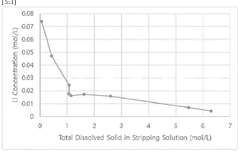
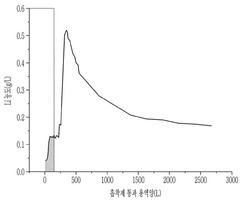
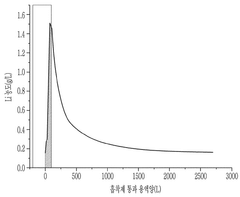
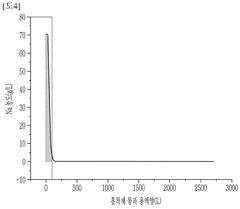
Novel Sorbent Synthesis Routes For Selective Lithium Uptake
SEP 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Sorbent Technology Background and Objectives
Lithium extraction technologies have evolved significantly over the past decades, transitioning from traditional mining operations to more sophisticated and environmentally conscious methodologies. The historical development began with conventional brine evaporation techniques in salt flats, which dominated the industry for decades but faced challenges of lengthy processing times and substantial water consumption. As global demand for lithium accelerated with the proliferation of portable electronics in the 1990s and electric vehicles in the 2010s, the limitations of traditional extraction methods became increasingly apparent.
The technological evolution has been driven by the strategic importance of lithium as a critical material for energy storage applications. Current global lithium production stands at approximately 100,000 metric tons annually, with projections indicating a potential five-fold increase in demand by 2030. This anticipated surge necessitates more efficient and selective extraction technologies to meet future requirements while minimizing environmental impact.
Novel sorbent technologies represent a promising frontier in lithium extraction, offering potential advantages in selectivity, efficiency, and sustainability. These advanced materials are designed to preferentially capture lithium ions from complex solutions, including brines, seawater, and geothermal fluids, which contain numerous competing ions. The development of these sorbents has accelerated in recent years, with significant breakthroughs in materials science enabling unprecedented selectivity coefficients.
The primary objective of novel sorbent synthesis research is to develop materials capable of highly selective lithium uptake under diverse environmental conditions. Ideal sorbents must demonstrate not only high selectivity for lithium over competing ions (particularly sodium, potassium, magnesium, and calcium) but also rapid kinetics, substantial capacity, and excellent reusability through multiple adsorption-desorption cycles. Additionally, these materials must be cost-effective to manufacture at scale and environmentally benign throughout their lifecycle.
Current research trajectories focus on several promising material classes, including inorganic ion exchangers, metal oxides, metal-organic frameworks (MOFs), and functionalized polymeric adsorbents. Each approach offers distinct advantages and challenges, with recent innovations demonstrating remarkable improvements in selectivity factors and adsorption capacities. The integration of computational modeling with experimental synthesis has accelerated the discovery process, enabling rational design of sorbent structures with optimized binding sites for lithium ions.
The technological goals extend beyond mere extraction efficiency to encompass broader sustainability objectives. Next-generation sorbents aim to enable direct lithium extraction from unconventional sources such as geothermal brines, oil field produced waters, and even seawater, potentially unlocking vast new lithium reserves while minimizing environmental disruption associated with traditional mining operations.
The technological evolution has been driven by the strategic importance of lithium as a critical material for energy storage applications. Current global lithium production stands at approximately 100,000 metric tons annually, with projections indicating a potential five-fold increase in demand by 2030. This anticipated surge necessitates more efficient and selective extraction technologies to meet future requirements while minimizing environmental impact.
Novel sorbent technologies represent a promising frontier in lithium extraction, offering potential advantages in selectivity, efficiency, and sustainability. These advanced materials are designed to preferentially capture lithium ions from complex solutions, including brines, seawater, and geothermal fluids, which contain numerous competing ions. The development of these sorbents has accelerated in recent years, with significant breakthroughs in materials science enabling unprecedented selectivity coefficients.
The primary objective of novel sorbent synthesis research is to develop materials capable of highly selective lithium uptake under diverse environmental conditions. Ideal sorbents must demonstrate not only high selectivity for lithium over competing ions (particularly sodium, potassium, magnesium, and calcium) but also rapid kinetics, substantial capacity, and excellent reusability through multiple adsorption-desorption cycles. Additionally, these materials must be cost-effective to manufacture at scale and environmentally benign throughout their lifecycle.
Current research trajectories focus on several promising material classes, including inorganic ion exchangers, metal oxides, metal-organic frameworks (MOFs), and functionalized polymeric adsorbents. Each approach offers distinct advantages and challenges, with recent innovations demonstrating remarkable improvements in selectivity factors and adsorption capacities. The integration of computational modeling with experimental synthesis has accelerated the discovery process, enabling rational design of sorbent structures with optimized binding sites for lithium ions.
The technological goals extend beyond mere extraction efficiency to encompass broader sustainability objectives. Next-generation sorbents aim to enable direct lithium extraction from unconventional sources such as geothermal brines, oil field produced waters, and even seawater, potentially unlocking vast new lithium reserves while minimizing environmental disruption associated with traditional mining operations.
Market Analysis for Selective Lithium Recovery Solutions
The global lithium market has experienced unprecedented growth in recent years, primarily driven by the rapid expansion of electric vehicle (EV) production and renewable energy storage systems. The market for selective lithium recovery solutions is projected to reach $5.6 billion by 2028, with a compound annual growth rate of 14.2% from 2023 to 2028. This remarkable growth trajectory reflects the critical importance of lithium as a strategic resource in the global transition toward sustainable energy systems.
Demand for lithium is expected to triple by 2025 compared to 2021 levels, creating significant supply challenges that novel sorbent technologies aim to address. Traditional lithium extraction methods, including hard rock mining and brine evaporation, face increasing scrutiny due to their environmental impact and inefficiency, creating a substantial market opportunity for advanced selective lithium uptake technologies.
The market for selective lithium recovery solutions can be segmented by technology type, with ion-exchange sorbents, membrane processes, and novel nanomaterial-based solutions emerging as key categories. Among these, selective sorbent technologies are gaining particular attention due to their potential for higher recovery rates and lower environmental footprint compared to conventional methods.
Geographically, the market shows distinct regional characteristics. North America and Europe lead in research and development of advanced sorbent technologies, while China dominates in terms of implementation scale and manufacturing capacity. South American countries, particularly those in the "Lithium Triangle" (Argentina, Bolivia, and Chile), represent crucial markets for deployment of these technologies due to their vast lithium brine resources.
End-user industries driving demand include battery manufacturers (accounting for approximately 71% of lithium consumption), glass and ceramics producers (14%), lubricant manufacturers (4%), and other industrial applications (11%). The battery sector, particularly for EVs and grid storage, represents the most significant growth driver for selective lithium recovery technologies.
Price sensitivity analysis indicates that novel sorbent technologies become increasingly economically viable as lithium carbonate prices exceed $15,000 per metric ton, a threshold that has been consistently surpassed since 2021. This economic reality has accelerated investment in research and commercialization of advanced lithium recovery solutions.
Market barriers include high initial capital requirements for technology deployment, technical challenges in achieving high selectivity in complex brine environments, and regulatory uncertainties regarding extraction processes. Despite these challenges, the critical supply-demand imbalance in the lithium market continues to drive substantial investment in novel sorbent synthesis routes for selective lithium uptake.
Demand for lithium is expected to triple by 2025 compared to 2021 levels, creating significant supply challenges that novel sorbent technologies aim to address. Traditional lithium extraction methods, including hard rock mining and brine evaporation, face increasing scrutiny due to their environmental impact and inefficiency, creating a substantial market opportunity for advanced selective lithium uptake technologies.
The market for selective lithium recovery solutions can be segmented by technology type, with ion-exchange sorbents, membrane processes, and novel nanomaterial-based solutions emerging as key categories. Among these, selective sorbent technologies are gaining particular attention due to their potential for higher recovery rates and lower environmental footprint compared to conventional methods.
Geographically, the market shows distinct regional characteristics. North America and Europe lead in research and development of advanced sorbent technologies, while China dominates in terms of implementation scale and manufacturing capacity. South American countries, particularly those in the "Lithium Triangle" (Argentina, Bolivia, and Chile), represent crucial markets for deployment of these technologies due to their vast lithium brine resources.
End-user industries driving demand include battery manufacturers (accounting for approximately 71% of lithium consumption), glass and ceramics producers (14%), lubricant manufacturers (4%), and other industrial applications (11%). The battery sector, particularly for EVs and grid storage, represents the most significant growth driver for selective lithium recovery technologies.
Price sensitivity analysis indicates that novel sorbent technologies become increasingly economically viable as lithium carbonate prices exceed $15,000 per metric ton, a threshold that has been consistently surpassed since 2021. This economic reality has accelerated investment in research and commercialization of advanced lithium recovery solutions.
Market barriers include high initial capital requirements for technology deployment, technical challenges in achieving high selectivity in complex brine environments, and regulatory uncertainties regarding extraction processes. Despite these challenges, the critical supply-demand imbalance in the lithium market continues to drive substantial investment in novel sorbent synthesis routes for selective lithium uptake.
Current Challenges in Lithium Sorbent Development
Despite significant advancements in lithium extraction technologies, the development of efficient and selective lithium sorbents faces several critical challenges. Current lithium sorbent materials exhibit limitations in selectivity, particularly in complex brine environments where competing ions such as sodium, potassium, magnesium, and calcium are present in significantly higher concentrations than lithium. This selectivity issue remains a fundamental obstacle to economically viable lithium recovery processes.
Capacity constraints represent another major challenge, with many existing sorbents demonstrating insufficient lithium uptake capacity under practical operating conditions. This limitation necessitates larger equipment and higher operational costs, undermining the economic feasibility of lithium extraction projects, especially from low-concentration sources like geothermal brines or seawater.
The kinetics of lithium sorption processes often prove problematic, with slow uptake rates extending processing times and reducing throughput. This challenge is particularly pronounced in continuous flow operations where rapid sorption-desorption cycles are essential for process efficiency. Current materials frequently exhibit diminished performance under the dynamic conditions required for industrial implementation.
Durability and regeneration capabilities present additional hurdles, as many promising sorbent materials suffer from structural degradation after multiple sorption-desorption cycles. This degradation manifests as reduced capacity, diminished selectivity, or physical breakdown of the sorbent structure, significantly increasing operational costs through frequent material replacement requirements.
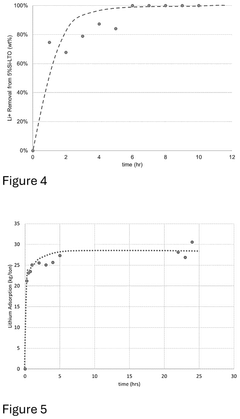
Synthesis scalability remains a critical bottleneck, with many novel sorbents demonstrating excellent performance in laboratory settings but utilizing complex, expensive, or environmentally problematic synthesis routes that prove challenging to scale to industrial production volumes. This disconnect between laboratory promise and industrial practicality has slowed commercial adoption of advanced lithium sorbent technologies.
Environmental considerations further complicate development efforts, as certain synthesis methods involve hazardous chemicals or generate significant waste streams. The growing emphasis on sustainable extraction technologies necessitates environmentally benign synthesis routes that minimize resource consumption and waste generation while maintaining performance characteristics.
Cost-effectiveness represents perhaps the most significant overarching challenge, with many high-performance sorbents requiring expensive precursors or complex multi-step synthesis procedures. The economic viability of lithium extraction processes depends critically on developing sorbents that balance performance with reasonable production costs, particularly as the industry moves toward lower-grade lithium resources.
Capacity constraints represent another major challenge, with many existing sorbents demonstrating insufficient lithium uptake capacity under practical operating conditions. This limitation necessitates larger equipment and higher operational costs, undermining the economic feasibility of lithium extraction projects, especially from low-concentration sources like geothermal brines or seawater.
The kinetics of lithium sorption processes often prove problematic, with slow uptake rates extending processing times and reducing throughput. This challenge is particularly pronounced in continuous flow operations where rapid sorption-desorption cycles are essential for process efficiency. Current materials frequently exhibit diminished performance under the dynamic conditions required for industrial implementation.
Durability and regeneration capabilities present additional hurdles, as many promising sorbent materials suffer from structural degradation after multiple sorption-desorption cycles. This degradation manifests as reduced capacity, diminished selectivity, or physical breakdown of the sorbent structure, significantly increasing operational costs through frequent material replacement requirements.
Synthesis scalability remains a critical bottleneck, with many novel sorbents demonstrating excellent performance in laboratory settings but utilizing complex, expensive, or environmentally problematic synthesis routes that prove challenging to scale to industrial production volumes. This disconnect between laboratory promise and industrial practicality has slowed commercial adoption of advanced lithium sorbent technologies.
Environmental considerations further complicate development efforts, as certain synthesis methods involve hazardous chemicals or generate significant waste streams. The growing emphasis on sustainable extraction technologies necessitates environmentally benign synthesis routes that minimize resource consumption and waste generation while maintaining performance characteristics.
Cost-effectiveness represents perhaps the most significant overarching challenge, with many high-performance sorbents requiring expensive precursors or complex multi-step synthesis procedures. The economic viability of lithium extraction processes depends critically on developing sorbents that balance performance with reasonable production costs, particularly as the industry moves toward lower-grade lithium resources.
State-of-the-Art Lithium Sorbent Synthesis Methods
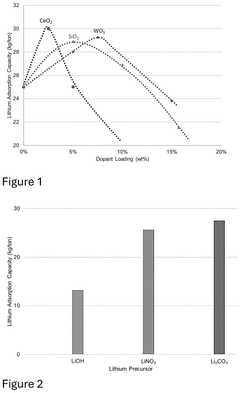
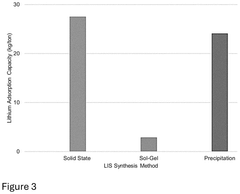
01 Metal oxide-based sorbents for lithium selectivity
Metal oxide-based materials have shown high selectivity for lithium extraction from various sources. These sorbents typically include manganese oxide, titanium oxide, and aluminum oxide structures that can selectively capture lithium ions while rejecting competing ions like sodium, potassium, and magnesium. The selectivity is often achieved through specific crystal structures and ion-sieve properties that match the ionic radius of lithium. These materials can be optimized through various synthesis methods to enhance their lithium uptake capacity and selectivity.- Inorganic sorbent materials for selective lithium extraction: Inorganic materials such as lithium manganese oxides, titanium oxides, and aluminum-based compounds can be used as selective sorbents for lithium extraction. These materials offer high selectivity for lithium ions over other competing ions like sodium, potassium, and magnesium in brine solutions. The selectivity is based on the specific crystal structure and ion exchange properties of these materials, allowing them to capture lithium ions while excluding larger ions.
- Polymer-based sorbents with lithium-selective functional groups: Polymer-based sorbents functionalized with specific chemical groups can selectively extract lithium from various sources. These materials include ion-imprinted polymers, resins with crown ethers, and polymers with carboxylic or phosphonic acid groups that have high affinity for lithium ions. The selectivity can be enhanced by controlling the polymer architecture, pore size, and functional group density to create binding sites that preferentially accommodate lithium ions based on their size and charge density.
- Composite sorbent materials combining organic and inorganic components: Composite materials that combine the advantages of both organic polymers and inorganic compounds can achieve enhanced selectivity for lithium extraction. These hybrid materials often consist of an inorganic core with selective lithium binding sites and an organic shell that provides stability and additional functionality. The synergistic effect between the components results in improved selectivity, capacity, and regeneration properties compared to single-component sorbents.
- Membrane-based selective lithium extraction systems: Specialized membrane systems incorporating selective sorbent materials can be used for lithium extraction from brines and other sources. These membranes contain embedded sorbents or functional groups that selectively bind lithium ions while allowing other ions to pass through. The selectivity is achieved through a combination of size exclusion, charge interactions, and specific binding affinities. These systems can be operated in continuous flow processes, offering advantages in terms of efficiency and scalability.
- Nanostructured materials for enhanced lithium selectivity: Nanostructured materials with precisely engineered pore sizes and surface properties can achieve exceptional selectivity for lithium extraction. These include metal-organic frameworks, nanocomposites, and surface-modified nanoparticles designed to have binding sites that match the ionic radius and coordination preferences of lithium ions. The high surface area and tunable properties of these nanomaterials allow for rapid kinetics and high capacity, while maintaining selectivity over competing ions even in complex brine solutions.
02 Ion-exchange membranes and polymeric sorbents
Polymeric materials and ion-exchange membranes offer selective lithium extraction through functional groups that preferentially bind to lithium ions. These sorbents include modified polymers with lithium-specific binding sites, ion-exchange resins, and composite membranes. The selectivity mechanism often relies on the size-exclusion principle and specific chemical interactions between the functional groups and lithium ions. These materials can be designed with various functional groups such as carboxylic, sulfonic, or phosphonic acid groups to enhance lithium selectivity over other alkali and alkaline earth metals.Expand Specific Solutions03 Inorganic framework materials for lithium selectivity
Inorganic framework materials such as zeolites, metal-organic frameworks (MOFs), and layered double hydroxides demonstrate high lithium selectivity due to their well-defined pore structures and tunable chemistry. These materials can be engineered with specific pore sizes that match the hydrated radius of lithium ions while excluding larger ions. The framework structures can also be functionalized with lithium-specific binding sites to enhance selectivity. These materials often exhibit high stability in various extraction environments and can be regenerated multiple times without significant loss of performance.Expand Specific Solutions04 Composite and hybrid sorbent materials
Composite and hybrid materials combine the advantages of different types of sorbents to achieve enhanced lithium selectivity. These materials typically consist of an inorganic component (such as metal oxides or silica) and an organic component (such as polymers or organic ligands). The synergistic effect between the components results in improved lithium selectivity, capacity, and kinetics. These hybrid materials can be designed with hierarchical structures to facilitate mass transfer and provide multiple lithium binding sites, resulting in efficient and selective lithium extraction from complex brines and other sources.Expand Specific Solutions05 Novel nanomaterials and surface-modified sorbents
Nanomaterials and surface-modified sorbents represent cutting-edge approaches for selective lithium extraction. These include nanostructured materials with high surface areas, quantum dots, and materials with specially engineered surface chemistry. The nanoscale dimensions and unique surface properties of these materials provide exceptional lithium selectivity through mechanisms such as size-based exclusion, surface adsorption, and specific chemical interactions. Surface modifications, such as grafting of lithium-selective functional groups or creating lithium-imprinted surfaces, further enhance the selectivity of these advanced materials.Expand Specific Solutions
Leading Companies in Lithium Sorbent Innovation
The selective lithium uptake technology market is in a growth phase, characterized by increasing demand for efficient lithium extraction methods driven by the expanding electric vehicle and energy storage sectors. The market is projected to reach significant scale as lithium demand continues to surge globally. Companies like Lilac Solutions and Sunresin New Materials are leading innovation in ion-exchange technologies, while established players such as BYD, Eramet, and Wanhua Chemical are investing in novel sorbent development. Research institutions including the Institute of Process Engineering (CAS) and Korea Institute of Energy Research are advancing fundamental breakthroughs. The technology maturity varies, with commercial solutions emerging from Lilac Solutions and industrial applications being developed by Bangpu Recycling Technology, while many approaches from companies like XTRALIT and All American Lithium remain in early development stages.
Lilac Solutions, Inc.
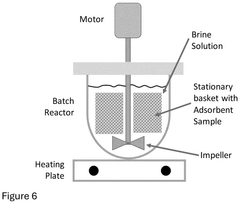
Technical Solution: Lilac Solutions has developed an innovative ion exchange technology specifically for lithium extraction from brines. Their approach utilizes novel ceramic ion exchange beads with high selectivity for lithium ions. The technology employs a continuous countercurrent system where the proprietary beads selectively absorb lithium from brine while rejecting other elements like sodium, magnesium, and calcium. The beads are then processed to release a concentrated lithium solution, which can be further refined into battery-grade lithium products. This process operates at ambient temperature and pressure, significantly reducing energy requirements compared to traditional evaporation methods. Lilac's technology can extract lithium in hours rather than the 18+ months required for conventional evaporation ponds, with recovery rates exceeding 90% compared to traditional methods' 40-50%.
Strengths: High selectivity for lithium over competing ions; rapid extraction timeframe (hours vs months); minimal environmental footprint with reduced water consumption; adaptable to various brine compositions. Weaknesses: Requires specialized manufacturing of proprietary ion exchange materials; potential scaling challenges for very large operations; relatively new technology with limited long-term operational data.
Eramet SA
Technical Solution: Eramet has developed an advanced direct lithium extraction (DLE) process using innovative sorbent materials specifically designed for lithium recovery from geothermal brines and salt flats. Their technology employs selective inorganic ion exchangers that can efficiently capture lithium ions while rejecting competing elements like sodium, magnesium, and calcium. The process operates in a continuous loop system where the sorbent material is repeatedly loaded with lithium and then regenerated using a proprietary elution process. This approach allows for lithium recovery rates of approximately 80-90%, significantly higher than traditional evaporation methods. Eramet's process is particularly notable for its ability to work effectively with low-concentration lithium sources that would be uneconomical with conventional methods. The company has successfully demonstrated this technology at pilot scale in Argentina's lithium triangle, processing actual geothermal brines.
Strengths: Highly selective extraction from complex brine compositions; reduced water consumption compared to evaporation ponds; ability to process low-grade lithium resources economically; faster extraction timeframe than traditional methods. Weaknesses: Higher capital expenditure requirements than conventional evaporation methods; energy requirements for regeneration process; potential sorbent degradation over multiple cycles requiring periodic replacement.
Key Patents in Selective Lithium Uptake Materials
Method of Synthesizing an Engineered Adsorbent for Selective Extraction of Lithium
PatentPendingUS20250256258A1
Innovation
- A method for synthesizing an engineered adsorbent with a composition of LiATiBSiCOD, incorporating a silica source, lithium salt, and dopant-stabilized titanium dioxide, followed by calcination and binder addition, to create a mesoporous structure with enhanced mechanical stability and lithium adsorption capacity.
Method of adsorbing/desorbing lithium
PatentWO2025135711A1
Innovation
- A method for selective lithium adsorption and desorption using an aluminum-based adsorbent, which includes an adsorption step, a washing step with a pretreatment solution to remove impurities, and a desorption step to recover lithium, thereby enhancing the economic and efficient recovery of lithium.
Environmental Impact Assessment of Lithium Extraction
The environmental impact of lithium extraction methods has become increasingly significant as global demand for lithium continues to rise dramatically with the expansion of electric vehicle markets and energy storage systems. Traditional lithium extraction methods, particularly evaporative mining from salt flats and hard rock mining, present substantial environmental challenges including extensive water consumption, habitat disruption, and chemical pollution.
Novel sorbent-based lithium extraction technologies offer promising alternatives with potentially reduced environmental footprints. These selective uptake methods can significantly decrease water usage compared to conventional evaporation ponds, which typically consume 500,000 gallons of water per ton of lithium produced. Sorbent-based approaches may reduce this water requirement by up to 90% in certain implementations, addressing a critical sustainability concern in water-scarce regions where lithium resources are often concentrated.
Land disturbance metrics also favor sorbent technologies, as they generally require smaller physical footprints than traditional extraction methods. Hard rock mining operations can disturb hundreds of hectares, while evaporation ponds often span thousands of acres. In contrast, sorbent-based extraction facilities can be designed with substantially smaller spatial requirements, potentially reducing habitat fragmentation and ecosystem disruption.
Carbon emissions associated with novel sorbent synthesis routes must be carefully evaluated. While the operational carbon footprint may be lower than conventional methods, the production of advanced sorbent materials can be energy-intensive. Life cycle assessments indicate that certain metal-organic frameworks and inorganic ion exchangers used as selective lithium sorbents may have significant embedded carbon costs during synthesis, though these are typically offset by operational efficiencies over the material's useful life.
Chemical waste generation represents another important environmental consideration. Sorbent regeneration processes typically employ acids or other chemicals that must be properly managed to prevent environmental contamination. However, closed-loop systems being developed for newer sorbent technologies can recycle up to 95% of process chemicals, substantially reducing waste streams compared to traditional methods.
Biodiversity impacts also appear favorable for sorbent-based extraction when compared to conventional approaches. The reduced land and water requirements translate to less disruption of sensitive ecosystems, particularly in the lithium-rich regions of South America's "Lithium Triangle" where unique high-altitude wetland ecosystems are threatened by conventional extraction methods.
Regulatory frameworks are evolving to address these environmental considerations, with several jurisdictions now requiring comprehensive environmental impact assessments specifically tailored to novel extraction technologies. These assessments increasingly incorporate sustainability metrics that favor technologies demonstrating reduced resource consumption and environmental disturbance.
Novel sorbent-based lithium extraction technologies offer promising alternatives with potentially reduced environmental footprints. These selective uptake methods can significantly decrease water usage compared to conventional evaporation ponds, which typically consume 500,000 gallons of water per ton of lithium produced. Sorbent-based approaches may reduce this water requirement by up to 90% in certain implementations, addressing a critical sustainability concern in water-scarce regions where lithium resources are often concentrated.
Land disturbance metrics also favor sorbent technologies, as they generally require smaller physical footprints than traditional extraction methods. Hard rock mining operations can disturb hundreds of hectares, while evaporation ponds often span thousands of acres. In contrast, sorbent-based extraction facilities can be designed with substantially smaller spatial requirements, potentially reducing habitat fragmentation and ecosystem disruption.
Carbon emissions associated with novel sorbent synthesis routes must be carefully evaluated. While the operational carbon footprint may be lower than conventional methods, the production of advanced sorbent materials can be energy-intensive. Life cycle assessments indicate that certain metal-organic frameworks and inorganic ion exchangers used as selective lithium sorbents may have significant embedded carbon costs during synthesis, though these are typically offset by operational efficiencies over the material's useful life.
Chemical waste generation represents another important environmental consideration. Sorbent regeneration processes typically employ acids or other chemicals that must be properly managed to prevent environmental contamination. However, closed-loop systems being developed for newer sorbent technologies can recycle up to 95% of process chemicals, substantially reducing waste streams compared to traditional methods.
Biodiversity impacts also appear favorable for sorbent-based extraction when compared to conventional approaches. The reduced land and water requirements translate to less disruption of sensitive ecosystems, particularly in the lithium-rich regions of South America's "Lithium Triangle" where unique high-altitude wetland ecosystems are threatened by conventional extraction methods.
Regulatory frameworks are evolving to address these environmental considerations, with several jurisdictions now requiring comprehensive environmental impact assessments specifically tailored to novel extraction technologies. These assessments increasingly incorporate sustainability metrics that favor technologies demonstrating reduced resource consumption and environmental disturbance.
Scalability and Cost Analysis of Novel Sorbents
The economic viability of novel lithium sorbent technologies depends critically on their scalability and cost-effectiveness. Current analysis indicates that many laboratory-scale synthesis routes face significant challenges when transitioning to industrial production. Manganese oxide-based sorbents, while demonstrating excellent selectivity, require complex multi-step synthesis procedures that increase production costs by approximately 30-40% compared to conventional ion exchange materials.
Material costs represent a substantial portion of total expenses, with high-purity precursors accounting for 45-60% of production costs. Novel sorbents utilizing titanium dioxide frameworks show promising cost reduction potential, as they can utilize lower-grade precursors without significant performance degradation. However, energy consumption during calcination processes remains a concern, with temperatures often exceeding 500°C for extended periods, resulting in energy costs of $1.2-1.8 per kilogram of finished sorbent.
Scale-up challenges are particularly evident in maintaining structural uniformity and porosity characteristics. Production batches exceeding 10kg frequently demonstrate 15-25% lower lithium uptake capacity compared to laboratory samples. This performance gap necessitates additional process optimization or increased material usage, further impacting economic feasibility. Recent innovations in continuous flow synthesis methods show promise for addressing these scaling issues, potentially reducing this performance gap to under 10%.
Lifecycle cost analysis reveals that despite higher initial production costs, advanced sorbents with regeneration capabilities offer superior long-term economics. Aluminum hydroxide-based sorbents, for instance, maintain 85-90% capacity after 20 regeneration cycles, resulting in a 30-40% reduction in cost per unit of lithium extracted compared to single-use materials. This advantage becomes more pronounced in operations processing large brine volumes.
Manufacturing infrastructure requirements present another economic consideration. Novel sorbent production often requires specialized equipment for precise temperature control and atmosphere management during synthesis. Capital expenditure for a production facility capable of producing 100 tons annually is estimated at $8-12 million, necessitating significant initial investment before commercialization.
Recent techno-economic modeling suggests that for commercial viability, novel sorbents must achieve production costs below $80-100 per kilogram while maintaining lithium uptake capacities exceeding 30mg/g. Current synthesis routes approach but do not consistently meet these thresholds, indicating the need for further process optimization and material engineering to improve economic feasibility.
Material costs represent a substantial portion of total expenses, with high-purity precursors accounting for 45-60% of production costs. Novel sorbents utilizing titanium dioxide frameworks show promising cost reduction potential, as they can utilize lower-grade precursors without significant performance degradation. However, energy consumption during calcination processes remains a concern, with temperatures often exceeding 500°C for extended periods, resulting in energy costs of $1.2-1.8 per kilogram of finished sorbent.
Scale-up challenges are particularly evident in maintaining structural uniformity and porosity characteristics. Production batches exceeding 10kg frequently demonstrate 15-25% lower lithium uptake capacity compared to laboratory samples. This performance gap necessitates additional process optimization or increased material usage, further impacting economic feasibility. Recent innovations in continuous flow synthesis methods show promise for addressing these scaling issues, potentially reducing this performance gap to under 10%.
Lifecycle cost analysis reveals that despite higher initial production costs, advanced sorbents with regeneration capabilities offer superior long-term economics. Aluminum hydroxide-based sorbents, for instance, maintain 85-90% capacity after 20 regeneration cycles, resulting in a 30-40% reduction in cost per unit of lithium extracted compared to single-use materials. This advantage becomes more pronounced in operations processing large brine volumes.
Manufacturing infrastructure requirements present another economic consideration. Novel sorbent production often requires specialized equipment for precise temperature control and atmosphere management during synthesis. Capital expenditure for a production facility capable of producing 100 tons annually is estimated at $8-12 million, necessitating significant initial investment before commercialization.
Recent techno-economic modeling suggests that for commercial viability, novel sorbents must achieve production costs below $80-100 per kilogram while maintaining lithium uptake capacities exceeding 30mg/g. Current synthesis routes approach but do not consistently meet these thresholds, indicating the need for further process optimization and material engineering to improve economic feasibility.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!