel phosphorus coatings: Heat treatment significantly affects the properties of nickel phosphorus coatings by transforming their amorphous structure into crystalline phases. Post-deposition heat treatment at specific temperatures (typically 300-400°C) can substantially increase hardness and wear resistance through the precipitation of nickel phosphide (Ni3P) phases. The heat treatment process must be carefully controlled to achieve optimal properties without causing embrittlement or substrate damage.
- Corrosion resistance enhancement techniques: Various methods can enhance the corrosion resistance of nickel phosphorus coatings, including increasing phosphorus content (high-phosphorus coatings typically offer better corrosion protection), incorporating additional elements like molybdenum or tungsten, applying sealants or topcoats, and using multi-layer coating systems. Surface preparation and post-treatment processes also significantly impact the coating's ability to protect against different corrosive environments.
- Composite nickel phosphorus coatings with particles: Composite nickel phosphorus coatings incorporate various particles such as silicon carbide, diamond, PTFE, or ceramic materials to enhance specific properties. These particles are co-deposited during the electroless plating process, resulting in coatings with improved wear resistance, self-lubrication, hardness, or thermal properties. The concentration and distribution of particles can be controlled by adjusting bath composition, agitation, and deposition parameters.
- Application-specific nickel phosphorus coating processes: Specialized nickel phosphorus coating processes have been developed for specific applications such as electronic components, automotive parts, aerospace components, and precision machinery. These processes involve tailored pre-treatments, custom bath formulations, and specific post-treatments to meet industry requirements. Parameters like coating thickness, phosphorus content, and surface finish are optimized based on the intended application's performance needs.
02 Heat treatment of nickel-phosphorus coatings
Heat treatment significantly affects the properties of nickel-phosphorus coatings. When heated at specific temperatures (typically 300-400°C), the amorphous structure transforms into crystalline Ni and Ni3P phases, resulting in increased hardness and wear resistance. The heat treatment process can be optimized by controlling temperature, duration, and atmosphere to achieve desired mechanical properties while minimizing oxidation or other undesirable effects.Expand Specific Solutions03 Corrosion resistance enhancement techniques
Various methods can enhance the corrosion resistance of nickel-phosphorus coatings, including increasing phosphorus content, incorporating additional elements (such as molybdenum, tungsten, or rare earth elements), applying multilayer structures, and post-treatment processes. Surface passivation treatments and sealants can further improve the coating's barrier properties against aggressive environments, making them suitable for applications in marine, chemical, and petroleum industries.Expand Specific Solutions04 Composite nickel-phosphorus coatings with particles
Incorporating particles such as silicon carbide, diamond, PTFE, or various nanoparticles into nickel-phosphorus matrices creates composite coatings with enhanced properties. These composite coatings exhibit improved wear resistance, self-lubrication, hardness, or thermal conductivity depending on the particles used. The co-deposition process requires careful control of particle concentration, agitation, and deposition parameters to achieve uniform particle distribution throughout the coating.Expand Specific Solutions05 Application-specific nickel-phosphorus coating formulations
Specialized nickel-phosphorus coating formulations are developed for specific applications such as electronic components, automotive parts, aerospace components, and chemical processing equipment. These tailored formulations may include modified bath compositions, specific phosphorus content ranges, incorporation of additional elements, or post-treatment processes to meet particular requirements like solderability, electromagnetic shielding, high-temperature stability, or resistance to specific chemicals.Expand Specific Solutions
Leading Manufacturers and Research Institutions in Ni-P Coating
The nickel phosphorus coating formation market is currently in a growth phase, characterized by increasing demand across automotive, electronics, and industrial sectors. The market size is expanding due to the coating's superior corrosion resistance and hardness properties, with projections showing continued growth driven by technological advancements. In terms of technical maturity, established players like Henkel AG, Nihon Parkerizing, and Chemetall GmbH lead with advanced chemical formulations and process control technologies. Companies including Toyota Motor Corp., ThyssenKrupp, and DuPont are investing in research to enhance coating performance and environmental sustainability. Academic institutions such as Zhejiang University and Wuhan Research Institute of Materials Protection are contributing significant research to advance fundamental understanding of chemical mechanisms, creating a competitive landscape balanced between industrial innovation and academic research.
Henkel Corporation
Technical Solution: Henkel Corporation has developed advanced electroless nickel phosphorus (ENP) coating technologies that focus on optimizing the chemical reduction mechanisms. Their approach involves a hypophosphite-based reduction process where sodium hypophosphite (NaH2PO2) acts as the reducing agent in an aqueous solution containing nickel ions. The reaction mechanism involves the catalytic oxidation of hypophosphite on activated substrate surfaces, releasing electrons that reduce nickel ions to metallic nickel while simultaneously incorporating phosphorus into the deposit. Henkel's proprietary stabilizer systems control the reaction kinetics to prevent spontaneous decomposition while maintaining high deposition rates[1]. Their formulations typically operate at pH ranges of 4.5-5.5 and temperatures between 85-92°C, optimized for maximum coating uniformity and phosphorus content control (typically 7-12% P). The company has also developed mid-phosphorus formulations that balance corrosion resistance with mechanical properties through precise control of the reduction kinetics and phosphorus co-deposition mechanisms.
Strengths: Superior bath stability allowing for longer solution life and consistent phosphorus content throughout multiple plating cycles. Their formulations achieve excellent thickness uniformity even on complex geometries due to the throwing power of the electroless process. Weaknesses: Higher operational costs compared to conventional electroplating processes and relatively slower deposition rates (15-25 μm/hour) compared to some competing technologies, limiting throughput in high-volume production environments.
General Electric Company
Technical Solution: General Electric has developed advanced electroless nickel phosphorus coating technologies through their GE Research division, focusing on understanding and controlling the fundamental chemical mechanisms. Their approach involves a sophisticated understanding of the redox reactions between nickel ions and hypophosphite reducing agents. GE's process begins with surface activation using proprietary catalysts, followed by controlled deposition where the hypophosphite anion (H2PO2-) undergoes oxidation at catalytic surfaces, releasing electrons that reduce nickel ions to metallic nickel. Simultaneously, a portion of the hypophosphite is converted to elemental phosphorus through a parallel reduction pathway, resulting in phosphorus co-deposition. GE has developed specialized complexing agents that maintain nickel solubility across a wide pH range while preventing hydroxide precipitation[4]. Their technology incorporates advanced stabilizer systems that selectively inhibit homogeneous nucleation while permitting surface-catalyzed deposition. GE's formulations precisely control the critical factors affecting phosphorus content, including temperature (85-95°C), pH (4.2-5.0), and hypophosphite-to-nickel ratio. Their research has elucidated the mechanism by which hydrogen gas evolution occurs as a side reaction, allowing them to minimize hydrogen embrittlement risks in critical applications like aerospace components and power generation equipment.
Strengths: Superior coating uniformity on complex geometries due to the solution-based deposition mechanism, and exceptional hardness (up to 1100 HV after heat treatment) for wear-resistant applications. Their high-phosphorus formulations (10-13% P) achieve outstanding corrosion resistance in aggressive environments. Weaknesses: Higher operational costs compared to conventional plating processes and relatively complex process control requirements necessitating sophisticated monitoring equipment. Their systems typically require more precise temperature and pH control than competing technologies.
Key Patents and Scientific Literature on Ni-P Coating Formation
Multi-layered nickel-phosphorous coatings and processes for forming the same
PatentInactiveUS20090286104A1
Innovation
- A multi-layered nickel phosphorous coating process involving an initial layer with 4-6 weight percent phosphorous, metallurgically bonded to a base metal substrate through heat treatment, followed by a second layer with 8-12 weight percent phosphorous, both formed by electroless plating, to enhance adhesion and mechanical properties without degrading the second layer.
Thermally stable nickel-phosphorus alloy for high temperature applications
PatentInactiveUS20210254232A1
Innovation
- A nickel-phosphorus alloy coating with a phosphorus content between 15.0 wt. percent to 20.9 wt. percent is developed, which includes a base layer and a nickel strike layer, maintaining hardness above 800 HV even after heat treatment, enabling wear and hot corrosion protection at temperatures higher than 900 degrees Fahrenheit.
Environmental Impact and Sustainability Considerations
The environmental impact of nickel phosphorus coating processes has become increasingly significant as regulatory frameworks and sustainability goals evolve globally. Traditional electroless nickel phosphorus plating operations utilize chemicals that pose substantial environmental concerns, particularly nickel compounds which are classified as carcinogenic and toxic to aquatic organisms. The plating baths typically contain sodium hypophosphite, complexing agents, and stabilizers that contribute to high chemical oxygen demand (COD) and biological oxygen demand (BOD) in wastewater streams.
Waste management represents a critical challenge in this industry, with spent plating solutions requiring specialized treatment before disposal. The high concentrations of heavy metals, particularly nickel, necessitate advanced precipitation, ion exchange, or membrane filtration technologies to meet increasingly stringent discharge regulations. Additionally, the reducing agents used in the process generate hydrogen gas as a byproduct, raising both safety and greenhouse gas emission concerns.
Energy consumption presents another significant environmental consideration. Conventional nickel phosphorus plating processes operate at elevated temperatures (85-95°C), requiring substantial energy inputs for bath heating and maintenance. This energy demand translates directly to carbon emissions when fossil fuel-derived electricity is utilized, contributing to the overall carbon footprint of coated products.
Recent sustainability innovations have focused on developing lower-temperature plating processes, reducing hazardous chemical usage, and implementing closed-loop recycling systems. Several research groups have demonstrated viable low-temperature (50-65°C) plating formulations that maintain acceptable deposition rates while reducing energy consumption by up to 40%. Additionally, alternative complexing agents derived from renewable resources are being investigated to replace traditional chemicals like ethylenediamine and citric acid.
Recovery and recycling technologies have advanced significantly, with selective ion exchange systems now capable of recovering over 95% of nickel from spent plating solutions. These recovered materials can be reintroduced into the manufacturing cycle, reducing both raw material demand and waste generation. Some facilities have implemented evaporation and crystallization techniques to recover valuable chemicals from rinse waters, approaching zero liquid discharge operations.
Life cycle assessment (LCA) studies comparing nickel phosphorus coatings with alternative surface treatments have yielded mixed results, indicating that while the production phase carries significant environmental burdens, the extended service life and corrosion protection provided by these coatings may offset initial impacts through reduced replacement frequency and associated resource consumption.
Waste management represents a critical challenge in this industry, with spent plating solutions requiring specialized treatment before disposal. The high concentrations of heavy metals, particularly nickel, necessitate advanced precipitation, ion exchange, or membrane filtration technologies to meet increasingly stringent discharge regulations. Additionally, the reducing agents used in the process generate hydrogen gas as a byproduct, raising both safety and greenhouse gas emission concerns.
Energy consumption presents another significant environmental consideration. Conventional nickel phosphorus plating processes operate at elevated temperatures (85-95°C), requiring substantial energy inputs for bath heating and maintenance. This energy demand translates directly to carbon emissions when fossil fuel-derived electricity is utilized, contributing to the overall carbon footprint of coated products.
Recent sustainability innovations have focused on developing lower-temperature plating processes, reducing hazardous chemical usage, and implementing closed-loop recycling systems. Several research groups have demonstrated viable low-temperature (50-65°C) plating formulations that maintain acceptable deposition rates while reducing energy consumption by up to 40%. Additionally, alternative complexing agents derived from renewable resources are being investigated to replace traditional chemicals like ethylenediamine and citric acid.
Recovery and recycling technologies have advanced significantly, with selective ion exchange systems now capable of recovering over 95% of nickel from spent plating solutions. These recovered materials can be reintroduced into the manufacturing cycle, reducing both raw material demand and waste generation. Some facilities have implemented evaporation and crystallization techniques to recover valuable chemicals from rinse waters, approaching zero liquid discharge operations.
Life cycle assessment (LCA) studies comparing nickel phosphorus coatings with alternative surface treatments have yielded mixed results, indicating that while the production phase carries significant environmental burdens, the extended service life and corrosion protection provided by these coatings may offset initial impacts through reduced replacement frequency and associated resource consumption.
Quality Control and Performance Testing Methodologies
Quality control in nickel phosphorus coating processes requires systematic methodologies to ensure consistent performance and reliability. The industry has established several standardized testing protocols that evaluate both the physical and chemical properties of these coatings. Thickness measurement represents a fundamental quality parameter, commonly assessed through X-ray fluorescence (XRF), coulometric methods, or cross-sectional microscopy. These techniques provide accurate measurements down to the micrometer level, enabling precise control over coating specifications.
Adhesion testing constitutes another critical quality control measure, typically performed using tape tests (ASTM D3359), bend tests, or thermal shock evaluations. These methods assess the coating's ability to maintain integrity under mechanical stress, which directly correlates with service life in industrial applications. For more quantitative assessment, scratch adhesion testing with progressive loading provides numerical values for coating-substrate bond strength.
Corrosion resistance testing forms the cornerstone of performance evaluation for nickel phosphorus coatings. Salt spray testing (ASTM B117), electrochemical impedance spectroscopy (EIS), and potentiodynamic polarization measurements offer complementary insights into coating protection mechanisms. Modern quality control protocols increasingly incorporate accelerated corrosion testing to predict long-term performance within compressed timeframes.
Hardness and wear resistance measurements have become standardized through microhardness testing (typically Vickers or Knoop methods) and various tribological assessments including pin-on-disk, Taber abrasion, and scratch testing. These properties directly influence the coating's suitability for applications involving mechanical contact and friction. Post-heat treatment effects on hardness are particularly important for quality control, as they can significantly alter performance characteristics.
Phosphorus content determination represents a specialized quality parameter unique to these coatings, typically measured through energy-dispersive X-ray spectroscopy (EDS), X-ray photoelectron spectroscopy (XPS), or wet chemical analysis. The phosphorus percentage critically influences crystallinity, hardness, and corrosion behavior, making its precise measurement essential for quality assurance.
Advanced characterization techniques including scanning electron microscopy (SEM), atomic force microscopy (AFM), and X-ray diffraction (XRD) provide deeper insights into coating microstructure, surface morphology, and phase composition. These methods, while not always part of routine quality control, offer valuable diagnostic capabilities for troubleshooting coating defects and optimizing process parameters.
Adhesion testing constitutes another critical quality control measure, typically performed using tape tests (ASTM D3359), bend tests, or thermal shock evaluations. These methods assess the coating's ability to maintain integrity under mechanical stress, which directly correlates with service life in industrial applications. For more quantitative assessment, scratch adhesion testing with progressive loading provides numerical values for coating-substrate bond strength.
Corrosion resistance testing forms the cornerstone of performance evaluation for nickel phosphorus coatings. Salt spray testing (ASTM B117), electrochemical impedance spectroscopy (EIS), and potentiodynamic polarization measurements offer complementary insights into coating protection mechanisms. Modern quality control protocols increasingly incorporate accelerated corrosion testing to predict long-term performance within compressed timeframes.
Hardness and wear resistance measurements have become standardized through microhardness testing (typically Vickers or Knoop methods) and various tribological assessments including pin-on-disk, Taber abrasion, and scratch testing. These properties directly influence the coating's suitability for applications involving mechanical contact and friction. Post-heat treatment effects on hardness are particularly important for quality control, as they can significantly alter performance characteristics.
Phosphorus content determination represents a specialized quality parameter unique to these coatings, typically measured through energy-dispersive X-ray spectroscopy (EDS), X-ray photoelectron spectroscopy (XPS), or wet chemical analysis. The phosphorus percentage critically influences crystallinity, hardness, and corrosion behavior, making its precise measurement essential for quality assurance.
Advanced characterization techniques including scanning electron microscopy (SEM), atomic force microscopy (AFM), and X-ray diffraction (XRD) provide deeper insights into coating microstructure, surface morphology, and phase composition. These methods, while not always part of routine quality control, offer valuable diagnostic capabilities for troubleshooting coating defects and optimizing process parameters.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!