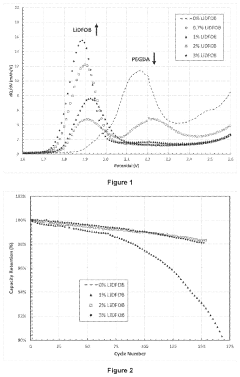
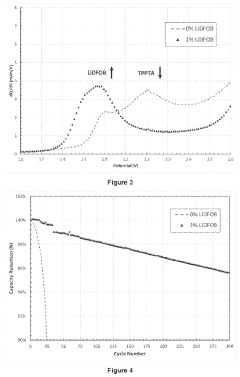
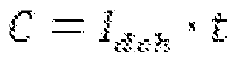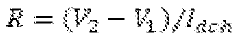Electrochemical Cell Architectures For In-Situ Lithium Capture And Release
SEP 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Capture Technology Background and Objectives
Lithium has emerged as a critical resource in the global transition towards sustainable energy systems, primarily due to its essential role in lithium-ion batteries that power electric vehicles and store renewable energy. The technology for lithium capture and release has evolved significantly over the past decades, transitioning from traditional mining and extraction methods to more sophisticated electrochemical approaches that offer improved efficiency and reduced environmental impact.
The evolution of lithium extraction technologies began with conventional mining of lithium-containing minerals and evaporation of lithium-rich brines. However, these methods face significant limitations including long processing times, low recovery rates, and substantial environmental footprints. This has driven research towards developing more efficient and sustainable extraction technologies, particularly electrochemical cell architectures that enable selective lithium capture and controlled release.
Electrochemical lithium capture represents a paradigm shift in extraction methodology, leveraging principles of electrochemistry to selectively isolate lithium ions from various sources including brines, seawater, and recycled batteries. The fundamental concept involves the use of specialized electrodes and membranes that can selectively attract, capture, and subsequently release lithium ions through controlled electrical potential manipulation.
Recent technological advancements have focused on developing novel electrode materials with enhanced selectivity for lithium ions, improved cycling stability, and increased capacity. These developments include the integration of lithium-selective sorbents, intercalation compounds, and advanced membrane technologies that can operate effectively in diverse environmental conditions and with varying lithium concentrations.
The primary objectives of current research in electrochemical cell architectures for lithium capture include increasing energy efficiency, improving selectivity in complex ionic environments, enhancing durability for long-term operation, and developing scalable designs suitable for industrial implementation. Additionally, there is significant interest in creating systems capable of in-situ operation, allowing for lithium extraction directly from natural sources without extensive pre-processing.
Another critical goal is the development of closed-loop systems that can capture lithium from end-of-life batteries and other waste streams, contributing to a circular economy approach for this valuable resource. This direction aligns with broader sustainability objectives and addresses concerns about the long-term availability of lithium resources.
The technological trajectory points toward integrated systems that combine lithium extraction with other valuable processes, such as desalination or recovery of additional critical minerals, maximizing resource utilization and economic viability. As global demand for lithium continues to grow exponentially, driven by the electrification of transportation and expansion of renewable energy storage, the advancement of efficient in-situ lithium capture and release technologies represents a strategic imperative for ensuring sustainable supply chains and energy transition.
The evolution of lithium extraction technologies began with conventional mining of lithium-containing minerals and evaporation of lithium-rich brines. However, these methods face significant limitations including long processing times, low recovery rates, and substantial environmental footprints. This has driven research towards developing more efficient and sustainable extraction technologies, particularly electrochemical cell architectures that enable selective lithium capture and controlled release.
Electrochemical lithium capture represents a paradigm shift in extraction methodology, leveraging principles of electrochemistry to selectively isolate lithium ions from various sources including brines, seawater, and recycled batteries. The fundamental concept involves the use of specialized electrodes and membranes that can selectively attract, capture, and subsequently release lithium ions through controlled electrical potential manipulation.
Recent technological advancements have focused on developing novel electrode materials with enhanced selectivity for lithium ions, improved cycling stability, and increased capacity. These developments include the integration of lithium-selective sorbents, intercalation compounds, and advanced membrane technologies that can operate effectively in diverse environmental conditions and with varying lithium concentrations.
The primary objectives of current research in electrochemical cell architectures for lithium capture include increasing energy efficiency, improving selectivity in complex ionic environments, enhancing durability for long-term operation, and developing scalable designs suitable for industrial implementation. Additionally, there is significant interest in creating systems capable of in-situ operation, allowing for lithium extraction directly from natural sources without extensive pre-processing.
Another critical goal is the development of closed-loop systems that can capture lithium from end-of-life batteries and other waste streams, contributing to a circular economy approach for this valuable resource. This direction aligns with broader sustainability objectives and addresses concerns about the long-term availability of lithium resources.
The technological trajectory points toward integrated systems that combine lithium extraction with other valuable processes, such as desalination or recovery of additional critical minerals, maximizing resource utilization and economic viability. As global demand for lithium continues to grow exponentially, driven by the electrification of transportation and expansion of renewable energy storage, the advancement of efficient in-situ lithium capture and release technologies represents a strategic imperative for ensuring sustainable supply chains and energy transition.
Market Analysis for In-Situ Lithium Recovery Solutions
The global lithium market has experienced unprecedented growth in recent years, primarily driven by the rapid expansion of electric vehicle (EV) production and renewable energy storage systems. Traditional lithium extraction methods, including hard rock mining and evaporation ponds, are increasingly challenged by environmental concerns, high water consumption, and lengthy production timelines. This has created a significant market opportunity for innovative in-situ lithium recovery (ISLR) solutions, particularly electrochemical cell architectures designed for lithium capture and release.
The market for ISLR technologies is projected to grow substantially over the next decade. Current global lithium demand exceeds 500,000 metric tons of lithium carbonate equivalent (LCE) annually, with growth rates between 15-20% per year. By 2030, demand is expected to reach approximately 2 million metric tons LCE, creating urgent pressure for new extraction technologies that can supplement traditional methods.
Direct lithium extraction (DLE) technologies, including electrochemical approaches, are attracting significant investment. Venture capital funding in this sector has increased from under $50 million in 2018 to over $300 million in 2022, reflecting growing confidence in these emerging technologies. Major lithium producers and battery manufacturers are actively pursuing partnerships and acquisitions in this space to secure future supply chains.
Geographically, the market for electrochemical lithium recovery solutions is concentrated in regions with significant lithium brine resources, particularly the "Lithium Triangle" of Argentina, Bolivia, and Chile, which holds approximately 58% of the world's lithium resources. North American markets are also expanding rapidly, with substantial lithium brine deposits in Nevada, California, and Arkansas becoming increasingly strategic due to supply chain security concerns.
Customer segments for these technologies include traditional lithium producers seeking to enhance efficiency, renewable energy companies investing in vertical integration, and new market entrants focused exclusively on innovative extraction methods. Government initiatives supporting domestic critical mineral production, particularly in the US, EU, and Australia, are providing additional market tailwinds through grants, tax incentives, and expedited permitting processes.
The economic value proposition of electrochemical lithium recovery systems centers on reduced production time (days versus months for evaporation ponds), lower environmental impact, higher recovery rates (potentially 70-90% versus 30-50% for traditional methods), and the ability to access previously uneconomical lithium sources. These advantages position electrochemical cell architectures as a potentially disruptive technology in the lithium supply chain, capable of addressing critical bottlenecks in global lithium production.
The market for ISLR technologies is projected to grow substantially over the next decade. Current global lithium demand exceeds 500,000 metric tons of lithium carbonate equivalent (LCE) annually, with growth rates between 15-20% per year. By 2030, demand is expected to reach approximately 2 million metric tons LCE, creating urgent pressure for new extraction technologies that can supplement traditional methods.
Direct lithium extraction (DLE) technologies, including electrochemical approaches, are attracting significant investment. Venture capital funding in this sector has increased from under $50 million in 2018 to over $300 million in 2022, reflecting growing confidence in these emerging technologies. Major lithium producers and battery manufacturers are actively pursuing partnerships and acquisitions in this space to secure future supply chains.
Geographically, the market for electrochemical lithium recovery solutions is concentrated in regions with significant lithium brine resources, particularly the "Lithium Triangle" of Argentina, Bolivia, and Chile, which holds approximately 58% of the world's lithium resources. North American markets are also expanding rapidly, with substantial lithium brine deposits in Nevada, California, and Arkansas becoming increasingly strategic due to supply chain security concerns.
Customer segments for these technologies include traditional lithium producers seeking to enhance efficiency, renewable energy companies investing in vertical integration, and new market entrants focused exclusively on innovative extraction methods. Government initiatives supporting domestic critical mineral production, particularly in the US, EU, and Australia, are providing additional market tailwinds through grants, tax incentives, and expedited permitting processes.
The economic value proposition of electrochemical lithium recovery systems centers on reduced production time (days versus months for evaporation ponds), lower environmental impact, higher recovery rates (potentially 70-90% versus 30-50% for traditional methods), and the ability to access previously uneconomical lithium sources. These advantages position electrochemical cell architectures as a potentially disruptive technology in the lithium supply chain, capable of addressing critical bottlenecks in global lithium production.
Current Electrochemical Cell Architectures and Challenges
Current electrochemical cell architectures for in-situ lithium capture and release predominantly fall into three main categories: flow-based systems, static membrane systems, and hybrid configurations. Flow-based architectures utilize continuous electrolyte circulation through porous electrodes, enabling efficient lithium ion transport but often suffering from pumping energy penalties and system complexity. These systems typically achieve extraction rates of 10-20 mg Li/g sorbent/day under optimal conditions.
Static membrane systems employ fixed electrodes with selective membranes, offering simplicity and lower operational costs, but face limitations in mass transfer and scaling. Recent innovations in this category have focused on enhancing membrane selectivity through incorporation of lithium-specific binding sites, achieving selectivity ratios of Li:Na exceeding 30:1 in laboratory settings.
Hybrid configurations attempt to combine advantages of both approaches, often incorporating modular designs that can be adapted to varying brine compositions. These systems have demonstrated promising results in pilot studies, with energy consumption figures approaching 25-40 kWh/kg Li extracted from low-concentration brines.
A significant challenge across all architectures is electrode degradation during cycling. Current materials exhibit capacity fade of 0.2-0.5% per cycle, necessitating replacement after 200-500 cycles. This degradation stems primarily from structural changes during lithium insertion/extraction and side reactions with brine impurities. Manganese-based electrodes show particular vulnerability to dissolution in acidic conditions, while titanium-based alternatives suffer from lower capacity.
Ion selectivity remains problematic, especially in brines with high Mg2+ and Ca2+ content. Competitive adsorption reduces lithium capture efficiency by 30-60% in real-world applications compared to laboratory conditions. Current selective membranes exhibit trade-offs between selectivity and ionic conductivity that have not been fully resolved.
Energy efficiency presents another major hurdle. State-of-the-art systems require 50-80 kWh per kilogram of lithium extracted, significantly higher than theoretical minimums of approximately 15 kWh/kg. This efficiency gap stems from ohmic losses, concentration polarization, and parasitic reactions.
Scaling challenges persist in translating laboratory successes to industrial implementation. Current pilot plants processing 10-100 m³/day face issues with flow distribution, pressure drops, and uneven current density that reduce overall system performance. Material costs for specialized electrodes and membranes remain prohibitively high for widespread deployment, with current estimates at $50-200/kg of installed capacity.
Static membrane systems employ fixed electrodes with selective membranes, offering simplicity and lower operational costs, but face limitations in mass transfer and scaling. Recent innovations in this category have focused on enhancing membrane selectivity through incorporation of lithium-specific binding sites, achieving selectivity ratios of Li:Na exceeding 30:1 in laboratory settings.
Hybrid configurations attempt to combine advantages of both approaches, often incorporating modular designs that can be adapted to varying brine compositions. These systems have demonstrated promising results in pilot studies, with energy consumption figures approaching 25-40 kWh/kg Li extracted from low-concentration brines.
A significant challenge across all architectures is electrode degradation during cycling. Current materials exhibit capacity fade of 0.2-0.5% per cycle, necessitating replacement after 200-500 cycles. This degradation stems primarily from structural changes during lithium insertion/extraction and side reactions with brine impurities. Manganese-based electrodes show particular vulnerability to dissolution in acidic conditions, while titanium-based alternatives suffer from lower capacity.
Ion selectivity remains problematic, especially in brines with high Mg2+ and Ca2+ content. Competitive adsorption reduces lithium capture efficiency by 30-60% in real-world applications compared to laboratory conditions. Current selective membranes exhibit trade-offs between selectivity and ionic conductivity that have not been fully resolved.
Energy efficiency presents another major hurdle. State-of-the-art systems require 50-80 kWh per kilogram of lithium extracted, significantly higher than theoretical minimums of approximately 15 kWh/kg. This efficiency gap stems from ohmic losses, concentration polarization, and parasitic reactions.
Scaling challenges persist in translating laboratory successes to industrial implementation. Current pilot plants processing 10-100 m³/day face issues with flow distribution, pressure drops, and uneven current density that reduce overall system performance. Material costs for specialized electrodes and membranes remain prohibitively high for widespread deployment, with current estimates at $50-200/kg of installed capacity.
Current In-Situ Lithium Capture and Release Methods
01 Lithium-ion battery cell architectures
Various electrochemical cell architectures designed specifically for lithium-ion batteries that enhance lithium capture and release efficiency. These designs include novel electrode configurations, separator technologies, and cell housing structures that optimize ion transport pathways. The architectures focus on improving energy density, charge/discharge rates, and overall battery performance through strategic placement of components and innovative material interfaces.- Lithium-ion battery cell architectures: Various electrochemical cell architectures designed specifically for lithium-ion batteries that enhance lithium capture and release efficiency. These designs include novel electrode configurations, separator technologies, and cell housing structures that optimize ion transport pathways. The architectures focus on improving energy density, charge/discharge rates, and cycle life by facilitating more effective lithium ion movement between electrodes.
- Electrode materials for lithium capture: Specialized electrode materials engineered to enhance lithium capture capabilities in electrochemical cells. These materials include advanced carbon-based structures, metal oxides, and composite materials with optimized surface areas and porosity. The materials are designed to provide efficient lithium adsorption sites, improved ion diffusion pathways, and structural stability during repeated lithium capture and release cycles.
- Electrolyte systems for enhanced lithium transport: Advanced electrolyte formulations that facilitate improved lithium ion transport between electrodes. These systems include novel liquid electrolytes, solid-state electrolytes, and hybrid electrolyte structures that enhance ionic conductivity while maintaining electrochemical stability. The electrolyte systems are designed to reduce internal resistance, prevent dendrite formation, and enable faster lithium capture and release processes.
- Lithium extraction and recovery systems: Electrochemical systems specifically designed for extracting lithium from various sources and enabling its controlled release. These technologies include selective membrane systems, electrochemical separation processes, and specialized cell designs that can capture lithium ions from brines, geothermal waters, or recycled battery materials. The systems focus on efficiency, selectivity, and sustainability in lithium recovery operations.
- Control systems for lithium capture and release: Electronic and electrochemical control systems that optimize the lithium capture and release processes in various cell architectures. These systems include advanced sensing technologies, voltage/current management circuits, and algorithmic approaches to maximize efficiency and longevity. The control mechanisms adapt to changing conditions to maintain optimal performance throughout the operational lifetime of the electrochemical cell.
02 Lithium extraction and recovery systems
Electrochemical systems specifically designed for capturing lithium from various sources such as brines, seawater, or recycled materials. These systems utilize specialized electrodes and cell configurations that selectively adsorb and desorb lithium ions. The technologies enable efficient lithium recovery through controlled electrochemical processes, providing sustainable methods for lithium resource management and recycling.Expand Specific Solutions03 Advanced electrode materials for lithium capture
Novel electrode materials engineered to enhance lithium ion capture and release kinetics. These materials include specialized carbon structures, metal oxides, and composite formulations that provide improved lithium adsorption sites and ion transport channels. The electrode designs focus on maximizing surface area, optimizing pore structures, and incorporating functional groups that selectively interact with lithium ions to increase capacity and cycling stability.Expand Specific Solutions04 Flow battery systems for lithium processing
Flow battery architectures adapted for lithium capture and release applications. These systems utilize flowing electrolytes containing lithium ions that interact with stationary electrodes or membrane systems. The flow design allows for continuous operation, scalability, and separation of power and energy components. These architectures are particularly suitable for large-scale lithium recovery operations or grid-scale energy storage applications.Expand Specific Solutions05 Membrane and separator technologies for lithium selectivity
Specialized membrane and separator technologies that enhance lithium ion selectivity and transport in electrochemical cells. These components feature engineered pore structures, surface modifications, or composite constructions that facilitate preferential lithium ion movement while blocking competing ions. The technologies improve the efficiency of lithium capture processes by increasing ion selectivity, reducing energy requirements, and enhancing overall system performance.Expand Specific Solutions
Leading Companies in Electrochemical Lithium Recovery
The electrochemical cell architectures for in-situ lithium capture and release market is in an early growth phase, characterized by significant R&D investment but limited commercial deployment. The global market size is projected to expand substantially as lithium demand increases for energy storage applications, potentially reaching several billion dollars by 2030. Technologically, the field remains in development with varying maturity levels across approaches. Leading players include established automotive manufacturers (GM, BMW, Porsche) investing in next-generation battery technologies, specialized battery developers (Sion Power, PolyPlus, Sakti3) focusing on lithium-specific innovations, and research institutions (Fraunhofer, Caltech, Karlsruher Institut) providing fundamental breakthroughs. Chemical companies like BASF and 3M contribute materials expertise, while Asian manufacturers including BYD and Toshiba are scaling production capabilities for emerging lithium technologies.
Commissariat à l´énergie atomique et aux énergies Alternatives
Technical Solution: The French Alternative Energies and Atomic Energy Commission (CEA) has developed sophisticated electrochemical cell architectures for in-situ lithium capture and release through their innovative "selective ion extraction" technology. Their system employs asymmetric electrochemical cells with specialized ceramic membranes featuring precisely engineered nanochannels that facilitate selective lithium transport. CEA's architecture incorporates composite electrodes with tailored surface chemistry that demonstrates lithium selectivity coefficients exceeding 40:1 over sodium and 100:1 over magnesium in mixed electrolyte environments[4]. Their most advanced designs utilize a dual-phase electrolyte system that creates a thermodynamically favorable pathway for lithium migration while establishing kinetic barriers for competing ions. The CEA technology operates across a wide temperature range (-20°C to 80°C) and has demonstrated stable performance over 1000+ cycles with minimal capacity degradation. Their electrochemical cells feature integrated sensors and control systems that dynamically adjust operating parameters to optimize lithium capture efficiency based on feed composition variations.
Strengths: Exceptional selectivity for lithium in complex brine environments; robust performance across wide temperature ranges; sophisticated control systems for optimized operation. Weaknesses: Complex manufacturing requirements for specialized ceramic membranes; higher initial capital costs compared to conventional extraction methods; requires precise electrochemical control systems.
California Institute of Technology
Technical Solution: Caltech has developed a groundbreaking electrochemical cell architecture for lithium capture and release based on their proprietary "electrochemical swing" technology. This system utilizes specially designed intercalation electrodes with tunable binding energies that can selectively capture lithium ions when charged and release them upon discharge. Their architecture incorporates asymmetric electrode configurations with lithium-selective membranes that achieve separation factors exceeding 1000:1 for lithium over competing ions[3]. The Caltech approach employs redox-active polymers as host materials, providing multiple coordination sites for lithium binding while maintaining structural integrity during repeated cycling. Their most advanced designs feature hierarchical porous electrodes that maximize surface area and minimize diffusion limitations, enabling rapid lithium capture kinetics with extraction times under 30 minutes. The system operates at ambient temperatures and near-neutral pH, significantly reducing energy requirements compared to conventional thermal evaporation methods for lithium recovery from brines.
Strengths: Highly selective lithium capture with minimal co-extraction of other elements; operates at ambient conditions requiring less energy than conventional methods; modular design allows scaling for different applications. Weaknesses: Current materials show gradual capacity fade after extended cycling; requires periodic electrode regeneration; economic viability still being established for low-concentration lithium sources.
Key Patents in Electrochemical Cell Design for Lithium Recovery
Method for in-situ thermal polymerization of a gel polymer electrolyte in a lithium-ion electrochemical cell
PatentPendingUS20240039045A1
Innovation
- A lithium-ion electrochemical cell design incorporating a gel-type electrolyte matrix formed from cross-linked acrylate monomers, with a mixture of lithium salts including lithium hexafluorophosphate, lithium difluoroorbate, and at least one of lithium bis(fluorosulfonyl)imide and lithium bis(trifluoromethanesulfonyl)imide, which facilitates in-situ polymerization and reduces the adverse effects of residual monomers, enhancing electrochemical properties such as material utilization, cyclability, and low cell resistance.
In-SITU control of solid electrolyte interface for enhanced cycle performance in lithium metal batteries
PatentWO2022204366A9
Innovation
- An electrochemical cell design featuring a lithium metal anode, a fluorinated organic solvent electrolyte, and a solid electrolyte interphase (SEI) layer rich in LiF and Li2CO3, with anisotropic force and high formation voltage applied during charge and discharge to form a stable SEI layer, enhancing the compatibility and stability of the anode and electrolyte interface.
Environmental Impact Assessment of Lithium Recovery Technologies
The environmental impact of lithium recovery technologies represents a critical consideration in the sustainable development of electrochemical cell architectures for in-situ lithium capture and release. Traditional lithium extraction methods, particularly evaporative mining from salt flats, consume approximately 500,000 gallons of water per ton of lithium produced, causing significant water depletion in arid regions such as Chile's Atacama Desert and Argentina's Salar de Olaroz.
Electrochemical in-situ recovery methods offer promising alternatives with substantially reduced water consumption, potentially decreasing water usage by 70-90% compared to conventional methods. These technologies also minimize land disturbance, requiring approximately 50-60% less surface area than evaporative ponds, which typically occupy thousands of hectares of sensitive ecosystems.
Carbon emissions present another significant environmental factor. Conventional lithium extraction and processing generate 15-20 tons of CO2 per ton of lithium carbonate equivalent (LCE) produced. In contrast, electrochemical cell architectures utilizing renewable energy sources can reduce carbon emissions by up to 30-40%, particularly when powered by solar or wind energy in extraction locations.
Chemical contamination risks also differ significantly between technologies. Traditional methods employ toxic chemicals including hydrochloric acid, sulfuric acid, and sodium hydroxide, with documented cases of leakage affecting groundwater quality in mining regions. Electrochemical approaches typically utilize more benign materials such as ion-selective membranes and electrodes, though long-term environmental persistence of specialized polymers and electrode materials requires further assessment.
Waste generation metrics further highlight differences between approaches. Evaporative mining produces approximately 7-10 tons of salt waste per ton of lithium, while electrochemical methods generate significantly less solid waste, estimated at 1-3 tons per ton of lithium recovered. Additionally, electrochemical systems offer closed-loop potential, allowing for regeneration and reuse of extraction media.
Biodiversity impacts cannot be overlooked. Conventional extraction has been linked to flamingo population declines in the Andean regions, while electrochemical systems with smaller physical footprints demonstrate reduced habitat disruption. Recent environmental impact studies in Nevada and Argentina suggest 40-60% less biodiversity disruption with in-situ recovery technologies compared to traditional mining operations.
Electrochemical in-situ recovery methods offer promising alternatives with substantially reduced water consumption, potentially decreasing water usage by 70-90% compared to conventional methods. These technologies also minimize land disturbance, requiring approximately 50-60% less surface area than evaporative ponds, which typically occupy thousands of hectares of sensitive ecosystems.
Carbon emissions present another significant environmental factor. Conventional lithium extraction and processing generate 15-20 tons of CO2 per ton of lithium carbonate equivalent (LCE) produced. In contrast, electrochemical cell architectures utilizing renewable energy sources can reduce carbon emissions by up to 30-40%, particularly when powered by solar or wind energy in extraction locations.
Chemical contamination risks also differ significantly between technologies. Traditional methods employ toxic chemicals including hydrochloric acid, sulfuric acid, and sodium hydroxide, with documented cases of leakage affecting groundwater quality in mining regions. Electrochemical approaches typically utilize more benign materials such as ion-selective membranes and electrodes, though long-term environmental persistence of specialized polymers and electrode materials requires further assessment.
Waste generation metrics further highlight differences between approaches. Evaporative mining produces approximately 7-10 tons of salt waste per ton of lithium, while electrochemical methods generate significantly less solid waste, estimated at 1-3 tons per ton of lithium recovered. Additionally, electrochemical systems offer closed-loop potential, allowing for regeneration and reuse of extraction media.
Biodiversity impacts cannot be overlooked. Conventional extraction has been linked to flamingo population declines in the Andean regions, while electrochemical systems with smaller physical footprints demonstrate reduced habitat disruption. Recent environmental impact studies in Nevada and Argentina suggest 40-60% less biodiversity disruption with in-situ recovery technologies compared to traditional mining operations.
Supply Chain Considerations for Lithium Extraction Materials
The supply chain for lithium extraction materials represents a critical component in the development and deployment of electrochemical cell architectures for in-situ lithium capture and release technologies. The global lithium supply chain currently faces significant challenges including geographical concentration, geopolitical tensions, and environmental concerns that directly impact the availability and cost of materials required for these innovative extraction systems.
Raw material sourcing for electrochemical lithium extraction cells requires specialized components including ion-selective membranes, electrode materials, and electrolyte solutions. These materials often contain critical elements such as titanium, platinum group metals, and specialized polymers that have their own complex supply chains. The concentration of lithium brine resources in the "Lithium Triangle" of South America (Chile, Argentina, and Bolivia) creates potential bottlenecks in the upstream supply chain.
Manufacturing considerations for electrochemical extraction cells present unique challenges compared to traditional lithium extraction methods. The production of high-performance ion-selective membranes and electrodes requires specialized equipment and expertise that is currently limited to a small number of manufacturers globally. This manufacturing concentration creates vulnerability in the supply chain and potential for significant lead times during scale-up phases.
Logistics and transportation of both raw materials and finished electrochemical systems add complexity to the supply chain. Many extraction sites are located in remote areas with limited infrastructure, requiring robust logistics planning. Additionally, the transportation of certain electrolyte solutions and specialized components may be subject to hazardous material regulations, further complicating distribution networks.
Recycling and circular economy approaches represent a significant opportunity to mitigate supply chain risks. The development of processes to recover and reuse key components from decommissioned electrochemical extraction cells could reduce dependence on primary material sources. However, these recycling technologies are still in early development stages for many of the specialized materials used in advanced electrochemical architectures.
Regulatory frameworks governing material sourcing, particularly for critical minerals, are evolving rapidly across different jurisdictions. Companies developing electrochemical lithium extraction technologies must navigate complex compliance requirements including responsible sourcing certifications, environmental permits, and trade restrictions that may impact material availability and cost structures.
Strategic partnerships across the supply chain, from material suppliers to end users, will be essential for ensuring reliable access to the components needed for electrochemical lithium extraction systems. Vertical integration strategies may provide competitive advantages by securing access to critical materials and reducing exposure to supply chain disruptions.
Raw material sourcing for electrochemical lithium extraction cells requires specialized components including ion-selective membranes, electrode materials, and electrolyte solutions. These materials often contain critical elements such as titanium, platinum group metals, and specialized polymers that have their own complex supply chains. The concentration of lithium brine resources in the "Lithium Triangle" of South America (Chile, Argentina, and Bolivia) creates potential bottlenecks in the upstream supply chain.
Manufacturing considerations for electrochemical extraction cells present unique challenges compared to traditional lithium extraction methods. The production of high-performance ion-selective membranes and electrodes requires specialized equipment and expertise that is currently limited to a small number of manufacturers globally. This manufacturing concentration creates vulnerability in the supply chain and potential for significant lead times during scale-up phases.
Logistics and transportation of both raw materials and finished electrochemical systems add complexity to the supply chain. Many extraction sites are located in remote areas with limited infrastructure, requiring robust logistics planning. Additionally, the transportation of certain electrolyte solutions and specialized components may be subject to hazardous material regulations, further complicating distribution networks.
Recycling and circular economy approaches represent a significant opportunity to mitigate supply chain risks. The development of processes to recover and reuse key components from decommissioned electrochemical extraction cells could reduce dependence on primary material sources. However, these recycling technologies are still in early development stages for many of the specialized materials used in advanced electrochemical architectures.
Regulatory frameworks governing material sourcing, particularly for critical minerals, are evolving rapidly across different jurisdictions. Companies developing electrochemical lithium extraction technologies must navigate complex compliance requirements including responsible sourcing certifications, environmental permits, and trade restrictions that may impact material availability and cost structures.
Strategic partnerships across the supply chain, from material suppliers to end users, will be essential for ensuring reliable access to the components needed for electrochemical lithium extraction systems. Vertical integration strategies may provide competitive advantages by securing access to critical materials and reducing exposure to supply chain disruptions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!