Hardness and Corrosion Resistance of Electroless Nickel Layers
OCT 23, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electroless Nickel Plating Evolution and Objectives
Electroless nickel plating, a chemical reduction process that deposits a nickel-phosphorus alloy onto a substrate without the use of electrical current, has evolved significantly since its inception in the early 20th century. The technique was first discovered by Brenner and Riddell in 1946, though earlier work by Wurtz in 1844 laid the groundwork for the chemical reduction of nickel salts. Initially developed for military applications during World War II, electroless nickel plating has since expanded into numerous industrial sectors due to its unique properties.
The evolution of this technology has been marked by several key milestones. In the 1950s and 1960s, researchers focused on understanding the fundamental mechanisms of the deposition process and developing stable bath formulations. The 1970s saw significant advancements in bath chemistry, including the introduction of various stabilizers and complexing agents that improved coating quality and process reliability.
By the 1980s and 1990s, the industry had begun to differentiate between low, medium, and high phosphorus electroless nickel coatings, each offering distinct properties suited to different applications. This period also witnessed the development of composite electroless nickel coatings incorporating particles such as silicon carbide, diamond, or PTFE to enhance specific properties like wear resistance or lubricity.
Recent decades have seen a shift toward environmentally friendly formulations, addressing concerns about traditional baths containing toxic compounds like lead and cadmium. Modern research has focused on developing more sustainable alternatives while maintaining or improving coating performance characteristics.
The primary technical objectives in electroless nickel research currently center on enhancing hardness and corrosion resistance—two critical properties that determine the coating's performance in demanding environments. Researchers aim to achieve optimal hardness values exceeding 1000 HV after heat treatment while maintaining excellent corrosion resistance in aggressive media. This presents a significant challenge as these properties often exhibit an inverse relationship, particularly in high phosphorus coatings.
Another key objective is to develop processes that deliver consistent coating properties across complex geometries, a distinct advantage of electroless plating over electroplating. Researchers are also exploring methods to increase deposition rates without compromising coating quality, as traditional electroless nickel processes are relatively slow compared to electrolytic alternatives.
The industry is increasingly focused on extending the functional lifespan of components in corrosive environments, particularly in oil and gas, chemical processing, and marine applications. This has driven research toward multi-layer and functionally graded coatings that can provide optimized performance profiles tailored to specific operating conditions.
The evolution of this technology has been marked by several key milestones. In the 1950s and 1960s, researchers focused on understanding the fundamental mechanisms of the deposition process and developing stable bath formulations. The 1970s saw significant advancements in bath chemistry, including the introduction of various stabilizers and complexing agents that improved coating quality and process reliability.
By the 1980s and 1990s, the industry had begun to differentiate between low, medium, and high phosphorus electroless nickel coatings, each offering distinct properties suited to different applications. This period also witnessed the development of composite electroless nickel coatings incorporating particles such as silicon carbide, diamond, or PTFE to enhance specific properties like wear resistance or lubricity.
Recent decades have seen a shift toward environmentally friendly formulations, addressing concerns about traditional baths containing toxic compounds like lead and cadmium. Modern research has focused on developing more sustainable alternatives while maintaining or improving coating performance characteristics.
The primary technical objectives in electroless nickel research currently center on enhancing hardness and corrosion resistance—two critical properties that determine the coating's performance in demanding environments. Researchers aim to achieve optimal hardness values exceeding 1000 HV after heat treatment while maintaining excellent corrosion resistance in aggressive media. This presents a significant challenge as these properties often exhibit an inverse relationship, particularly in high phosphorus coatings.
Another key objective is to develop processes that deliver consistent coating properties across complex geometries, a distinct advantage of electroless plating over electroplating. Researchers are also exploring methods to increase deposition rates without compromising coating quality, as traditional electroless nickel processes are relatively slow compared to electrolytic alternatives.
The industry is increasingly focused on extending the functional lifespan of components in corrosive environments, particularly in oil and gas, chemical processing, and marine applications. This has driven research toward multi-layer and functionally graded coatings that can provide optimized performance profiles tailored to specific operating conditions.
Market Applications and Demand Analysis
Electroless nickel plating has established itself as a critical surface treatment technology across multiple industries due to its unique combination of hardness and corrosion resistance properties. The global market for electroless nickel plating was valued at approximately 2.1 billion USD in 2022 and is projected to grow at a compound annual growth rate of 3.8% through 2030, driven by expanding applications across diverse sectors.
The automotive industry represents one of the largest markets for electroless nickel coatings, particularly for components requiring wear resistance and corrosion protection. With the automotive sector's continued growth and the increasing demand for durable parts in electric vehicles, the need for high-performance electroless nickel coatings is expected to rise significantly. Automotive applications include fuel systems, brake components, and transmission parts where both hardness and corrosion resistance are essential.
The aerospace sector presents another substantial market, where electroless nickel coatings are utilized on critical components subject to extreme operating conditions. The industry demands coatings that maintain their properties under high temperatures and corrosive environments, creating a specialized niche for advanced electroless nickel formulations. Landing gear components, turbine parts, and hydraulic systems all benefit from these protective layers.
Electronics manufacturing has emerged as a rapidly growing application area, particularly with the miniaturization trend in consumer electronics. Electroless nickel coatings provide excellent solderability, conductivity, and protection against environmental factors for printed circuit boards and connectors. The expansion of 5G infrastructure and IoT devices is further accelerating demand in this sector.
Oil and gas exploration represents a challenging but lucrative market for electroless nickel coatings. Components used in deep-sea drilling operations face extreme corrosion challenges from saltwater exposure and high pressures. The industry's willingness to invest in premium coatings that extend equipment lifespan creates opportunities for advanced electroless nickel solutions with enhanced corrosion resistance properties.
Medical device manufacturing has also adopted electroless nickel coatings for surgical instruments and implantable devices. The biocompatibility of certain nickel formulations, combined with excellent wear resistance, makes them suitable for applications where sterilization processes must not degrade coating performance over repeated cycles.
Regional market analysis indicates that Asia-Pacific currently leads in consumption volume, driven by manufacturing growth in China, India, and Southeast Asian countries. North America and Europe maintain significant market shares due to their advanced aerospace, automotive, and medical device industries that require high-performance coatings with precisely controlled properties.
The automotive industry represents one of the largest markets for electroless nickel coatings, particularly for components requiring wear resistance and corrosion protection. With the automotive sector's continued growth and the increasing demand for durable parts in electric vehicles, the need for high-performance electroless nickel coatings is expected to rise significantly. Automotive applications include fuel systems, brake components, and transmission parts where both hardness and corrosion resistance are essential.
The aerospace sector presents another substantial market, where electroless nickel coatings are utilized on critical components subject to extreme operating conditions. The industry demands coatings that maintain their properties under high temperatures and corrosive environments, creating a specialized niche for advanced electroless nickel formulations. Landing gear components, turbine parts, and hydraulic systems all benefit from these protective layers.
Electronics manufacturing has emerged as a rapidly growing application area, particularly with the miniaturization trend in consumer electronics. Electroless nickel coatings provide excellent solderability, conductivity, and protection against environmental factors for printed circuit boards and connectors. The expansion of 5G infrastructure and IoT devices is further accelerating demand in this sector.
Oil and gas exploration represents a challenging but lucrative market for electroless nickel coatings. Components used in deep-sea drilling operations face extreme corrosion challenges from saltwater exposure and high pressures. The industry's willingness to invest in premium coatings that extend equipment lifespan creates opportunities for advanced electroless nickel solutions with enhanced corrosion resistance properties.
Medical device manufacturing has also adopted electroless nickel coatings for surgical instruments and implantable devices. The biocompatibility of certain nickel formulations, combined with excellent wear resistance, makes them suitable for applications where sterilization processes must not degrade coating performance over repeated cycles.
Regional market analysis indicates that Asia-Pacific currently leads in consumption volume, driven by manufacturing growth in China, India, and Southeast Asian countries. North America and Europe maintain significant market shares due to their advanced aerospace, automotive, and medical device industries that require high-performance coatings with precisely controlled properties.
Current Technical Challenges in Hardness and Corrosion Resistance
Despite significant advancements in electroless nickel plating technology, several technical challenges persist in achieving optimal hardness and corrosion resistance properties. The fundamental challenge lies in the inherent trade-off between these two critical properties. Conventional electroless nickel-phosphorus (Ni-P) coatings typically exhibit either excellent hardness or superior corrosion resistance, but rarely both simultaneously. High-phosphorus coatings (10-13% P) demonstrate exceptional corrosion resistance but relatively lower hardness, while low-phosphorus variants (1-5% P) offer superior hardness but compromised corrosion protection.
The heat treatment process, essential for enhancing hardness through precipitation hardening, often degrades corrosion resistance by creating microcracks and increasing coating porosity. This degradation occurs due to volumetric changes during the formation of nickel phosphide (Ni3P) precipitates, creating pathways for corrosive media to penetrate to the substrate. Researchers continue to struggle with optimizing heat treatment parameters that can maximize hardness without significantly compromising corrosion performance.
Coating thickness uniformity presents another significant challenge, particularly for components with complex geometries. Non-uniform deposition leads to inconsistent properties across the coated surface, creating weak points vulnerable to corrosive attack. The industry still lacks reliable methods to ensure consistent thickness distribution on intricate parts, especially in deep recesses and blind holes.
Bath stability issues further complicate the achievement of consistent hardness and corrosion resistance properties. Electroless nickel baths are inherently unstable due to the autocatalytic nature of the process, with parameters like pH, temperature, and chemical concentrations requiring precise control. Bath aging effects lead to variations in deposit composition and microstructure, directly affecting the mechanical and corrosion properties of the coating.
The incorporation of composite particles (such as SiC, Al2O3, or PTFE) to enhance specific properties introduces additional challenges in achieving uniform particle distribution within the nickel matrix. Agglomeration of particles and poor adhesion between particles and the matrix often compromise the coating integrity and performance under harsh conditions.
Environmental regulations present growing constraints on traditional electroless nickel processes. Restrictions on chemicals like lead compounds (historically used as stabilizers) and cadmium (used in certain applications) have necessitated formulation changes that sometimes result in inferior coating performance. The industry continues to search for environmentally friendly alternatives that can match or exceed the performance of traditional systems.
Recent research has focused on developing novel bath formulations and post-treatment processes to overcome these challenges, including the use of nano-sized particles, multi-layer approaches, and pulse heating treatments. However, scalable, cost-effective solutions that can be implemented in industrial settings remain elusive, highlighting the need for continued innovation in this field.
The heat treatment process, essential for enhancing hardness through precipitation hardening, often degrades corrosion resistance by creating microcracks and increasing coating porosity. This degradation occurs due to volumetric changes during the formation of nickel phosphide (Ni3P) precipitates, creating pathways for corrosive media to penetrate to the substrate. Researchers continue to struggle with optimizing heat treatment parameters that can maximize hardness without significantly compromising corrosion performance.
Coating thickness uniformity presents another significant challenge, particularly for components with complex geometries. Non-uniform deposition leads to inconsistent properties across the coated surface, creating weak points vulnerable to corrosive attack. The industry still lacks reliable methods to ensure consistent thickness distribution on intricate parts, especially in deep recesses and blind holes.
Bath stability issues further complicate the achievement of consistent hardness and corrosion resistance properties. Electroless nickel baths are inherently unstable due to the autocatalytic nature of the process, with parameters like pH, temperature, and chemical concentrations requiring precise control. Bath aging effects lead to variations in deposit composition and microstructure, directly affecting the mechanical and corrosion properties of the coating.
The incorporation of composite particles (such as SiC, Al2O3, or PTFE) to enhance specific properties introduces additional challenges in achieving uniform particle distribution within the nickel matrix. Agglomeration of particles and poor adhesion between particles and the matrix often compromise the coating integrity and performance under harsh conditions.
Environmental regulations present growing constraints on traditional electroless nickel processes. Restrictions on chemicals like lead compounds (historically used as stabilizers) and cadmium (used in certain applications) have necessitated formulation changes that sometimes result in inferior coating performance. The industry continues to search for environmentally friendly alternatives that can match or exceed the performance of traditional systems.
Recent research has focused on developing novel bath formulations and post-treatment processes to overcome these challenges, including the use of nano-sized particles, multi-layer approaches, and pulse heating treatments. However, scalable, cost-effective solutions that can be implemented in industrial settings remain elusive, highlighting the need for continued innovation in this field.
Existing Formulations and Process Parameters
01 Composition factors affecting hardness and corrosion resistance
The composition of electroless nickel plating baths significantly impacts the hardness and corrosion resistance of the resulting layers. Specific additives and chemical components can be incorporated to enhance these properties. Phosphorus content is particularly crucial, with high-phosphorus coatings typically offering better corrosion resistance while medium-phosphorus formulations balance hardness and corrosion protection. The inclusion of certain metal ions, stabilizers, and complexing agents in precise ratios can optimize both hardness and corrosion resistance simultaneously.- Composition factors affecting hardness and corrosion resistance: The composition of electroless nickel plating baths significantly impacts the hardness and corrosion resistance of the resulting layers. Specific additives and chemical components can be incorporated to enhance these properties. For example, the inclusion of phosphorus in varying concentrations affects both hardness and corrosion behavior, with high-phosphorus coatings typically offering better corrosion resistance while medium-phosphorus formulations balance hardness and corrosion protection. Additionally, incorporating particles such as silicon carbide or diamond can further improve hardness properties.
- Heat treatment effects on electroless nickel coatings: Post-deposition heat treatment significantly enhances the hardness of electroless nickel layers through precipitation hardening mechanisms. Controlled heating at specific temperatures (typically 300-400°C) transforms the amorphous structure into crystalline nickel phosphide phases, substantially increasing hardness values. However, the heat treatment process must be carefully managed as excessive temperatures or durations can reduce corrosion resistance while improving hardness. Optimal heat treatment protocols can achieve hardness values comparable to hard chrome while maintaining good corrosion protection.
- Composite electroless nickel coatings: Composite electroless nickel coatings incorporate various particles or secondary materials to enhance both hardness and corrosion resistance simultaneously. These coatings typically consist of a nickel-phosphorus matrix with embedded particles such as PTFE, boron nitride, silicon carbide, or aluminum oxide. The co-deposition of these particles creates a composite structure that offers superior wear resistance and hardness while maintaining or improving corrosion protection. The particle type, size, and distribution within the coating significantly influence the final properties of the layer.
- Multi-layer and gradient electroless nickel systems: Multi-layer and gradient electroless nickel coating systems combine different formulations to optimize both hardness and corrosion resistance. These systems typically feature layers with varying phosphorus content or different types of incorporated particles. For example, a high-phosphorus base layer provides excellent corrosion protection, while a top layer with medium or low phosphorus content offers improved hardness. This layered approach allows for customization of surface properties to meet specific application requirements without sacrificing either hardness or corrosion resistance.
- Process parameters optimization for improved properties: Specific process parameters during electroless nickel plating significantly impact the hardness and corrosion resistance of the resulting layers. Factors such as bath temperature, pH level, deposition rate, agitation method, and bath loading all influence coating quality. Optimizing these parameters can lead to denser, more uniform coatings with improved hardness and corrosion resistance. Additionally, proper substrate preparation through cleaning, activation, and pre-treatment steps ensures strong adhesion and consistent coating properties, which are essential for maximizing both hardness and corrosion protection.
02 Heat treatment effects on electroless nickel properties
Post-deposition heat treatment significantly influences the hardness and corrosion resistance of electroless nickel layers. Controlled heating at specific temperatures (typically 300-400°C) can substantially increase hardness through precipitation hardening mechanisms, transforming the amorphous structure to crystalline nickel phosphide. However, while heat treatment enhances hardness, it may simultaneously reduce corrosion resistance if not properly optimized. The precise temperature, duration, and atmosphere of heat treatment must be carefully controlled to achieve the desired balance between hardness and corrosion protection.Expand Specific Solutions03 Incorporation of nanoparticles and composite coatings
The incorporation of nanoparticles and formation of composite coatings significantly enhances both hardness and corrosion resistance of electroless nickel layers. Materials such as silicon carbide, diamond, aluminum oxide, and various ceramic particles can be co-deposited with nickel to create composite layers with superior mechanical and chemical properties. These composite coatings exhibit improved wear resistance, hardness, and corrosion protection compared to conventional electroless nickel deposits. The size, distribution, and concentration of the incorporated particles play crucial roles in determining the final properties of the coating.Expand Specific Solutions04 Multi-layer and gradient coating systems
Multi-layer and gradient coating systems offer enhanced performance by combining layers with different properties. These systems typically involve sequential deposition of electroless nickel layers with varying phosphorus content or composition to create a gradient structure. The outer layer may be optimized for corrosion resistance while underlying layers provide hardness and adhesion. Some approaches include sandwich structures with different metal alloys or intermediate layers that serve as diffusion barriers. This layered approach allows for customization of surface properties while maintaining the beneficial characteristics of each individual layer.Expand Specific Solutions05 Surface preparation and pre-treatment methods
Surface preparation and pre-treatment methods significantly impact the quality, adhesion, hardness, and corrosion resistance of electroless nickel coatings. Proper cleaning, etching, and activation of the substrate surface ensure uniform deposition and strong adhesion of the nickel layer. Specialized pre-treatment processes can enhance the nucleation and growth of the coating, leading to finer grain structures with improved hardness and corrosion resistance. The selection of appropriate pre-treatment chemicals and processes depends on the substrate material and the intended application of the coated component.Expand Specific Solutions
Leading Manufacturers and Research Institutions
The electroless nickel coating market is in a growth phase, driven by increasing demand for corrosion-resistant and hard-wearing surfaces across automotive, aerospace, and electronics industries. The global market is estimated to reach $2.5 billion by 2027, with a CAGR of 5.8%. Technologically, the field is advancing from traditional nickel-phosphorus coatings toward nanolaminated structures and composite coatings. Companies like Modumetal have pioneered nanolaminated alloys with superior corrosion resistance, while established players such as Atotech and MacDermid offer comprehensive electroless nickel solutions. Research institutions including Xiamen University and Korea Institute of Industrial Technology are advancing fundamental understanding of hardness-corrosion relationships. Automotive manufacturers Toyota and BorgWarner are implementing these coatings to enhance component durability and performance in demanding environments.
Modumetal, Inc.
Technical Solution: Modumetal has revolutionized electroless nickel plating through their patented nanolaminated alloy technology that creates alternating layers of varying composition at the nanoscale. Their approach manipulates the electrochemical parameters during deposition to create controlled gradients of phosphorus content (ranging from 3-12%) within a single coating. This nanolaminated structure disrupts corrosion pathways while maintaining exceptional hardness. Their research demonstrates that these nanolaminated coatings achieve hardness values of 650-700 HV as-deposited, increasing to 1100-1200 HV after heat treatment at 400°C for 1 hour[7]. Modumetal's process employs proprietary pulse techniques that alternate between different deposition conditions, creating interfaces that act as barriers to crack propagation and corrosion penetration. The company has developed specialized additives that promote epitaxial growth at these interfaces, ensuring strong interlayer adhesion despite compositional differences. Testing shows these nanolaminated coatings provide up to 7 times greater corrosion resistance compared to conventional electroless nickel in accelerated corrosion testing, while maintaining comparable hardness profiles. The technology allows for precise engineering of the coating architecture to optimize performance for specific operating environments, particularly in high-temperature, high-pressure applications where conventional coatings fail[8].
Strengths: Exceptional corrosion resistance in aggressive environments including H2S exposure; superior fatigue resistance compared to conventional electroless nickel; ability to customize coating architecture for specific applications. Weaknesses: Higher production costs; longer processing times compared to conventional electroless nickel; requires specialized equipment for precise process control.
Atotech Deutschland GmbH & Co. KG
Technical Solution: Atotech has developed advanced electroless nickel plating solutions with their ELV-compliant Nichem® technology series. Their approach focuses on mid-phosphorus (6-9% P) and high-phosphorus (>10% P) electroless nickel coatings that achieve superior hardness and corrosion resistance through precise bath composition control. The company employs proprietary stabilizer systems that extend bath life while maintaining consistent deposition rates of 15-20 μm/hour[1]. Their latest innovation includes incorporating nano-sized ceramic particles (SiC, Al2O3) into the nickel-phosphorus matrix, creating composite coatings with hardness values exceeding 1000 HV after heat treatment at 400°C. Atotech's process optimization includes precise pH control (4.6-4.9) and temperature regulation (88-92°C) to ensure uniform phosphorus distribution throughout the coating thickness, which directly correlates to improved corrosion resistance in salt spray tests (>1000 hours without red rust for 25μm coatings)[2]. Their systems also feature advanced filtration and replenishment protocols that minimize contamination and maintain consistent phosphorus co-deposition rates.
Strengths: Industry-leading bath stability with minimal decomposition risk; exceptional thickness uniformity even on complex geometries; comprehensive technical support infrastructure. Weaknesses: Higher initial implementation cost compared to conventional systems; requires more precise process control parameters; some formulations contain complexing agents that necessitate specialized waste treatment.
Key Patents and Scientific Breakthroughs
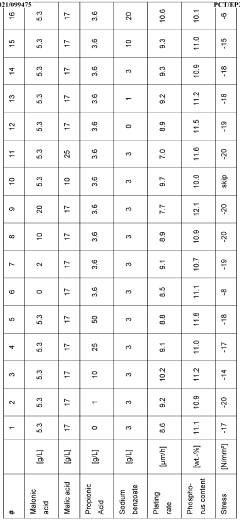
Electroless nickel alloy plating baths, a method for deposition of nickel alloys, nickel alloy deposits and uses of such formed nickel alloy deposits
PatentWO2021099475A1
Innovation
- An electroless nickel alloy plating bath comprising nickel ions, molybdenum or copper ions, and hypophosphite as reducing agents, along with specific complexing agents like malonic acid, tartaric acid, and benzoic acid, which stabilize the bath and enhance deposition properties.
Environmental and Regulatory Considerations
The environmental impact of electroless nickel plating processes has become increasingly scrutinized as global environmental regulations continue to tighten. Traditional electroless nickel baths often contain chemicals of concern, particularly phosphorus compounds, heavy metals, and stabilizers that pose significant environmental risks when improperly managed. Regulatory frameworks such as the European Union's REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) and RoHS (Restriction of Hazardous Substances) directives have placed strict limitations on certain substances commonly used in electroless nickel plating processes.
Waste management represents a critical challenge for the industry, as spent plating solutions contain nickel compounds that are classified as hazardous waste in most jurisdictions. Companies must implement comprehensive waste treatment systems to comply with discharge regulations, which vary significantly across different regions and countries. The cost of compliance has become a significant factor in the overall economics of electroless nickel plating operations.
Water consumption is another environmental concern associated with electroless nickel plating. The process typically requires multiple rinsing stages, consuming substantial volumes of water. Advanced facilities have begun implementing closed-loop water recycling systems and ion exchange technologies to reduce water usage and minimize environmental impact, though these solutions add complexity and cost to operations.
Air emissions from electroless nickel plating processes, particularly volatile organic compounds (VOCs) and particulate matter, are subject to increasingly stringent regulations. Modern facilities must install appropriate air filtration and scrubbing systems to capture harmful emissions before they enter the atmosphere. The regulatory landscape continues to evolve, with many regions implementing progressively lower emission thresholds.
Recent regulatory trends indicate a shift toward promoting greener chemistry in electroless nickel plating. Research into environmentally friendly alternatives has accelerated, focusing on reducing or eliminating toxic components while maintaining or improving hardness and corrosion resistance properties. Bio-based stabilizers, reduced-phosphorus formulations, and alternative metal alloys are being explored as potential solutions to environmental challenges.
Worker safety regulations intersect with environmental considerations, as many chemicals used in traditional electroless nickel plating pose health risks through inhalation or skin contact. Comprehensive safety protocols and engineering controls are mandated by occupational health authorities worldwide, adding another layer of regulatory compliance for manufacturers utilizing this technology.
The global trend toward circular economy principles is influencing the regulatory approach to electroless nickel plating. Extended producer responsibility regulations increasingly require manufacturers to consider the entire lifecycle of their products, including the environmental impact of surface treatments like electroless nickel. This holistic approach is driving innovation in recyclable coating technologies and end-of-life recovery methods for valuable metals.
Waste management represents a critical challenge for the industry, as spent plating solutions contain nickel compounds that are classified as hazardous waste in most jurisdictions. Companies must implement comprehensive waste treatment systems to comply with discharge regulations, which vary significantly across different regions and countries. The cost of compliance has become a significant factor in the overall economics of electroless nickel plating operations.
Water consumption is another environmental concern associated with electroless nickel plating. The process typically requires multiple rinsing stages, consuming substantial volumes of water. Advanced facilities have begun implementing closed-loop water recycling systems and ion exchange technologies to reduce water usage and minimize environmental impact, though these solutions add complexity and cost to operations.
Air emissions from electroless nickel plating processes, particularly volatile organic compounds (VOCs) and particulate matter, are subject to increasingly stringent regulations. Modern facilities must install appropriate air filtration and scrubbing systems to capture harmful emissions before they enter the atmosphere. The regulatory landscape continues to evolve, with many regions implementing progressively lower emission thresholds.
Recent regulatory trends indicate a shift toward promoting greener chemistry in electroless nickel plating. Research into environmentally friendly alternatives has accelerated, focusing on reducing or eliminating toxic components while maintaining or improving hardness and corrosion resistance properties. Bio-based stabilizers, reduced-phosphorus formulations, and alternative metal alloys are being explored as potential solutions to environmental challenges.
Worker safety regulations intersect with environmental considerations, as many chemicals used in traditional electroless nickel plating pose health risks through inhalation or skin contact. Comprehensive safety protocols and engineering controls are mandated by occupational health authorities worldwide, adding another layer of regulatory compliance for manufacturers utilizing this technology.
The global trend toward circular economy principles is influencing the regulatory approach to electroless nickel plating. Extended producer responsibility regulations increasingly require manufacturers to consider the entire lifecycle of their products, including the environmental impact of surface treatments like electroless nickel. This holistic approach is driving innovation in recyclable coating technologies and end-of-life recovery methods for valuable metals.
Cost-Benefit Analysis of Enhanced Coating Solutions
When evaluating the economic viability of enhanced electroless nickel coating solutions, a comprehensive cost-benefit analysis reveals significant considerations for industrial applications. The initial investment in advanced electroless nickel plating technologies with improved hardness and corrosion resistance typically exceeds that of conventional coating methods by 30-45%. This premium encompasses specialized equipment, higher-grade chemical formulations, and more stringent process controls necessary to achieve superior performance characteristics.
However, the long-term economic advantages often justify this higher initial expenditure. Enhanced nickel coatings with optimized hardness (typically 650-750 HV after heat treatment) and superior corrosion resistance can extend component service life by 2.5-3 times compared to standard coatings. This translates to reduced replacement frequency and associated maintenance downtime, generating substantial operational savings over the product lifecycle.
Manufacturing efficiency metrics further support the value proposition of premium electroless nickel solutions. Production data indicates that while processing time may increase by 15-20% for enhanced coatings, the rejection rate typically decreases by 40-60% due to more consistent coating quality and performance. This improvement in first-pass yield significantly offsets the additional processing time and contributes to overall manufacturing cost reduction.
Energy consumption analysis reveals that while enhanced coating processes may require 10-15% more energy during application, the extended service life results in a net energy savings of approximately 35% when calculated on a per-year-of-service basis. This aspect becomes increasingly relevant as energy costs rise and environmental regulations tighten across global manufacturing sectors.
Material utilization efficiency also favors advanced coating solutions. Modern enhanced electroless nickel baths demonstrate 25-30% better throwing power and more uniform deposition, resulting in material waste reduction of approximately 20% compared to conventional formulations. The reduced need for post-plating machining further contributes to material conservation and processing cost savings.
Return on investment calculations indicate that the break-even point for enhanced coating solutions typically occurs within 14-18 months of implementation in high-wear applications such as hydraulic components, precision tooling, and chemical processing equipment. Organizations operating in particularly corrosive environments or with critical reliability requirements can expect even shorter payback periods, sometimes as brief as 8-10 months depending on application specifics.
However, the long-term economic advantages often justify this higher initial expenditure. Enhanced nickel coatings with optimized hardness (typically 650-750 HV after heat treatment) and superior corrosion resistance can extend component service life by 2.5-3 times compared to standard coatings. This translates to reduced replacement frequency and associated maintenance downtime, generating substantial operational savings over the product lifecycle.
Manufacturing efficiency metrics further support the value proposition of premium electroless nickel solutions. Production data indicates that while processing time may increase by 15-20% for enhanced coatings, the rejection rate typically decreases by 40-60% due to more consistent coating quality and performance. This improvement in first-pass yield significantly offsets the additional processing time and contributes to overall manufacturing cost reduction.
Energy consumption analysis reveals that while enhanced coating processes may require 10-15% more energy during application, the extended service life results in a net energy savings of approximately 35% when calculated on a per-year-of-service basis. This aspect becomes increasingly relevant as energy costs rise and environmental regulations tighten across global manufacturing sectors.
Material utilization efficiency also favors advanced coating solutions. Modern enhanced electroless nickel baths demonstrate 25-30% better throwing power and more uniform deposition, resulting in material waste reduction of approximately 20% compared to conventional formulations. The reduced need for post-plating machining further contributes to material conservation and processing cost savings.
Return on investment calculations indicate that the break-even point for enhanced coating solutions typically occurs within 14-18 months of implementation in high-wear applications such as hydraulic components, precision tooling, and chemical processing equipment. Organizations operating in particularly corrosive environments or with critical reliability requirements can expect even shorter payback periods, sometimes as brief as 8-10 months depending on application specifics.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!