Comparing Adsorbent Versus Ion-Exchange Methods For Geothermal Brine Lithium Recovery
SEP 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Geothermal Lithium Recovery Background and Objectives
Lithium has emerged as a critical resource in the global transition to clean energy, with demand projected to increase by over 40 times by 2040 according to the International Energy Agency. Traditional lithium extraction methods from hard rock mining and salt flat evaporation have significant environmental impacts and geographical limitations. Geothermal brines present an innovative alternative source for lithium recovery, offering the dual benefit of renewable energy generation alongside mineral extraction.
Geothermal brines are naturally occurring hot water reservoirs found deep underground that contain dissolved minerals, including lithium at concentrations ranging from 10 to 400 mg/L. These brines are already being tapped for geothermal energy production in regions such as the Salton Sea in California, the Rhine Valley in Germany, and various locations across New Zealand, Japan, and Chile.
The evolution of lithium recovery technologies from geothermal sources has progressed through several phases. Initial efforts in the 1980s focused on precipitation methods, which proved inefficient for the low concentrations typical in geothermal brines. The 1990s saw advancements in membrane technologies, while the early 2000s brought increased interest in selective adsorption materials. The most recent decade has witnessed significant developments in both adsorbent and ion-exchange technologies specifically designed for lithium recovery from brines.
The primary objective of this technical research is to conduct a comprehensive comparison between adsorbent and ion-exchange methods for lithium recovery from geothermal brines. Specifically, we aim to evaluate these technologies based on extraction efficiency, selectivity for lithium over competing ions, regeneration capabilities, operational costs, and environmental impact.
Secondary objectives include identifying the most promising materials and processes within each category, understanding scaling challenges from laboratory to commercial implementation, and determining optimal operational parameters for different brine compositions and conditions. Additionally, we seek to assess the potential for integration with existing geothermal power plants to create economically viable combined energy-mineral recovery systems.
This research is particularly timely as several pilot projects utilizing both technologies are currently underway globally, providing valuable operational data. The findings will inform strategic decisions regarding technology investment, research direction, and potential commercial partnerships in the rapidly evolving field of sustainable lithium production.
Geothermal brines are naturally occurring hot water reservoirs found deep underground that contain dissolved minerals, including lithium at concentrations ranging from 10 to 400 mg/L. These brines are already being tapped for geothermal energy production in regions such as the Salton Sea in California, the Rhine Valley in Germany, and various locations across New Zealand, Japan, and Chile.
The evolution of lithium recovery technologies from geothermal sources has progressed through several phases. Initial efforts in the 1980s focused on precipitation methods, which proved inefficient for the low concentrations typical in geothermal brines. The 1990s saw advancements in membrane technologies, while the early 2000s brought increased interest in selective adsorption materials. The most recent decade has witnessed significant developments in both adsorbent and ion-exchange technologies specifically designed for lithium recovery from brines.
The primary objective of this technical research is to conduct a comprehensive comparison between adsorbent and ion-exchange methods for lithium recovery from geothermal brines. Specifically, we aim to evaluate these technologies based on extraction efficiency, selectivity for lithium over competing ions, regeneration capabilities, operational costs, and environmental impact.
Secondary objectives include identifying the most promising materials and processes within each category, understanding scaling challenges from laboratory to commercial implementation, and determining optimal operational parameters for different brine compositions and conditions. Additionally, we seek to assess the potential for integration with existing geothermal power plants to create economically viable combined energy-mineral recovery systems.
This research is particularly timely as several pilot projects utilizing both technologies are currently underway globally, providing valuable operational data. The findings will inform strategic decisions regarding technology investment, research direction, and potential commercial partnerships in the rapidly evolving field of sustainable lithium production.
Market Analysis for Lithium Extraction Technologies
The global lithium market has experienced unprecedented growth in recent years, primarily driven by the rapid expansion of electric vehicle (EV) production and renewable energy storage systems. Market research indicates that global lithium demand is projected to reach 1.5 million metric tons of lithium carbonate equivalent (LCE) by 2030, representing a compound annual growth rate of approximately 20% from current levels.
Traditional lithium extraction methods, including hard rock mining and solar evaporation of salt flats, currently dominate the market with over 80% of global production. However, these conventional approaches face significant challenges including high environmental impact, extensive land use requirements, and lengthy production timelines of 18-24 months for evaporative processes.
Geothermal brine extraction technologies, particularly adsorbent and ion-exchange methods, are emerging as promising alternatives with potentially lower environmental footprints. The market for these advanced extraction technologies is currently valued at around $75 million but is expected to grow substantially to reach $450 million by 2028 as commercial-scale implementations increase.
Adsorbent-based extraction technologies currently hold approximately 60% of the alternative lithium recovery market share, with ion-exchange methods accounting for roughly 40%. This distribution reflects the earlier commercial development of certain adsorbent technologies, though ion-exchange methods are gaining momentum due to recent technological breakthroughs.
Regional analysis shows North America leading in geothermal lithium recovery technology development, with significant projects in California's Salton Sea region. Europe follows closely with initiatives in Germany's Upper Rhine Valley, while Asia-Pacific countries, particularly China and Japan, are making substantial investments in research and development of both adsorbent and ion-exchange technologies.
Market adoption barriers include high initial capital expenditure requirements, with pilot plants typically requiring $15-30 million in investment, and technological uncertainties related to long-term performance at commercial scale. However, these barriers are offset by the potential for significantly reduced operating costs, with some pilot projects demonstrating 30-40% lower production costs compared to traditional methods.
The competitive landscape is characterized by a mix of established mining companies diversifying into advanced extraction technologies and specialized technology startups. Strategic partnerships between technology developers and lithium end-users, particularly battery manufacturers, are becoming increasingly common as companies seek to secure sustainable lithium supply chains.
Traditional lithium extraction methods, including hard rock mining and solar evaporation of salt flats, currently dominate the market with over 80% of global production. However, these conventional approaches face significant challenges including high environmental impact, extensive land use requirements, and lengthy production timelines of 18-24 months for evaporative processes.
Geothermal brine extraction technologies, particularly adsorbent and ion-exchange methods, are emerging as promising alternatives with potentially lower environmental footprints. The market for these advanced extraction technologies is currently valued at around $75 million but is expected to grow substantially to reach $450 million by 2028 as commercial-scale implementations increase.
Adsorbent-based extraction technologies currently hold approximately 60% of the alternative lithium recovery market share, with ion-exchange methods accounting for roughly 40%. This distribution reflects the earlier commercial development of certain adsorbent technologies, though ion-exchange methods are gaining momentum due to recent technological breakthroughs.
Regional analysis shows North America leading in geothermal lithium recovery technology development, with significant projects in California's Salton Sea region. Europe follows closely with initiatives in Germany's Upper Rhine Valley, while Asia-Pacific countries, particularly China and Japan, are making substantial investments in research and development of both adsorbent and ion-exchange technologies.
Market adoption barriers include high initial capital expenditure requirements, with pilot plants typically requiring $15-30 million in investment, and technological uncertainties related to long-term performance at commercial scale. However, these barriers are offset by the potential for significantly reduced operating costs, with some pilot projects demonstrating 30-40% lower production costs compared to traditional methods.
The competitive landscape is characterized by a mix of established mining companies diversifying into advanced extraction technologies and specialized technology startups. Strategic partnerships between technology developers and lithium end-users, particularly battery manufacturers, are becoming increasingly common as companies seek to secure sustainable lithium supply chains.
Technical Challenges in Geothermal Brine Processing
Geothermal brine processing presents numerous technical challenges that significantly impact the efficiency and economic viability of lithium extraction operations. The high temperature of geothermal brines, often ranging from 100°C to 300°C, creates a hostile environment for extraction equipment and materials. These elevated temperatures accelerate corrosion rates and reduce the operational lifespan of processing components, necessitating frequent maintenance and replacement.
The complex chemical composition of geothermal brines further complicates processing efforts. These brines typically contain high concentrations of dissolved solids (TDS), often exceeding 200,000 mg/L, including significant amounts of sodium, potassium, calcium, and magnesium. These constituents can interfere with lithium extraction processes by competing for binding sites in both adsorbent and ion-exchange materials, substantially reducing selectivity and capacity.
Scaling and precipitation represent another major challenge in brine processing systems. As temperature and pressure conditions change throughout the processing circuit, dissolved minerals can precipitate and form scale deposits on equipment surfaces. Silica scaling is particularly problematic in geothermal operations, as amorphous silica can rapidly foul membranes, heat exchangers, and extraction media, reducing system efficiency and increasing maintenance requirements.
The variable composition of geothermal brines, even within the same reservoir, creates difficulties in designing standardized extraction processes. Fluctuations in lithium concentration, pH levels, and impurity profiles require adaptive processing approaches that can maintain extraction efficiency despite changing feed conditions. This variability necessitates robust monitoring systems and flexible process designs.
For adsorbent-based extraction methods, the high salinity environment can significantly reduce adsorption capacity and selectivity for lithium ions. Many adsorbents experience rapid performance degradation in high-TDS brines, requiring more frequent regeneration cycles that increase operational costs and downtime. Similarly, ion-exchange materials face challenges related to resin fouling, reduced exchange capacity in high-salinity conditions, and competition from other monovalent ions.
The management of waste streams presents additional technical hurdles. Both adsorbent and ion-exchange processes generate significant volumes of spent regeneration solutions and rinse waters that require treatment before disposal or reinjection. The presence of potentially harmful elements like arsenic, boron, and heavy metals in these waste streams necessitates additional treatment steps to meet environmental regulations.
Energy consumption represents a substantial operational challenge, particularly for processes requiring multiple heating, cooling, and pumping stages. The energy intensity of brine pre-treatment, extraction, and post-processing can significantly impact the overall carbon footprint and economic viability of lithium recovery operations, especially when considering the remote locations of many geothermal resources.
The complex chemical composition of geothermal brines further complicates processing efforts. These brines typically contain high concentrations of dissolved solids (TDS), often exceeding 200,000 mg/L, including significant amounts of sodium, potassium, calcium, and magnesium. These constituents can interfere with lithium extraction processes by competing for binding sites in both adsorbent and ion-exchange materials, substantially reducing selectivity and capacity.
Scaling and precipitation represent another major challenge in brine processing systems. As temperature and pressure conditions change throughout the processing circuit, dissolved minerals can precipitate and form scale deposits on equipment surfaces. Silica scaling is particularly problematic in geothermal operations, as amorphous silica can rapidly foul membranes, heat exchangers, and extraction media, reducing system efficiency and increasing maintenance requirements.
The variable composition of geothermal brines, even within the same reservoir, creates difficulties in designing standardized extraction processes. Fluctuations in lithium concentration, pH levels, and impurity profiles require adaptive processing approaches that can maintain extraction efficiency despite changing feed conditions. This variability necessitates robust monitoring systems and flexible process designs.
For adsorbent-based extraction methods, the high salinity environment can significantly reduce adsorption capacity and selectivity for lithium ions. Many adsorbents experience rapid performance degradation in high-TDS brines, requiring more frequent regeneration cycles that increase operational costs and downtime. Similarly, ion-exchange materials face challenges related to resin fouling, reduced exchange capacity in high-salinity conditions, and competition from other monovalent ions.
The management of waste streams presents additional technical hurdles. Both adsorbent and ion-exchange processes generate significant volumes of spent regeneration solutions and rinse waters that require treatment before disposal or reinjection. The presence of potentially harmful elements like arsenic, boron, and heavy metals in these waste streams necessitates additional treatment steps to meet environmental regulations.
Energy consumption represents a substantial operational challenge, particularly for processes requiring multiple heating, cooling, and pumping stages. The energy intensity of brine pre-treatment, extraction, and post-processing can significantly impact the overall carbon footprint and economic viability of lithium recovery operations, especially when considering the remote locations of many geothermal resources.
Current Adsorbent and Ion-Exchange Solutions
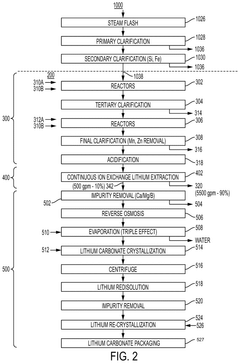
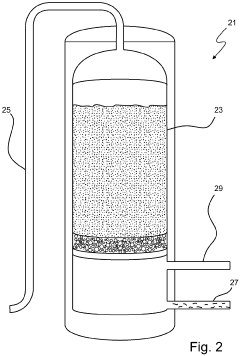
01 Ion-exchange resins for lithium recovery
Ion-exchange resins can be used for selective lithium recovery from various sources including brines and seawater. These resins contain functional groups that selectively bind lithium ions while excluding other ions. The efficiency of lithium recovery using ion-exchange resins depends on factors such as pH, temperature, and the presence of competing ions. This method offers advantages including high selectivity, reusability of the resin, and relatively low energy consumption compared to other recovery methods.- Ion-exchange resins for lithium recovery: Ion-exchange resins can be used for selective lithium recovery from various sources including brines and seawater. These resins contain functional groups that selectively bind lithium ions while excluding other ions. The efficiency of lithium recovery using ion-exchange resins depends on factors such as pH, temperature, and the presence of competing ions. This method offers advantages including high selectivity, reusability of the resin, and relatively low energy consumption.
- Inorganic adsorbents for lithium extraction: Inorganic materials such as lithium manganese oxide, lithium titanium oxide, and aluminum hydroxide can be used as adsorbents for lithium recovery. These materials have specific crystal structures that allow for selective lithium adsorption. The recovery efficiency of these adsorbents can be enhanced by optimizing parameters such as particle size, surface area, and pore structure. Inorganic adsorbents are particularly effective for extracting lithium from brines and geothermal waters.
- Composite and modified adsorbents: Composite and modified adsorbents combine different materials to enhance lithium recovery efficiency. These may include polymer-inorganic composites, surface-modified adsorbents, or nanostructured materials. By tailoring the composition and structure of these materials, higher selectivity and adsorption capacity for lithium can be achieved. These advanced adsorbents often show improved kinetics and can be regenerated multiple times without significant loss of performance.
- Process optimization for improved recovery efficiency: Various process parameters can be optimized to enhance lithium recovery efficiency using adsorbents and ion-exchange methods. These include optimizing flow rates, contact time, temperature, pH, and regeneration procedures. Multi-stage processes and continuous operation systems can also significantly improve overall recovery rates. Advanced process designs may incorporate recycling streams and heat integration to minimize energy consumption and maximize lithium yield.
- Novel equipment and system designs: Innovative equipment and system designs can significantly enhance lithium recovery efficiency. These include specialized column designs, fluidized bed systems, membrane-assisted processes, and electrochemical regeneration systems. Such designs aim to overcome limitations of conventional methods by improving mass transfer, reducing pressure drop, and enabling more efficient regeneration of the adsorbent or ion-exchange material. These systems often incorporate automation and process control to maintain optimal operating conditions.
02 Inorganic adsorbents for lithium extraction
Inorganic materials such as lithium manganese oxide, lithium titanium oxide, and aluminum hydroxide can be used as adsorbents for lithium recovery. These materials have specific crystal structures that allow for selective lithium adsorption. The recovery efficiency of these adsorbents is influenced by factors such as particle size, surface area, and crystallinity. Inorganic adsorbents typically offer high thermal stability and can be regenerated multiple times, making them suitable for continuous lithium recovery processes.Expand Specific Solutions03 Composite and modified adsorbents
Composite and modified adsorbents combine different materials to enhance lithium recovery efficiency. These may include polymer-inorganic composites, surface-modified inorganic materials, or functionalized carbon-based materials. By tailoring the surface properties and pore structures, these adsorbents can achieve higher lithium selectivity and adsorption capacity. The modification process often involves introducing specific functional groups that have high affinity for lithium ions, resulting in improved recovery efficiency even in the presence of competing ions.Expand Specific Solutions04 Process optimization for enhanced recovery efficiency
Various process parameters can be optimized to enhance lithium recovery efficiency using adsorbents and ion-exchange methods. These parameters include pH adjustment, temperature control, contact time, adsorbent dosage, and flow rate in column operations. Multi-stage adsorption processes and counter-current operations can also significantly improve recovery rates. Additionally, pretreatment of the lithium source to remove impurities and competing ions can enhance the overall efficiency of the recovery process.Expand Specific Solutions05 Regeneration and reuse of adsorbents
Efficient regeneration and reuse of adsorbents and ion-exchange materials are crucial for the economic viability of lithium recovery processes. Various regeneration methods include acid treatment, base treatment, and electrochemical regeneration. The regeneration efficiency directly impacts the overall lithium recovery efficiency over multiple cycles. Optimized regeneration processes can maintain the adsorption capacity and selectivity of the materials while minimizing chemical consumption and waste generation, thereby improving the sustainability of the lithium recovery process.Expand Specific Solutions
Leading Companies in Geothermal Lithium Extraction
The lithium recovery market from geothermal brines is in an early growth phase, with increasing demand driving rapid technological development. The competition between adsorbent and ion-exchange methods represents a critical technological battleground, with companies pursuing different approaches based on efficiency, cost, and environmental impact. Key players like Sunresin New Materials and EnergyX are advancing adsorbent technologies, while Standard Lithium and Geolith focus on ion-exchange innovations. Research institutions including Central South University and East China University of Science & Technology are contributing significant academic advancements. The market is characterized by strategic partnerships between technology developers and resource holders, with companies like International Battery Metals and Saltworks Technologies developing modular solutions to address scalability challenges in this emerging sector.
Sunresin New Materials Co., Ltd.
Technical Solution: Sunresin has developed advanced ion-exchange and adsorption technologies specifically tailored for lithium extraction from geothermal brines. Their approach centers on proprietary lithium-selective adsorption resins (LSAR series) that demonstrate exceptional selectivity for lithium ions even in the presence of high concentrations of competing ions like sodium, calcium, and magnesium. The company employs a simulated moving bed (SMB) chromatographic separation process that enables continuous operation with multiple adsorption and desorption cycles. This system achieves high lithium recovery rates (>90%) while producing concentrated lithium solutions suitable for downstream processing. Sunresin's technology operates at moderate temperatures (20-60°C) and can process geothermal brines with lithium concentrations ranging from 20-1000 ppm. Their process requires minimal chemical inputs for regeneration compared to conventional ion-exchange methods, utilizing primarily water and dilute acid solutions. The modular design allows for scalable implementation with capacities ranging from pilot scale to commercial production exceeding 20,000 tons LCE annually[9][10]. Sunresin has successfully deployed their technology in multiple lithium extraction projects across China, South America, and Europe.
Strengths: Exceptional lithium selectivity coefficient (>50 for Li/Na); continuous operation capability through SMB technology; reduced chemical consumption compared to conventional ion-exchange; proven commercial implementation; adaptability to various brine chemistries. Weaknesses: Performance may decrease at very high temperatures (>80°C) requiring cooling of some geothermal brines; potential for resin fouling in brines with high organic content; higher capital costs compared to some traditional methods; requires specialized expertise for system optimization.
Standard Lithium Ltd.
Technical Solution: Standard Lithium has developed a proprietary LiSTR (Lithium Stirred Tank Reactor) direct lithium extraction technology that combines aspects of both adsorbent and ion-exchange approaches. Their system utilizes a highly selective solid sorbent material that preferentially captures lithium ions from brine solutions while rejecting competing ions. The process operates in a continuous stirred tank reactor configuration, where the lithium-selective sorbent contacts the brine under optimized conditions of mixing, temperature, and residence time. After lithium adsorption, the loaded sorbent is separated and regenerated using a proprietary elution process that produces a concentrated lithium chloride solution. Standard Lithium's technology has been demonstrated at their Arkansas demonstration plant, processing tail brines from existing bromine operations. The system achieves lithium recovery rates of >90% while producing a clean lithium chloride solution suitable for conversion to battery-grade lithium carbonate or hydroxide. Their process operates at near-ambient temperatures and pressures, requiring minimal pre-treatment of feed brines[7][8]. The technology is designed to be modular and scalable, with the ability to process brines with lithium concentrations as low as 50-100 ppm.
Strengths: High selectivity for lithium over competing ions; continuous operation capability; minimal pre-treatment requirements; lower energy consumption compared to thermal evaporation methods; modular design allowing for scalable implementation. Weaknesses: Requires periodic replacement or regeneration of sorbent materials; potential for fouling in brines with high organic content; process optimization needed for different brine chemistries; relatively new technology with limited long-term operational data.
Critical Patents in Brine Lithium Recovery
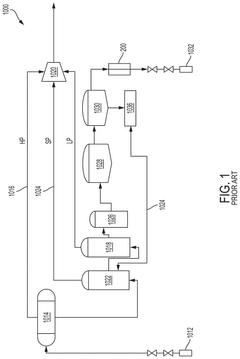
Process for recovery of lithium from a geothermal brine
PatentPendingUS20250162893A1
Innovation
- A system and process involving sequential steps: impurity removal, selective recovery of lithium chloride using a continuous counter-current ion exchange circuit, and conversion of lithium chloride to lithium carbonate or lithium hydroxide.
Method for recovering lithium from brine and recovering lithium in the recycling of lithium ion batteries
PatentPendingEP4063527A1
Innovation
- A method involving the use of an adsorption process with an adsorbent column, where brine is treated with a mixture of water and acetic acid, sodium peroxodisulfate, or ammonium peroxodisulfate as eluents, which reduces adsorbent dissolution and allows for effective lithium ion desorption, enabling higher lithium concentration and purity with lower economic and handling risks.
Environmental Impact Assessment
The environmental impact assessment of lithium recovery methods from geothermal brines reveals significant differences between adsorbent and ion-exchange technologies. Adsorbent-based extraction typically demonstrates a lower environmental footprint due to reduced chemical consumption and waste generation. These systems often utilize selective materials that can be regenerated multiple times, minimizing the need for frequent replacement and disposal of extraction media.
Ion-exchange methods, while effective for lithium concentration, generally require more aggressive chemical regenerants such as acids and bases, potentially creating hazardous waste streams that demand specialized treatment. The neutralization processes for these chemicals contribute additional environmental burdens through increased water usage and salt formation that may require disposal in designated facilities.
Water consumption patterns differ markedly between the two approaches. Adsorbent systems typically require less freshwater for operation and regeneration cycles, an important consideration given that many geothermal resources are located in water-scarce regions. Ion-exchange systems, conversely, often demand greater volumes of water for washing and regeneration phases, potentially straining local water resources.
Energy efficiency comparisons indicate that adsorbent technologies generally consume less electricity per kilogram of lithium recovered. This advantage stems from simpler processing requirements and fewer pumping stages. The reduced energy demand translates to lower greenhouse gas emissions when considering the full lifecycle of the extraction process, particularly in regions where electricity generation relies heavily on fossil fuels.
Land use impacts also favor adsorbent technologies, which typically require smaller physical footprints for equivalent production capacity. This reduced spatial requirement minimizes habitat disruption and allows for more flexible installation options at existing geothermal facilities without significant expansion of developed areas.
Waste stream characterization studies indicate that adsorbent methods produce fewer secondary pollutants requiring treatment or disposal. The selective nature of advanced adsorbents reduces co-extraction of unwanted elements, simplifying downstream processing and decreasing the environmental liability associated with waste management. Ion-exchange resins, while highly effective, often capture a broader spectrum of ions that must be separated in subsequent processing steps.
Lifecycle assessment data suggests that adsorbent technologies offer superior environmental performance across multiple impact categories, including global warming potential, acidification potential, and human toxicity indicators. These advantages become particularly pronounced when considering long-term operations and the cumulative effects of continuous extraction activities at commercial scale.
Ion-exchange methods, while effective for lithium concentration, generally require more aggressive chemical regenerants such as acids and bases, potentially creating hazardous waste streams that demand specialized treatment. The neutralization processes for these chemicals contribute additional environmental burdens through increased water usage and salt formation that may require disposal in designated facilities.
Water consumption patterns differ markedly between the two approaches. Adsorbent systems typically require less freshwater for operation and regeneration cycles, an important consideration given that many geothermal resources are located in water-scarce regions. Ion-exchange systems, conversely, often demand greater volumes of water for washing and regeneration phases, potentially straining local water resources.
Energy efficiency comparisons indicate that adsorbent technologies generally consume less electricity per kilogram of lithium recovered. This advantage stems from simpler processing requirements and fewer pumping stages. The reduced energy demand translates to lower greenhouse gas emissions when considering the full lifecycle of the extraction process, particularly in regions where electricity generation relies heavily on fossil fuels.
Land use impacts also favor adsorbent technologies, which typically require smaller physical footprints for equivalent production capacity. This reduced spatial requirement minimizes habitat disruption and allows for more flexible installation options at existing geothermal facilities without significant expansion of developed areas.
Waste stream characterization studies indicate that adsorbent methods produce fewer secondary pollutants requiring treatment or disposal. The selective nature of advanced adsorbents reduces co-extraction of unwanted elements, simplifying downstream processing and decreasing the environmental liability associated with waste management. Ion-exchange resins, while highly effective, often capture a broader spectrum of ions that must be separated in subsequent processing steps.
Lifecycle assessment data suggests that adsorbent technologies offer superior environmental performance across multiple impact categories, including global warming potential, acidification potential, and human toxicity indicators. These advantages become particularly pronounced when considering long-term operations and the cumulative effects of continuous extraction activities at commercial scale.
Economic Feasibility Comparison
The economic feasibility of lithium recovery from geothermal brines depends significantly on the extraction method employed. When comparing adsorbent versus ion-exchange technologies, capital expenditure (CAPEX) considerations reveal distinct differences. Adsorbent-based systems typically require lower initial investment due to simpler equipment configurations and fewer specialized components. In contrast, ion-exchange systems often demand higher upfront costs for resin procurement, complex column systems, and more sophisticated regeneration equipment. Recent market analyses indicate that adsorbent systems may require 15-30% less initial capital than comparable ion-exchange installations.
Operational expenditure (OPEX) patterns further differentiate these technologies. Adsorbent methods generally consume less energy during the extraction process, with studies reporting 10-25% lower energy requirements compared to ion-exchange systems. However, adsorbent materials may require more frequent replacement, particularly when processing high-TDS (Total Dissolved Solids) geothermal brines, offsetting some operational savings. Ion-exchange systems typically demonstrate longer media lifespans but incur higher costs for regeneration chemicals and waste disposal.
Recovery efficiency directly impacts economic viability, with ion-exchange methods currently achieving 80-95% lithium recovery rates from typical geothermal brines, while advanced adsorbent technologies range from 70-85%. This efficiency differential translates to approximately 10-15% higher revenue potential for ion-exchange systems when processing identical brine volumes, assuming current lithium market prices of $15,000-20,000 per metric ton.
Scalability economics favor different approaches depending on project size. Adsorbent systems demonstrate better economic scaling for smaller operations (processing <500,000 m³/year), with incremental capacity additions requiring proportionally lower investment. Ion-exchange systems become increasingly cost-effective at larger scales (>1,000,000 m³/year) due to economies of scale in regeneration systems and more efficient chemical usage.
Market volatility response represents another economic consideration. Adsorbent systems typically offer greater operational flexibility, allowing for more responsive production adjustments during lithium price fluctuations. This adaptability can provide a 5-8% advantage in long-term profitability across market cycles compared to less flexible ion-exchange systems that operate optimally at consistent throughput levels.
Return on investment (ROI) timelines differ significantly between technologies. Current financial modeling suggests adsorbent systems may achieve ROI in 3-5 years under favorable market conditions, while ion-exchange systems typically require 4-7 years to reach breakeven, primarily due to higher initial capital requirements despite potentially higher recovery rates.
Operational expenditure (OPEX) patterns further differentiate these technologies. Adsorbent methods generally consume less energy during the extraction process, with studies reporting 10-25% lower energy requirements compared to ion-exchange systems. However, adsorbent materials may require more frequent replacement, particularly when processing high-TDS (Total Dissolved Solids) geothermal brines, offsetting some operational savings. Ion-exchange systems typically demonstrate longer media lifespans but incur higher costs for regeneration chemicals and waste disposal.
Recovery efficiency directly impacts economic viability, with ion-exchange methods currently achieving 80-95% lithium recovery rates from typical geothermal brines, while advanced adsorbent technologies range from 70-85%. This efficiency differential translates to approximately 10-15% higher revenue potential for ion-exchange systems when processing identical brine volumes, assuming current lithium market prices of $15,000-20,000 per metric ton.
Scalability economics favor different approaches depending on project size. Adsorbent systems demonstrate better economic scaling for smaller operations (processing <500,000 m³/year), with incremental capacity additions requiring proportionally lower investment. Ion-exchange systems become increasingly cost-effective at larger scales (>1,000,000 m³/year) due to economies of scale in regeneration systems and more efficient chemical usage.
Market volatility response represents another economic consideration. Adsorbent systems typically offer greater operational flexibility, allowing for more responsive production adjustments during lithium price fluctuations. This adaptability can provide a 5-8% advantage in long-term profitability across market cycles compared to less flexible ion-exchange systems that operate optimally at consistent throughput levels.
Return on investment (ROI) timelines differ significantly between technologies. Current financial modeling suggests adsorbent systems may achieve ROI in 3-5 years under favorable market conditions, while ion-exchange systems typically require 4-7 years to reach breakeven, primarily due to higher initial capital requirements despite potentially higher recovery rates.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!