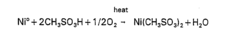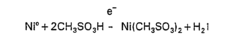Electroless Nickel Plating Fundamentals and Applications
OCT 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electroless Nickel Plating Evolution and Objectives
Electroless nickel plating, a chemical reduction process discovered in the early 1940s, has evolved significantly over the past eight decades. Initially developed as an alternative to electrolytic plating methods, this technology gained prominence during World War II when it was utilized for military applications requiring uniform metal deposition on complex geometries. The fundamental breakthrough came from the discovery that nickel ions could be reduced to metallic nickel without external electrical current, using reducing agents such as sodium hypophosphite.
The 1950s and 1960s witnessed substantial refinement of the basic chemistry, with researchers establishing optimal bath compositions and operating parameters. During this period, the correlation between phosphorus content and deposit properties became better understood, leading to the development of low, medium, and high phosphorus electroless nickel coatings tailored for specific applications.
By the 1970s and 1980s, the technology expanded beyond traditional nickel-phosphorus deposits to include nickel-boron and composite coatings incorporating particles such as silicon carbide, diamond, and PTFE. This diversification significantly broadened the application spectrum across industries including automotive, electronics, aerospace, and chemical processing.
The 1990s marked a turning point with increasing environmental concerns driving research toward more sustainable formulations. Traditional baths containing cadmium, lead, and other heavy metals as stabilizers were gradually replaced with environmentally benign alternatives. Concurrently, the microelectronics industry's growth created demand for highly specialized electroless nickel plating solutions capable of meeting stringent requirements for uniformity and purity.
The early 2000s saw significant advancements in bath stability and process control, enabled by improved analytical techniques and automation. The integration of computational modeling allowed for more precise prediction of deposit properties based on bath composition and operating conditions, reducing empirical trial-and-error approaches.
Current research focuses on several key objectives: developing more energy-efficient processes with lower operating temperatures; creating more environmentally friendly formulations with reduced waste generation; improving deposit performance characteristics such as corrosion resistance, hardness, and wear resistance; and enhancing process reliability and reproducibility for advanced manufacturing applications.
The emergence of nanotechnology has opened new frontiers, with research exploring electroless nickel nanocomposite coatings incorporating nanoscale particles to achieve unprecedented performance characteristics. Additionally, there is growing interest in applying electroless nickel plating to novel substrate materials, including advanced polymers, ceramics, and carbon-based materials, to enable next-generation applications in fields ranging from renewable energy to biomedical devices.
The 1950s and 1960s witnessed substantial refinement of the basic chemistry, with researchers establishing optimal bath compositions and operating parameters. During this period, the correlation between phosphorus content and deposit properties became better understood, leading to the development of low, medium, and high phosphorus electroless nickel coatings tailored for specific applications.
By the 1970s and 1980s, the technology expanded beyond traditional nickel-phosphorus deposits to include nickel-boron and composite coatings incorporating particles such as silicon carbide, diamond, and PTFE. This diversification significantly broadened the application spectrum across industries including automotive, electronics, aerospace, and chemical processing.
The 1990s marked a turning point with increasing environmental concerns driving research toward more sustainable formulations. Traditional baths containing cadmium, lead, and other heavy metals as stabilizers were gradually replaced with environmentally benign alternatives. Concurrently, the microelectronics industry's growth created demand for highly specialized electroless nickel plating solutions capable of meeting stringent requirements for uniformity and purity.
The early 2000s saw significant advancements in bath stability and process control, enabled by improved analytical techniques and automation. The integration of computational modeling allowed for more precise prediction of deposit properties based on bath composition and operating conditions, reducing empirical trial-and-error approaches.
Current research focuses on several key objectives: developing more energy-efficient processes with lower operating temperatures; creating more environmentally friendly formulations with reduced waste generation; improving deposit performance characteristics such as corrosion resistance, hardness, and wear resistance; and enhancing process reliability and reproducibility for advanced manufacturing applications.
The emergence of nanotechnology has opened new frontiers, with research exploring electroless nickel nanocomposite coatings incorporating nanoscale particles to achieve unprecedented performance characteristics. Additionally, there is growing interest in applying electroless nickel plating to novel substrate materials, including advanced polymers, ceramics, and carbon-based materials, to enable next-generation applications in fields ranging from renewable energy to biomedical devices.
Market Demand Analysis for Electroless Nickel Coatings
The global market for electroless nickel coatings has experienced significant growth over the past decade, driven primarily by increasing demand from automotive, electronics, aerospace, and oil & gas industries. These sectors value electroless nickel plating for its uniform deposition properties, excellent corrosion resistance, and ability to coat complex geometries with consistent thickness.
In the automotive industry, electroless nickel coatings are increasingly utilized for fuel system components, brake parts, and transmission systems where corrosion resistance and wear properties are critical. The transition toward electric vehicles has created new applications for these coatings in battery components and electrical systems, further expanding market potential.
The electronics sector represents another major demand driver, with electroless nickel-phosphorus and electroless nickel-boron coatings being essential for printed circuit boards, connectors, and electromagnetic shielding applications. The miniaturization trend in consumer electronics has heightened the need for uniform, thin coatings that can be applied to increasingly complex micro-components.
Aerospace applications continue to grow steadily, with electroless nickel coatings being specified for landing gear components, turbine parts, and hydraulic systems. The superior hardness and wear resistance of these coatings, particularly after heat treatment, make them ideal for components subjected to extreme operating conditions.
The oil and gas industry remains a substantial consumer of electroless nickel coatings, particularly for downhole tools, valves, and offshore equipment exposed to highly corrosive environments. The ability of these coatings to provide protection in harsh chemical and high-temperature conditions drives their adoption in this sector.
Regionally, Asia-Pacific dominates the market share, with China, Japan, and South Korea being major contributors due to their robust manufacturing bases. North America and Europe follow, with steady demand from established industrial sectors and increasing adoption in emerging applications.
Environmental regulations have significantly influenced market dynamics, with growing restrictions on traditional plating processes containing cadmium and hexavalent chromium. This regulatory landscape has accelerated the adoption of electroless nickel as a more environmentally acceptable alternative, though challenges remain regarding the management of nickel-containing waste streams.
The market is also witnessing increased demand for specialized variants, including composite electroless nickel coatings incorporating particles like silicon carbide or PTFE to enhance specific properties such as lubricity or wear resistance. These value-added coatings command premium pricing and represent a growing segment within the broader electroless nickel market.
In the automotive industry, electroless nickel coatings are increasingly utilized for fuel system components, brake parts, and transmission systems where corrosion resistance and wear properties are critical. The transition toward electric vehicles has created new applications for these coatings in battery components and electrical systems, further expanding market potential.
The electronics sector represents another major demand driver, with electroless nickel-phosphorus and electroless nickel-boron coatings being essential for printed circuit boards, connectors, and electromagnetic shielding applications. The miniaturization trend in consumer electronics has heightened the need for uniform, thin coatings that can be applied to increasingly complex micro-components.
Aerospace applications continue to grow steadily, with electroless nickel coatings being specified for landing gear components, turbine parts, and hydraulic systems. The superior hardness and wear resistance of these coatings, particularly after heat treatment, make them ideal for components subjected to extreme operating conditions.
The oil and gas industry remains a substantial consumer of electroless nickel coatings, particularly for downhole tools, valves, and offshore equipment exposed to highly corrosive environments. The ability of these coatings to provide protection in harsh chemical and high-temperature conditions drives their adoption in this sector.
Regionally, Asia-Pacific dominates the market share, with China, Japan, and South Korea being major contributors due to their robust manufacturing bases. North America and Europe follow, with steady demand from established industrial sectors and increasing adoption in emerging applications.
Environmental regulations have significantly influenced market dynamics, with growing restrictions on traditional plating processes containing cadmium and hexavalent chromium. This regulatory landscape has accelerated the adoption of electroless nickel as a more environmentally acceptable alternative, though challenges remain regarding the management of nickel-containing waste streams.
The market is also witnessing increased demand for specialized variants, including composite electroless nickel coatings incorporating particles like silicon carbide or PTFE to enhance specific properties such as lubricity or wear resistance. These value-added coatings command premium pricing and represent a growing segment within the broader electroless nickel market.
Global Technical Status and Challenges in ENP Technology
Electroless Nickel Plating (ENP) technology has evolved significantly since its discovery in the 1940s. Currently, the global ENP market is dominated by three main variants: nickel-phosphorus, nickel-boron, and composite coatings. The Asia-Pacific region leads market implementation, accounting for approximately 45% of global ENP applications, followed by North America (30%) and Europe (20%). This regional distribution reflects both industrial concentration and technological advancement patterns in manufacturing sectors.
The current state-of-the-art in ENP technology focuses on bath stability enhancement, with significant improvements in solution longevity from historical 3-5 metal turnovers to modern systems achieving 8-10 turnovers. Advanced stabilizer systems incorporating both organic and inorganic components have substantially reduced spontaneous decomposition issues that previously plagued industrial applications.
Despite these advancements, several critical challenges persist in ENP technology. Bath stability remains a primary concern, particularly for high-production environments where maintaining consistent deposition rates throughout bath life presents significant difficulties. The trade-off between deposition speed and coating quality continues to challenge manufacturers seeking both efficiency and performance.
Environmental considerations represent another major challenge, as traditional ENP processes utilize chemicals of increasing regulatory concern. Specifically, the presence of lead compounds as stabilizers, formaldehyde as a reducing agent in some formulations, and challenges in wastewater treatment present significant environmental compliance hurdles across global markets.
Technical limitations in coating thickness uniformity on complex geometries remain problematic, with variations exceeding 10% on intricate parts. Additionally, the industry faces challenges in achieving consistent phosphorus content distribution throughout the coating thickness, which directly impacts corrosion resistance and hardness properties.
Recent research has focused on developing more environmentally benign processes, with significant progress in formaldehyde-free systems using sodium hypophosphite, aminoborane, or hydrazine-based reducing agents. However, these alternatives often present their own challenges in terms of bath stability, cost-effectiveness, or performance characteristics.
The integration of nanotechnology represents an emerging frontier, with nano-composite ENP coatings incorporating silicon carbide, diamond, or aluminum oxide particles showing promising enhancements in wear resistance and hardness. However, particle distribution uniformity and bath stability with suspended nanoparticles remain technically challenging.
Automation and process control technologies have advanced significantly, with real-time monitoring systems for pH, temperature, and chemical constituent concentrations becoming increasingly sophisticated. These systems help maintain bath parameters within increasingly narrow operating windows required for high-performance applications in aerospace, electronics, and automotive industries.
The current state-of-the-art in ENP technology focuses on bath stability enhancement, with significant improvements in solution longevity from historical 3-5 metal turnovers to modern systems achieving 8-10 turnovers. Advanced stabilizer systems incorporating both organic and inorganic components have substantially reduced spontaneous decomposition issues that previously plagued industrial applications.
Despite these advancements, several critical challenges persist in ENP technology. Bath stability remains a primary concern, particularly for high-production environments where maintaining consistent deposition rates throughout bath life presents significant difficulties. The trade-off between deposition speed and coating quality continues to challenge manufacturers seeking both efficiency and performance.
Environmental considerations represent another major challenge, as traditional ENP processes utilize chemicals of increasing regulatory concern. Specifically, the presence of lead compounds as stabilizers, formaldehyde as a reducing agent in some formulations, and challenges in wastewater treatment present significant environmental compliance hurdles across global markets.
Technical limitations in coating thickness uniformity on complex geometries remain problematic, with variations exceeding 10% on intricate parts. Additionally, the industry faces challenges in achieving consistent phosphorus content distribution throughout the coating thickness, which directly impacts corrosion resistance and hardness properties.
Recent research has focused on developing more environmentally benign processes, with significant progress in formaldehyde-free systems using sodium hypophosphite, aminoborane, or hydrazine-based reducing agents. However, these alternatives often present their own challenges in terms of bath stability, cost-effectiveness, or performance characteristics.
The integration of nanotechnology represents an emerging frontier, with nano-composite ENP coatings incorporating silicon carbide, diamond, or aluminum oxide particles showing promising enhancements in wear resistance and hardness. However, particle distribution uniformity and bath stability with suspended nanoparticles remain technically challenging.
Automation and process control technologies have advanced significantly, with real-time monitoring systems for pH, temperature, and chemical constituent concentrations becoming increasingly sophisticated. These systems help maintain bath parameters within increasingly narrow operating windows required for high-performance applications in aerospace, electronics, and automotive industries.
Current ENP Formulations and Implementation Methodologies
01 Composition of electroless nickel plating baths
Electroless nickel plating baths typically contain nickel salts, reducing agents, complexing agents, stabilizers, and pH adjusters. The composition of these baths significantly affects the quality and properties of the resulting nickel coating. Different formulations can be used to achieve specific coating characteristics such as hardness, corrosion resistance, and uniformity. The balance of components in the bath is critical for controlling the deposition rate and preventing bath decomposition.- Composition of electroless nickel plating baths: Electroless nickel plating baths typically contain nickel salts, reducing agents, complexing agents, stabilizers, and pH adjusters. The composition of these baths significantly affects the quality and properties of the resulting nickel coating. Various formulations have been developed to optimize deposition rate, coating uniformity, and physical properties such as hardness and corrosion resistance.
- Process parameters and optimization techniques: The electroless nickel plating process is influenced by several parameters including temperature, pH, bath concentration, and agitation. Optimizing these parameters is crucial for achieving desired coating properties. Advanced techniques involve precise control of bath chemistry, temperature regulation systems, and monitoring methods to maintain bath stability over extended periods of use.
- Substrate preparation and pretreatment methods: Proper substrate preparation is essential for successful electroless nickel plating. This includes cleaning, degreasing, etching, and activation steps to ensure good adhesion of the nickel coating. Various pretreatment methods have been developed for different substrate materials including metals, plastics, and ceramics, each requiring specific chemical treatments to create an active surface for nickel deposition.
- Composite and alloy electroless nickel coatings: Advanced electroless nickel plating techniques include the co-deposition of particles or alloying elements to enhance specific properties. Nickel-phosphorus, nickel-boron, and composite coatings containing dispersed particles such as silicon carbide, PTFE, or diamond can be produced. These specialized coatings offer improved wear resistance, lubricity, hardness, or corrosion protection compared to standard electroless nickel deposits.
- Post-treatment and heat treatment processes: After electroless nickel plating, various post-treatment processes can be applied to enhance coating properties. Heat treatment at specific temperatures can significantly increase the hardness and wear resistance of nickel-phosphorus deposits through precipitation hardening. Other post-treatments include passivation for improved corrosion resistance, sealing treatments, and application of topcoats for specialized applications.
02 Surface preparation techniques for electroless nickel plating
Proper surface preparation is essential for successful electroless nickel plating. This includes cleaning, degreasing, etching, and activation of the substrate surface. These steps ensure good adhesion of the nickel coating to the substrate and uniform deposition. Different substrate materials may require specific preparation methods to achieve optimal results. The quality of surface preparation directly impacts the performance and durability of the final plated product.Expand Specific Solutions03 Process parameters and control methods
Control of process parameters such as temperature, pH, bath concentration, and agitation is crucial for consistent electroless nickel plating results. Monitoring and maintaining these parameters within specific ranges ensures uniform coating thickness and quality. Advanced control systems can be implemented to automate the process and improve reproducibility. Proper filtration and replenishment strategies help extend bath life and maintain plating performance over time.Expand Specific Solutions04 Specialized electroless nickel coatings with enhanced properties
Various additives and co-deposited materials can be incorporated into electroless nickel coatings to enhance specific properties. These include phosphorus, boron, and composite particles that can improve hardness, wear resistance, corrosion protection, and lubricity. Heat treatment of electroless nickel deposits can further modify their properties, such as increasing hardness through precipitation hardening. These specialized coatings can be tailored for specific industrial applications with demanding requirements.Expand Specific Solutions05 Environmental and efficiency improvements in electroless nickel plating
Recent developments in electroless nickel plating focus on environmental sustainability and process efficiency. This includes the development of lead-free formulations, reduced chemical consumption, improved bath stability, and waste reduction methods. Recovery and recycling of nickel from spent plating solutions help minimize environmental impact. Energy-efficient processes and equipment designs contribute to cost reduction and sustainability in industrial applications.Expand Specific Solutions
Leading Companies and Competitive Landscape in ENP Industry
The electroless nickel plating market is currently in a mature growth phase, characterized by established technologies and steady expansion driven by increasing applications in electronics, automotive, and industrial sectors. The global market size is estimated to exceed $2 billion, with projected annual growth of 5-7% through 2025. Leading players include Atotech Deutschland, MacDermid Enthone, and MacDermid Inc., who have developed proprietary formulations offering enhanced corrosion resistance and uniformity. Technology maturity varies across applications, with companies like AT&S and Texas Instruments advancing specialized implementations for semiconductor and PCB applications. Asian manufacturers such as EEJA and Jiangmen REACH are rapidly gaining market share through cost-effective solutions, while research institutions like Korea Institute of Industrial Technology and Hunan University are pioneering next-generation environmentally friendly formulations with reduced phosphorus content.
Atotech Deutschland GmbH & Co. KG
Technical Solution: Atotech has developed advanced electroless nickel plating solutions with their Nichem® product line, which offers mid-phosphorus (5-9%) and high-phosphorus (>10%) options for various industrial applications. Their technology incorporates proprietary stabilizer systems that extend bath life up to 10 metal turnovers while maintaining consistent deposition rates of 15-20 μm/hour. Atotech's electroless nickel processes feature enhanced corrosion protection with salt spray resistance exceeding 1,000 hours for 25μm coatings. Their systems include specialized pre-treatment chemicals that ensure optimal adhesion on difficult substrates like aluminum and stainless steel. Notably, their ENIG (Electroless Nickel Immersion Gold) process for electronics applications achieves thickness uniformity within ±10% across complex geometries, making it ideal for high-reliability electronic components. Atotech has also pioneered environmentally compliant formulations that reduce chemical consumption by approximately 30% compared to conventional processes.
Strengths: Industry-leading bath stability and longevity; excellent thickness distribution even on complex parts; comprehensive process solutions from pre-treatment to final coating. Weaknesses: Higher initial investment costs compared to basic systems; requires more sophisticated process control equipment; some formulations may have more stringent waste treatment requirements.
MacDermid, Inc.
Technical Solution: MacDermid has developed the Enthone ENPLATE® system for electroless nickel plating, featuring proprietary stabilizer technology that enables exceptional deposit uniformity across complex geometries. Their mid-phosphorus (6-9%) formulations achieve hardness values of 550-650 HV as-deposited, with post-heat treatment values reaching 950-1000 HV. MacDermid's electroless nickel solutions incorporate advanced wetting agents that facilitate deposition in deep recesses and blind holes with aspect ratios exceeding 10:1. Their process chemistry maintains consistent deposition rates of 12-18 μm/hour throughout the bath life, with minimal rate decay (less than 15%) even after 8 metal turnovers. The company has also developed specialized low-stress formulations that deposit coatings with internal stress below 10 MPa, critical for precision components. MacDermid's systems feature automated replenishment technology that monitors and adjusts bath chemistry in real-time, maintaining optimal plating conditions and extending bath life by up to 40% compared to manually controlled processes.
Strengths: Exceptional deposit uniformity on complex parts; advanced bath monitoring and control systems; specialized formulations for demanding applications like electronics and aerospace. Weaknesses: Higher operational costs compared to basic systems; requires more technical expertise to maintain optimal performance; some formulations have narrower operating windows requiring precise control.
Key Patents and Technical Innovations in Electroless Nickel
Electroless nickel plating solutions
PatentInactiveEP1378584A1
Innovation
- The use of nickel salts of alkyl sulfonic acids as the source of nickel ions in electroless nickel plating solutions, combined with hypophosphorous acid or its soluble salts, without added nickel hypophosphite or alkali/alkaline earth metal ions, to maintain solution stability and prevent orthophosphite precipitation.
Environmental Impact and Sustainability Considerations
Electroless nickel plating processes, while offering significant technical advantages, present notable environmental challenges that demand careful consideration in modern industrial applications. The traditional formulations contain several hazardous chemicals, including nickel compounds which are classified as carcinogens, and reducing agents such as sodium hypophosphite that contribute to wastewater contamination. These plating baths typically operate at pH levels between 4.5-5.5, requiring substantial neutralization before discharge to meet environmental regulations.
Water consumption represents another critical environmental concern, with conventional processes requiring 15-20 liters of water per square meter of plated surface for rinsing operations. This high water usage translates to approximately 2,000-3,000 liters daily for a medium-sized plating facility, placing significant pressure on local water resources. Additionally, the energy requirements for maintaining bath temperatures at 85-95°C contribute substantially to the carbon footprint of these operations.
Recent sustainability initiatives have focused on developing more environmentally compatible alternatives. Low-temperature electroless nickel plating processes operating at 60-70°C have emerged, reducing energy consumption by approximately 30% compared to conventional methods. Similarly, recovery systems for nickel from spent baths have achieved recycling rates of up to 85%, significantly reducing waste generation and raw material requirements.
The industry has also witnessed the development of nickel-free alternatives based on copper, tin, or cobalt alloys for applications where nickel's specific properties are not critical. These alternatives have demonstrated a 40-60% reduction in environmental impact according to life cycle assessment studies, though they typically offer reduced corrosion resistance compared to traditional nickel coatings.
Regulatory frameworks worldwide continue to evolve, with the European Union's REACH regulations and similar initiatives in North America imposing increasingly stringent requirements on nickel plating operations. Companies implementing closed-loop systems for bath maintenance have reported 70-80% reductions in wastewater discharge volumes and associated treatment costs, demonstrating the economic viability of environmentally responsible approaches.
Future sustainability directions include the development of biodegradable complexing agents to replace conventional chelators, and advanced filtration technologies capable of removing contaminants at the molecular level. Research into catalytic recovery systems shows promise for reducing chemical consumption by up to 50% while extending bath life by 2-3 times compared to current practices.
Water consumption represents another critical environmental concern, with conventional processes requiring 15-20 liters of water per square meter of plated surface for rinsing operations. This high water usage translates to approximately 2,000-3,000 liters daily for a medium-sized plating facility, placing significant pressure on local water resources. Additionally, the energy requirements for maintaining bath temperatures at 85-95°C contribute substantially to the carbon footprint of these operations.
Recent sustainability initiatives have focused on developing more environmentally compatible alternatives. Low-temperature electroless nickel plating processes operating at 60-70°C have emerged, reducing energy consumption by approximately 30% compared to conventional methods. Similarly, recovery systems for nickel from spent baths have achieved recycling rates of up to 85%, significantly reducing waste generation and raw material requirements.
The industry has also witnessed the development of nickel-free alternatives based on copper, tin, or cobalt alloys for applications where nickel's specific properties are not critical. These alternatives have demonstrated a 40-60% reduction in environmental impact according to life cycle assessment studies, though they typically offer reduced corrosion resistance compared to traditional nickel coatings.
Regulatory frameworks worldwide continue to evolve, with the European Union's REACH regulations and similar initiatives in North America imposing increasingly stringent requirements on nickel plating operations. Companies implementing closed-loop systems for bath maintenance have reported 70-80% reductions in wastewater discharge volumes and associated treatment costs, demonstrating the economic viability of environmentally responsible approaches.
Future sustainability directions include the development of biodegradable complexing agents to replace conventional chelators, and advanced filtration technologies capable of removing contaminants at the molecular level. Research into catalytic recovery systems shows promise for reducing chemical consumption by up to 50% while extending bath life by 2-3 times compared to current practices.
Quality Control and Testing Standards for ENP Coatings
Quality control and testing standards play a crucial role in ensuring the reliability and performance of Electroless Nickel Plating (ENP) coatings across various industrial applications. The industry has developed comprehensive testing protocols that evaluate multiple aspects of coating quality, with standards primarily governed by organizations such as ASTM, ISO, and military specifications.
Thickness measurement represents one of the most fundamental quality parameters for ENP coatings, typically assessed through methods including X-ray fluorescence, beta backscatter, magnetic induction, and microscopic cross-sectional analysis. ASTM B733 and ISO 4527 provide detailed guidelines for thickness measurement procedures, with tolerances generally ranging from ±10% to ±15% depending on application requirements.
Adhesion testing constitutes another critical quality parameter, commonly evaluated through bend tests, thermal shock tests, and tape tests as outlined in ASTM B571. For high-performance applications, more rigorous methods such as the pull-off adhesion test (ASTM D4541) may be employed to quantify adhesion strength in megapascals.
Corrosion resistance testing follows standardized procedures including neutral salt spray (ASTM B117), corrodkote (ASTM B380), and electrochemical impedance spectroscopy. The expected performance criteria vary by phosphorus content, with high-phosphorus ENP coatings typically required to withstand 500-1000 hours of salt spray exposure without significant base metal corrosion.
Hardness testing for ENP coatings presents unique challenges due to the thin nature of deposits. Microhardness testing using Vickers or Knoop methods (ASTM E384) with light loads (25-100g) has become the industry standard. As-deposited ENP typically exhibits 500-600 HV, while heat-treated coatings can reach 900-1100 HV.
Porosity assessment utilizes techniques such as ferroxyl testing for steel substrates (ASTM B733) and salt droplet testing, with acceptance criteria generally requiring less than 5 pores per square centimeter for critical applications. Modern quality systems increasingly incorporate statistical process control (SPC) methodologies to monitor bath parameters including pH, temperature, nickel concentration, and reducing agent levels.
Recent developments in quality control include the adoption of non-destructive evaluation techniques such as eddy current testing and ultrasonic methods for detecting coating defects without damaging components. Additionally, environmental compliance testing has gained prominence, with standards like RoHS and REACH imposing strict requirements on hazardous substance content in ENP processes and finished coatings.
Thickness measurement represents one of the most fundamental quality parameters for ENP coatings, typically assessed through methods including X-ray fluorescence, beta backscatter, magnetic induction, and microscopic cross-sectional analysis. ASTM B733 and ISO 4527 provide detailed guidelines for thickness measurement procedures, with tolerances generally ranging from ±10% to ±15% depending on application requirements.
Adhesion testing constitutes another critical quality parameter, commonly evaluated through bend tests, thermal shock tests, and tape tests as outlined in ASTM B571. For high-performance applications, more rigorous methods such as the pull-off adhesion test (ASTM D4541) may be employed to quantify adhesion strength in megapascals.
Corrosion resistance testing follows standardized procedures including neutral salt spray (ASTM B117), corrodkote (ASTM B380), and electrochemical impedance spectroscopy. The expected performance criteria vary by phosphorus content, with high-phosphorus ENP coatings typically required to withstand 500-1000 hours of salt spray exposure without significant base metal corrosion.
Hardness testing for ENP coatings presents unique challenges due to the thin nature of deposits. Microhardness testing using Vickers or Knoop methods (ASTM E384) with light loads (25-100g) has become the industry standard. As-deposited ENP typically exhibits 500-600 HV, while heat-treated coatings can reach 900-1100 HV.
Porosity assessment utilizes techniques such as ferroxyl testing for steel substrates (ASTM B733) and salt droplet testing, with acceptance criteria generally requiring less than 5 pores per square centimeter for critical applications. Modern quality systems increasingly incorporate statistical process control (SPC) methodologies to monitor bath parameters including pH, temperature, nickel concentration, and reducing agent levels.
Recent developments in quality control include the adoption of non-destructive evaluation techniques such as eddy current testing and ultrasonic methods for detecting coating defects without damaging components. Additionally, environmental compliance testing has gained prominence, with standards like RoHS and REACH imposing strict requirements on hazardous substance content in ENP processes and finished coatings.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!