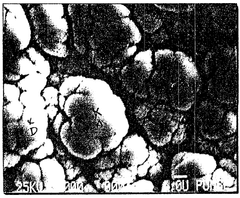
Wear Corrosion Synergy in Electroless Nickel Coatings
OCT 23, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electroless Nickel Coating Technology Evolution and Objectives
Electroless nickel (EN) coating technology has evolved significantly since its inception in the mid-20th century. Initially developed as an alternative to electrolytic nickel plating, the process gained prominence due to its ability to deposit uniform coatings on complex geometries without requiring electrical current. The early formulations primarily focused on achieving basic corrosion protection, with limited consideration for wear resistance properties.
The 1960s marked a pivotal shift when researchers discovered that incorporating phosphorus into nickel deposits significantly enhanced their corrosion resistance. This led to the development of various EN formulations with phosphorus content ranging from low (1-5%), medium (5-9%), to high (>9%), each offering distinct performance characteristics. By the 1970s, the technology expanded to include composite coatings incorporating hard particles such as silicon carbide and diamond to improve wear resistance.
The 1980s and 1990s witnessed significant advancements in understanding the synergistic relationship between wear and corrosion mechanisms in EN coatings. Researchers began to recognize that these phenomena were not isolated but rather interacted in complex ways, particularly in aggressive environments. This period saw the emergence of more sophisticated testing methodologies designed to evaluate this synergistic behavior.
Recent technological developments have focused on nano-composite EN coatings, incorporating nanoscale particles to achieve superior wear-corrosion resistance while maintaining or improving other coating properties. Advanced characterization techniques such as scanning electron microscopy (SEM), transmission electron microscopy (TEM), and atomic force microscopy (AFM) have enabled deeper insights into the microstructural features that influence wear-corrosion synergy.
The primary objectives in this field now center on developing EN coating systems that can withstand increasingly demanding operational environments where both mechanical wear and chemical/electrochemical corrosion occur simultaneously. This includes optimizing coating composition, structure, and post-treatment processes to enhance the synergistic wear-corrosion resistance while maintaining other critical properties such as ductility and adhesion.
Current research aims to establish comprehensive models that can predict the wear-corrosion behavior of EN coatings under various service conditions, enabling more targeted development of application-specific solutions. Additionally, there is growing interest in environmentally friendly EN formulations that reduce or eliminate hazardous chemicals while maintaining or improving performance characteristics, aligning with global sustainability initiatives and regulatory requirements.
The evolution of EN coating technology continues to be driven by increasing demands from industries such as aerospace, automotive, oil and gas, and electronics, where components face ever more challenging operating conditions requiring exceptional wear-corrosion resistance to ensure reliability and longevity.
The 1960s marked a pivotal shift when researchers discovered that incorporating phosphorus into nickel deposits significantly enhanced their corrosion resistance. This led to the development of various EN formulations with phosphorus content ranging from low (1-5%), medium (5-9%), to high (>9%), each offering distinct performance characteristics. By the 1970s, the technology expanded to include composite coatings incorporating hard particles such as silicon carbide and diamond to improve wear resistance.
The 1980s and 1990s witnessed significant advancements in understanding the synergistic relationship between wear and corrosion mechanisms in EN coatings. Researchers began to recognize that these phenomena were not isolated but rather interacted in complex ways, particularly in aggressive environments. This period saw the emergence of more sophisticated testing methodologies designed to evaluate this synergistic behavior.
Recent technological developments have focused on nano-composite EN coatings, incorporating nanoscale particles to achieve superior wear-corrosion resistance while maintaining or improving other coating properties. Advanced characterization techniques such as scanning electron microscopy (SEM), transmission electron microscopy (TEM), and atomic force microscopy (AFM) have enabled deeper insights into the microstructural features that influence wear-corrosion synergy.
The primary objectives in this field now center on developing EN coating systems that can withstand increasingly demanding operational environments where both mechanical wear and chemical/electrochemical corrosion occur simultaneously. This includes optimizing coating composition, structure, and post-treatment processes to enhance the synergistic wear-corrosion resistance while maintaining other critical properties such as ductility and adhesion.
Current research aims to establish comprehensive models that can predict the wear-corrosion behavior of EN coatings under various service conditions, enabling more targeted development of application-specific solutions. Additionally, there is growing interest in environmentally friendly EN formulations that reduce or eliminate hazardous chemicals while maintaining or improving performance characteristics, aligning with global sustainability initiatives and regulatory requirements.
The evolution of EN coating technology continues to be driven by increasing demands from industries such as aerospace, automotive, oil and gas, and electronics, where components face ever more challenging operating conditions requiring exceptional wear-corrosion resistance to ensure reliability and longevity.
Market Applications and Demand Analysis for Wear-Resistant Coatings
The global market for wear-resistant coatings has experienced significant growth in recent years, driven by increasing demands across multiple industries for solutions that extend component life and reduce maintenance costs. Electroless nickel coatings, particularly those engineered to address wear-corrosion synergy effects, represent a high-value segment within this market due to their unique combination of properties.
The automotive industry remains one of the largest consumers of wear-resistant coatings, with applications in engine components, transmission systems, and fuel delivery systems. The transition toward electric vehicles has created new demands for coatings that can withstand different operational conditions while maintaining excellent wear and corrosion resistance properties. Market research indicates that wear-resistant coating applications in the automotive sector are projected to grow steadily as manufacturers seek to improve vehicle efficiency and durability.
The aerospace industry presents another significant market for advanced wear-resistant coatings, particularly those that can withstand extreme operating conditions. Components such as landing gear, turbine blades, and hydraulic systems require exceptional protection against combined wear-corrosion mechanisms. The demand in this sector is characterized by stringent performance requirements and regulatory compliance needs.
Oil and gas extraction operations continue to drive substantial demand for wear-resistant coatings capable of withstanding harsh environments. Downhole tools, valves, pumps, and offshore structures face severe wear-corrosion synergy challenges due to exposure to corrosive fluids, abrasive particles, and high mechanical stresses. The industry's push toward deeper wells and more challenging extraction environments has intensified the need for superior coating solutions.
The medical device industry represents an emerging market for specialized electroless nickel coatings. Surgical instruments, implantable devices, and diagnostic equipment require coatings that combine wear resistance with biocompatibility and corrosion resistance in biological environments. This sector demands coatings with exceptional purity and consistency.
Regional market analysis reveals that North America and Europe currently lead in adoption of advanced wear-resistant coating technologies, while Asia-Pacific markets show the highest growth rates. This growth is attributed to rapid industrialization, increasing manufacturing sophistication, and growing awareness of lifecycle cost benefits associated with high-performance coatings.
Customer demand patterns indicate a shift toward customized coating solutions optimized for specific operational environments rather than general-purpose applications. End-users increasingly seek coatings that address the specific wear-corrosion mechanisms relevant to their applications, driving innovation in coating formulations and application techniques.
The automotive industry remains one of the largest consumers of wear-resistant coatings, with applications in engine components, transmission systems, and fuel delivery systems. The transition toward electric vehicles has created new demands for coatings that can withstand different operational conditions while maintaining excellent wear and corrosion resistance properties. Market research indicates that wear-resistant coating applications in the automotive sector are projected to grow steadily as manufacturers seek to improve vehicle efficiency and durability.
The aerospace industry presents another significant market for advanced wear-resistant coatings, particularly those that can withstand extreme operating conditions. Components such as landing gear, turbine blades, and hydraulic systems require exceptional protection against combined wear-corrosion mechanisms. The demand in this sector is characterized by stringent performance requirements and regulatory compliance needs.
Oil and gas extraction operations continue to drive substantial demand for wear-resistant coatings capable of withstanding harsh environments. Downhole tools, valves, pumps, and offshore structures face severe wear-corrosion synergy challenges due to exposure to corrosive fluids, abrasive particles, and high mechanical stresses. The industry's push toward deeper wells and more challenging extraction environments has intensified the need for superior coating solutions.
The medical device industry represents an emerging market for specialized electroless nickel coatings. Surgical instruments, implantable devices, and diagnostic equipment require coatings that combine wear resistance with biocompatibility and corrosion resistance in biological environments. This sector demands coatings with exceptional purity and consistency.
Regional market analysis reveals that North America and Europe currently lead in adoption of advanced wear-resistant coating technologies, while Asia-Pacific markets show the highest growth rates. This growth is attributed to rapid industrialization, increasing manufacturing sophistication, and growing awareness of lifecycle cost benefits associated with high-performance coatings.
Customer demand patterns indicate a shift toward customized coating solutions optimized for specific operational environments rather than general-purpose applications. End-users increasingly seek coatings that address the specific wear-corrosion mechanisms relevant to their applications, driving innovation in coating formulations and application techniques.
Current Challenges in Electroless Nickel Coating Durability
Despite significant advancements in electroless nickel (EN) coating technology, several persistent challenges continue to impact the durability and performance of these coatings in demanding industrial applications. The synergistic effect of wear and corrosion represents one of the most complex and difficult-to-address issues in this field, as these two degradation mechanisms often interact in ways that amplify overall material deterioration.
A primary challenge lies in the microstructural stability of EN coatings under combined wear-corrosion conditions. The phosphorus content, which typically ranges from 2-14% in commercial EN coatings, significantly influences both wear resistance and corrosion protection. However, optimizing phosphorus content presents an inherent trade-off: high-phosphorus coatings (10-14% P) offer superior corrosion resistance but reduced hardness, while low-phosphorus variants (2-5% P) provide better wear performance but diminished corrosion protection.
Heat treatment processes, while effective at increasing coating hardness through precipitation hardening, often compromise corrosion resistance by creating phosphorus-depleted zones and increasing coating porosity. This contradiction creates significant difficulties when designing EN systems for environments where both mechanical wear and chemical attack occur simultaneously.
The incorporation of particles (PTFE, SiC, Al₂O₃) to create composite EN coatings has shown promise in enhancing wear resistance, but these particles can create discontinuities in the coating matrix that become preferential sites for localized corrosion. Achieving uniform particle distribution without compromising the coating's integrity remains technically challenging.
Coating thickness uniformity presents another significant hurdle. While thicker coatings generally provide longer service life, excessive thickness can lead to internal stresses, cracking, and delamination under mechanical loading. Conversely, thin coatings may not provide adequate protection against aggressive environments where material loss occurs through both chemical and mechanical mechanisms.
The testing and prediction of wear-corrosion synergy represents perhaps the most fundamental challenge. Standard testing protocols typically evaluate wear and corrosion separately, failing to capture their synergistic interactions. The development of reliable accelerated testing methods that accurately simulate real-world combined wear-corrosion conditions remains elusive, making performance prediction difficult.
Additionally, environmental and regulatory pressures are driving the industry away from traditional EN formulations containing lead, cadmium, and other toxic stabilizers. Finding environmentally acceptable alternatives that maintain or improve coating performance under wear-corrosion conditions adds another layer of complexity to an already challenging technical landscape.
A primary challenge lies in the microstructural stability of EN coatings under combined wear-corrosion conditions. The phosphorus content, which typically ranges from 2-14% in commercial EN coatings, significantly influences both wear resistance and corrosion protection. However, optimizing phosphorus content presents an inherent trade-off: high-phosphorus coatings (10-14% P) offer superior corrosion resistance but reduced hardness, while low-phosphorus variants (2-5% P) provide better wear performance but diminished corrosion protection.
Heat treatment processes, while effective at increasing coating hardness through precipitation hardening, often compromise corrosion resistance by creating phosphorus-depleted zones and increasing coating porosity. This contradiction creates significant difficulties when designing EN systems for environments where both mechanical wear and chemical attack occur simultaneously.
The incorporation of particles (PTFE, SiC, Al₂O₃) to create composite EN coatings has shown promise in enhancing wear resistance, but these particles can create discontinuities in the coating matrix that become preferential sites for localized corrosion. Achieving uniform particle distribution without compromising the coating's integrity remains technically challenging.
Coating thickness uniformity presents another significant hurdle. While thicker coatings generally provide longer service life, excessive thickness can lead to internal stresses, cracking, and delamination under mechanical loading. Conversely, thin coatings may not provide adequate protection against aggressive environments where material loss occurs through both chemical and mechanical mechanisms.
The testing and prediction of wear-corrosion synergy represents perhaps the most fundamental challenge. Standard testing protocols typically evaluate wear and corrosion separately, failing to capture their synergistic interactions. The development of reliable accelerated testing methods that accurately simulate real-world combined wear-corrosion conditions remains elusive, making performance prediction difficult.
Additionally, environmental and regulatory pressures are driving the industry away from traditional EN formulations containing lead, cadmium, and other toxic stabilizers. Finding environmentally acceptable alternatives that maintain or improve coating performance under wear-corrosion conditions adds another layer of complexity to an already challenging technical landscape.
Contemporary Solutions for Wear-Corrosion Resistance Enhancement
01 Composition of electroless nickel coatings for enhanced wear and corrosion resistance
Electroless nickel coatings can be formulated with specific compositions to simultaneously enhance both wear and corrosion resistance. These coatings typically incorporate phosphorus, boron, or other alloying elements in precise amounts to create a synergistic effect. The microstructure and properties of these coatings can be tailored by controlling the chemical bath composition, which affects the deposition rate and the resulting coating characteristics. These specialized compositions provide superior protection in harsh environments where both mechanical wear and chemical attack are concerns.- Composition of electroless nickel coatings for enhanced wear and corrosion resistance: Electroless nickel coatings can be formulated with specific compositions to simultaneously enhance both wear and corrosion resistance. These compositions typically include phosphorus, boron, or other alloying elements that create a synergistic effect. The phosphorus content in particular plays a crucial role in determining the balance between wear and corrosion properties, with medium phosphorus content (5-9%) often providing the optimal combination. Certain additives and co-deposited particles can further improve the coating's performance in harsh environments.
- Heat treatment effects on electroless nickel coating properties: Heat treatment significantly influences the wear and corrosion resistance of electroless nickel coatings through microstructural changes. Controlled heating processes can transform the amorphous structure to crystalline, increasing hardness and wear resistance while potentially affecting corrosion resistance. Optimal heat treatment temperatures typically range between 300-400°C to achieve the best balance of properties. The duration and atmosphere of heat treatment also play important roles in developing the desired microstructure that provides synergistic wear and corrosion protection.
- Composite electroless nickel coatings with particle incorporation: Incorporating hard particles such as silicon carbide, diamond, or ceramic materials into electroless nickel matrices creates composite coatings with superior wear resistance while maintaining good corrosion protection. These particles act as reinforcements that resist abrasion and erosion while the nickel matrix provides a corrosion barrier. The size, distribution, and volume fraction of incorporated particles significantly influence the synergistic performance. Proper dispersion techniques and bath stability control are essential for achieving uniform particle distribution throughout the coating.
- Multi-layer and gradient electroless nickel coating systems: Multi-layer and gradient coating systems can be designed to optimize both wear and corrosion resistance by combining layers with different properties. These systems typically feature a corrosion-resistant base layer with higher phosphorus content and a wear-resistant top layer with lower phosphorus content or particle reinforcement. The transitional layers or gradual composition changes help minimize internal stresses and improve adhesion between layers. This approach allows for customization of coating properties to meet specific application requirements where both wear and corrosion resistance are needed.
- Bath formulation and process parameters for optimized coating performance: The electroless nickel plating bath formulation and process parameters significantly impact the wear-corrosion synergy of the resulting coatings. Key factors include pH control, temperature stability, complexing agents, stabilizers, and brighteners. Optimized bath chemistry ensures uniform deposition and desired alloy composition. Process parameters such as deposition rate, agitation, and substrate preparation also influence coating quality. Advanced bath formulations may include specific additives that promote the co-deposition of beneficial elements or compounds that enhance both wear resistance and corrosion protection simultaneously.
02 Heat treatment effects on electroless nickel coating performance
Heat treatment processes significantly influence the wear and corrosion resistance properties of electroless nickel coatings. Post-deposition thermal treatments at specific temperatures and durations can transform the amorphous structure of as-deposited coatings into crystalline phases, enhancing hardness and wear resistance while maintaining good corrosion protection. The formation of nickel phosphides during heat treatment contributes to improved mechanical properties. Optimized heat treatment protocols can create a balance between maximum wear resistance and adequate corrosion protection, addressing the often contradictory requirements for these properties.Expand Specific Solutions03 Multi-layer and composite electroless nickel coating systems
Multi-layer and composite electroless nickel coating systems offer enhanced synergistic protection against both wear and corrosion. These systems typically consist of layers with varying phosphorus content or incorporate particles such as silicon carbide, diamond, or PTFE within the nickel matrix. The layered approach allows for customization where the outer layer provides wear resistance while underlying layers offer corrosion protection. Composite coatings with embedded particles provide improved tribological properties while maintaining the inherent corrosion resistance of the nickel-phosphorus matrix. These sophisticated coating architectures can be tailored for specific industrial applications requiring both types of protection.Expand Specific Solutions04 Surface preparation and pre-treatment methods for improved coating performance
Surface preparation and pre-treatment methods significantly impact the performance of electroless nickel coatings in terms of wear and corrosion resistance. Proper cleaning, etching, and activation of the substrate surface ensure strong adhesion and uniform coating deposition. Specialized pre-treatment processes can enhance the interface between the substrate and coating, preventing delamination under mechanical stress and eliminating potential corrosion initiation sites. Advanced techniques such as double activation or specific strike layers can further improve the synergistic protection against wear and corrosion by creating an optimal foundation for the electroless nickel coating.Expand Specific Solutions05 Novel additives and bath formulations for enhanced coating properties
Innovative additives and bath formulations have been developed to enhance the synergistic wear and corrosion resistance of electroless nickel coatings. These formulations may include stabilizers, brighteners, complexing agents, and other functional additives that influence the deposition process and resulting coating properties. Certain additives can promote finer grain structures or more uniform phosphorus distribution, leading to improved hardness without sacrificing corrosion protection. Advanced bath chemistry can also enable the co-deposition of beneficial elements or compounds that enhance both mechanical durability and chemical stability, resulting in coatings with superior performance in demanding applications.Expand Specific Solutions
Leading Manufacturers and Research Institutions in Surface Engineering
The wear corrosion synergy in electroless nickel coatings market is currently in a growth phase, with increasing applications across automotive, aerospace, and electronics industries. The global market size is estimated to reach $2.5 billion by 2025, driven by demand for corrosion-resistant materials in harsh environments. Technologically, the field shows moderate maturity with ongoing innovation. Leading players include United Technologies Corp. and BorgWarner developing advanced coating solutions for aerospace and automotive applications, while specialized companies like Atotech Deutschland and NOF Metal Coating Europe focus on high-performance surface treatments. Academic institutions such as Xi'an Jiaotong University and Swansea University are advancing fundamental research, collaborating with industry partners like Tata Steel and NSK Ltd. to bridge theoretical understanding with practical applications.
United Technologies Corp.
Technical Solution: United Technologies Corp. has developed an advanced electroless nickel coating system called Pratt & Whitney EcoShield™ specifically designed for aerospace components facing severe wear-corrosion synergy challenges. Their technology utilizes a proprietary nickel-phosphorus-boron formulation with carefully controlled composition (7-9% P, 0.5-1.5% B) that creates an optimal microstructure for combined wear and corrosion resistance. The company's process incorporates specialized pre-treatment protocols that enhance coating adhesion through nano-scale surface modification of the substrate. Their coating technology features a unique dual-layer structure with an inner layer optimized for adhesion and corrosion protection (high P content) and an outer layer engineered for wear resistance (lower P content with nano-diamond particles). UTC's proprietary heat treatment process (300-350°C in controlled atmosphere) transforms the coating microstructure to achieve hardness values exceeding 900 HV while maintaining excellent corrosion resistance in salt spray testing (>1000 hours to white corrosion).
Strengths: Exceptional performance in high-temperature aerospace environments where traditional coatings degrade; superior adhesion properties prevent coating delamination under extreme mechanical stress. Weaknesses: Complex multi-stage process increases production time and costs; requires specialized equipment and strict quality control protocols.
NOF Metal Coating Europe SA
Technical Solution: NOF Metal Coating Europe has developed advanced electroless nickel coating solutions with proprietary PTFE co-deposition technology. Their Nickelos® series incorporates nano-dispersed PTFE particles within the nickel-phosphorus matrix, creating a self-lubricating surface that significantly enhances wear resistance while maintaining excellent corrosion protection. The company's process control system ensures uniform particle distribution throughout the coating thickness (typically 5-25μm), achieving optimal synergistic effects between wear and corrosion resistance. Their proprietary post-treatment processes include specialized heat treatments at controlled temperatures (300-400°C) that transform the amorphous Ni-P structure into a crystalline state with nickel phosphides, further enhancing hardness while preserving corrosion resistance properties.
Strengths: Superior particle distribution technology creates more uniform coatings with consistent performance; proprietary heat treatment processes optimize both wear and corrosion resistance simultaneously. Weaknesses: Higher production costs compared to standard electroless nickel; process requires more precise control parameters which increases complexity.
Key Patents and Research Breakthroughs in Electroless Nickel Composites
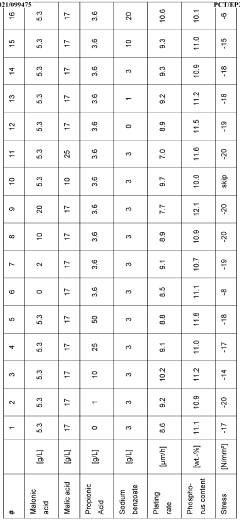
Electroless nickel alloy plating baths, a method for deposition of nickel alloys, nickel alloy deposits and uses of such formed nickel alloy deposits
PatentWO2021099475A1
Innovation
- An electroless nickel alloy plating bath comprising nickel ions, molybdenum or copper ions, and hypophosphite as reducing agents, along with specific complexing agents like malonic acid, tartaric acid, and benzoic acid, which stabilize the bath and enhance deposition properties.
Corrosion/wear-resistant metal coating compositions
PatentInactiveCA1269286A
Innovation
- A novel metal alloy coating composition comprising nickel, cobalt, boron, and thallium, applied through an electroless process using a borohydride reducing agent in an alkaline solution, creating a hard, ductile, and heterogeneous coating with amorphous nodular deposits that enhance wear and corrosion resistance.
Environmental Impact and Sustainability of Electroless Plating Processes
Electroless nickel plating processes, while offering significant technical advantages, present considerable environmental challenges that demand attention in sustainable manufacturing practices. Traditional electroless nickel baths contain several environmentally problematic components, including nickel salts, reducing agents like sodium hypophosphite, complexing agents, and stabilizers. These chemicals contribute to wastewater contamination with heavy metals and phosphorus compounds, which can lead to eutrophication and ecosystem disruption when improperly managed.
The energy consumption associated with maintaining electroless plating baths at elevated temperatures (typically 85-95°C) represents another significant environmental concern. This continuous heating requirement results in substantial carbon emissions, particularly in regions where electricity generation relies heavily on fossil fuels. Additionally, the relatively short lifespan of plating baths necessitates frequent disposal and replacement, further increasing the environmental footprint.
Recent sustainability initiatives in the electroless plating industry have focused on developing more environmentally friendly alternatives. Low-temperature plating processes that operate at 50-60°C have emerged, reducing energy consumption by up to 40% compared to conventional methods. These processes maintain comparable wear-corrosion resistance properties while significantly decreasing carbon emissions associated with bath heating.
Chemical recovery systems represent another important advancement in sustainable electroless plating. Ion exchange technologies and membrane filtration systems now enable the recovery of nickel from spent baths, reducing waste generation and raw material consumption. Some facilities report nickel recovery rates exceeding 95%, substantially reducing the environmental impact of their operations.
Biodegradable complexing agents are gradually replacing traditional chelating compounds like EDTA, which persist in the environment. These newer agents, often derived from natural sources such as citric acid and gluconic acid, maintain plating performance while degrading more readily in wastewater treatment systems. This innovation addresses one of the most persistent environmental challenges in electroless plating waste management.
Regulatory frameworks worldwide are increasingly stringent regarding electroless plating emissions and waste. The European Union's REACH regulations and similar initiatives in North America and Asia have accelerated the development of more sustainable plating technologies. Companies investing in environmentally responsible plating processes not only achieve regulatory compliance but often realize cost savings through reduced waste treatment requirements and improved resource efficiency.
The synergy between wear resistance and corrosion protection in electroless nickel coatings must now be balanced with environmental considerations, creating a three-way optimization challenge for modern coating developers. This balance will be critical in determining the future trajectory of electroless nickel technology in an increasingly sustainability-focused manufacturing landscape.
The energy consumption associated with maintaining electroless plating baths at elevated temperatures (typically 85-95°C) represents another significant environmental concern. This continuous heating requirement results in substantial carbon emissions, particularly in regions where electricity generation relies heavily on fossil fuels. Additionally, the relatively short lifespan of plating baths necessitates frequent disposal and replacement, further increasing the environmental footprint.
Recent sustainability initiatives in the electroless plating industry have focused on developing more environmentally friendly alternatives. Low-temperature plating processes that operate at 50-60°C have emerged, reducing energy consumption by up to 40% compared to conventional methods. These processes maintain comparable wear-corrosion resistance properties while significantly decreasing carbon emissions associated with bath heating.
Chemical recovery systems represent another important advancement in sustainable electroless plating. Ion exchange technologies and membrane filtration systems now enable the recovery of nickel from spent baths, reducing waste generation and raw material consumption. Some facilities report nickel recovery rates exceeding 95%, substantially reducing the environmental impact of their operations.
Biodegradable complexing agents are gradually replacing traditional chelating compounds like EDTA, which persist in the environment. These newer agents, often derived from natural sources such as citric acid and gluconic acid, maintain plating performance while degrading more readily in wastewater treatment systems. This innovation addresses one of the most persistent environmental challenges in electroless plating waste management.
Regulatory frameworks worldwide are increasingly stringent regarding electroless plating emissions and waste. The European Union's REACH regulations and similar initiatives in North America and Asia have accelerated the development of more sustainable plating technologies. Companies investing in environmentally responsible plating processes not only achieve regulatory compliance but often realize cost savings through reduced waste treatment requirements and improved resource efficiency.
The synergy between wear resistance and corrosion protection in electroless nickel coatings must now be balanced with environmental considerations, creating a three-way optimization challenge for modern coating developers. This balance will be critical in determining the future trajectory of electroless nickel technology in an increasingly sustainability-focused manufacturing landscape.
Cost-Benefit Analysis of Advanced Electroless Nickel Coating Systems
The implementation of advanced electroless nickel coating systems requires careful financial consideration to justify their adoption in industrial applications. When evaluating these systems, organizations must weigh initial capital expenditures against long-term operational benefits and cost savings derived from enhanced wear and corrosion resistance properties.
Initial investment costs for advanced electroless nickel coating systems typically include equipment acquisition, facility modifications, staff training, and process optimization. These capital expenditures can range from $50,000 for small-scale operations to several million dollars for comprehensive industrial implementations. However, these figures must be contextualized within the operational lifespan of the equipment, which typically extends 10-15 years with proper maintenance.
Operational expenses represent another significant cost factor, encompassing chemical consumables, energy requirements, waste treatment, and maintenance. Advanced electroless nickel baths containing phosphorus, boron, or composite materials command premium prices compared to conventional plating solutions, with costs approximately 30-40% higher. Nevertheless, these advanced formulations often deliver extended bath life and higher deposition efficiency, partially offsetting their increased cost.
The primary financial benefits of advanced electroless nickel coating systems manifest through enhanced component longevity and reduced maintenance requirements. Components treated with these coatings typically demonstrate 2-3 times longer service life in wear-corrosion synergistic environments compared to traditional coatings. This translates directly to decreased replacement frequency and associated downtime costs, which can represent savings of $10,000-$100,000 annually depending on application scale.
Quality improvements represent another significant value proposition. Advanced electroless nickel coatings provide more consistent thickness distribution and superior adhesion properties, reducing rejection rates in manufacturing processes. Industry data suggests quality-related savings of 5-15% in production environments where precise coating specifications are critical.
Return on investment (ROI) calculations for these systems typically indicate payback periods of 18-36 months for most industrial applications. However, in severe wear-corrosion environments such as offshore oil and gas, chemical processing, or mining operations, ROI can be achieved in as little as 6-12 months due to the extreme cost of equipment failure in these sectors.
Environmental compliance represents both a cost and benefit consideration. While advanced systems may require more sophisticated waste treatment processes, they typically produce less hazardous waste per unit of production and consume fewer resources over their operational lifetime, potentially reducing environmental compliance costs by 10-20% compared to conventional alternatives.
AI-powered predictive maintenance capabilities integrated with modern electroless nickel coating systems offer additional financial benefits through optimized chemical usage and process parameters, potentially reducing operational costs by an additional 5-10% while extending equipment service life.
Initial investment costs for advanced electroless nickel coating systems typically include equipment acquisition, facility modifications, staff training, and process optimization. These capital expenditures can range from $50,000 for small-scale operations to several million dollars for comprehensive industrial implementations. However, these figures must be contextualized within the operational lifespan of the equipment, which typically extends 10-15 years with proper maintenance.
Operational expenses represent another significant cost factor, encompassing chemical consumables, energy requirements, waste treatment, and maintenance. Advanced electroless nickel baths containing phosphorus, boron, or composite materials command premium prices compared to conventional plating solutions, with costs approximately 30-40% higher. Nevertheless, these advanced formulations often deliver extended bath life and higher deposition efficiency, partially offsetting their increased cost.
The primary financial benefits of advanced electroless nickel coating systems manifest through enhanced component longevity and reduced maintenance requirements. Components treated with these coatings typically demonstrate 2-3 times longer service life in wear-corrosion synergistic environments compared to traditional coatings. This translates directly to decreased replacement frequency and associated downtime costs, which can represent savings of $10,000-$100,000 annually depending on application scale.
Quality improvements represent another significant value proposition. Advanced electroless nickel coatings provide more consistent thickness distribution and superior adhesion properties, reducing rejection rates in manufacturing processes. Industry data suggests quality-related savings of 5-15% in production environments where precise coating specifications are critical.
Return on investment (ROI) calculations for these systems typically indicate payback periods of 18-36 months for most industrial applications. However, in severe wear-corrosion environments such as offshore oil and gas, chemical processing, or mining operations, ROI can be achieved in as little as 6-12 months due to the extreme cost of equipment failure in these sectors.
Environmental compliance represents both a cost and benefit consideration. While advanced systems may require more sophisticated waste treatment processes, they typically produce less hazardous waste per unit of production and consume fewer resources over their operational lifetime, potentially reducing environmental compliance costs by 10-20% compared to conventional alternatives.
AI-powered predictive maintenance capabilities integrated with modern electroless nickel coating systems offer additional financial benefits through optimized chemical usage and process parameters, potentially reducing operational costs by an additional 5-10% while extending equipment service life.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!