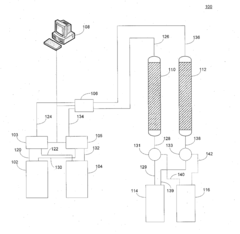
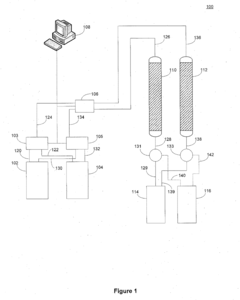
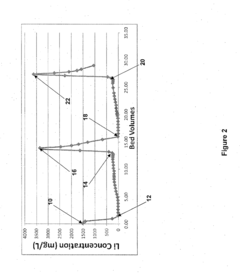
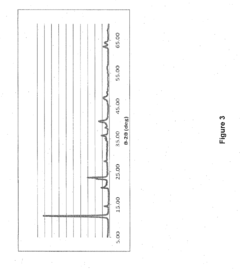
Optimizing Sorbent Selectivity For Low-Concentration Geothermal Lithium Streams
SEP 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Geothermal Lithium Extraction Background and Objectives
Lithium has emerged as a critical resource in the global transition to clean energy, primarily due to its essential role in rechargeable batteries for electric vehicles and energy storage systems. Traditional lithium extraction methods have predominantly relied on hard rock mining and solar evaporation of salt flats, both of which present significant environmental challenges and resource limitations. In this context, geothermal lithium extraction represents a promising alternative that aligns with sustainable development goals.
Geothermal brines, found in underground reservoirs associated with geothermal energy production, contain varying concentrations of dissolved lithium, typically ranging from 10 to 200 mg/L. These concentrations, while lower than those in traditional brine deposits, represent a substantial untapped resource that could significantly contribute to meeting the growing global demand for lithium, projected to increase by 400-500% by 2030.
The evolution of geothermal lithium extraction technology has progressed through several key phases since initial research began in the 1970s. Early efforts focused primarily on understanding the geochemistry of lithium in geothermal systems. The 2000s saw increased interest in direct lithium extraction (DLE) methods, with significant advancements in sorbent technologies and membrane processes. Recent years have witnessed accelerated development, driven by the urgent need for sustainable lithium sources to support the clean energy transition.
The primary technical objective in optimizing sorbent selectivity for low-concentration geothermal lithium streams is to develop materials that can efficiently and selectively capture lithium ions from complex brine solutions containing numerous competing ions, particularly sodium, potassium, calcium, and magnesium. This selectivity is crucial given the relatively low lithium concentrations in geothermal brines compared to other dissolved minerals.
Additional objectives include developing sorbents with rapid kinetics to enable efficient processing of large brine volumes, creating materials with high durability that can withstand repeated adsorption-desorption cycles in harsh geothermal environments, and ensuring economic viability through cost-effective manufacturing processes and materials.
The technological trajectory points toward increasingly sophisticated sorbent designs, incorporating advanced materials science approaches such as molecular imprinting, nanomaterial engineering, and biomimetic strategies. These developments aim to overcome the fundamental challenge of extracting lithium from dilute solutions while minimizing energy inputs and environmental impacts, ultimately establishing geothermal lithium extraction as a cornerstone of sustainable critical mineral production.
Geothermal brines, found in underground reservoirs associated with geothermal energy production, contain varying concentrations of dissolved lithium, typically ranging from 10 to 200 mg/L. These concentrations, while lower than those in traditional brine deposits, represent a substantial untapped resource that could significantly contribute to meeting the growing global demand for lithium, projected to increase by 400-500% by 2030.
The evolution of geothermal lithium extraction technology has progressed through several key phases since initial research began in the 1970s. Early efforts focused primarily on understanding the geochemistry of lithium in geothermal systems. The 2000s saw increased interest in direct lithium extraction (DLE) methods, with significant advancements in sorbent technologies and membrane processes. Recent years have witnessed accelerated development, driven by the urgent need for sustainable lithium sources to support the clean energy transition.
The primary technical objective in optimizing sorbent selectivity for low-concentration geothermal lithium streams is to develop materials that can efficiently and selectively capture lithium ions from complex brine solutions containing numerous competing ions, particularly sodium, potassium, calcium, and magnesium. This selectivity is crucial given the relatively low lithium concentrations in geothermal brines compared to other dissolved minerals.
Additional objectives include developing sorbents with rapid kinetics to enable efficient processing of large brine volumes, creating materials with high durability that can withstand repeated adsorption-desorption cycles in harsh geothermal environments, and ensuring economic viability through cost-effective manufacturing processes and materials.
The technological trajectory points toward increasingly sophisticated sorbent designs, incorporating advanced materials science approaches such as molecular imprinting, nanomaterial engineering, and biomimetic strategies. These developments aim to overcome the fundamental challenge of extracting lithium from dilute solutions while minimizing energy inputs and environmental impacts, ultimately establishing geothermal lithium extraction as a cornerstone of sustainable critical mineral production.
Market Analysis for Low-Concentration Lithium Recovery
The global lithium market has experienced unprecedented growth in recent years, primarily driven by the rapid expansion of electric vehicle (EV) production and renewable energy storage systems. The market value for lithium reached approximately $7.5 billion in 2022, with projections indicating potential growth to $18.9 billion by 2030, representing a compound annual growth rate (CAGR) of 12.3%. This remarkable growth trajectory underscores the critical importance of developing efficient lithium recovery technologies, particularly from unconventional sources.
Geothermal brines represent a significant yet largely untapped lithium resource. Unlike traditional mining operations or high-concentration salt flat extractions, geothermal lithium recovery offers substantial environmental advantages, including minimal land disturbance, reduced water consumption, and lower carbon emissions. Current estimates suggest that geothermal brines in the Salton Sea region of California alone could potentially supply up to 40% of global lithium demand.
The market for low-concentration lithium recovery technologies is still in its nascent stage but demonstrates enormous potential. Early commercial implementations have attracted substantial investment, with venture capital funding exceeding $1.2 billion in 2022 for companies specializing in direct lithium extraction (DLE) technologies. Major automotive manufacturers and battery producers are increasingly securing strategic partnerships with technology developers to ensure future supply chain stability.
Demand-side analysis reveals that battery manufacturers require increasingly higher purity lithium products, with battery-grade lithium carbonate or hydroxide typically requiring 99.5% purity or higher. This quality requirement creates a premium market segment for advanced extraction technologies that can deliver high-purity lithium from low-concentration sources. The price differential between technical-grade and battery-grade lithium compounds can exceed 20%, providing strong economic incentives for technology optimization.
Regional market assessment indicates that North America and Europe are positioning themselves as key growth markets for geothermal lithium recovery, driven by governmental initiatives to reduce dependency on foreign lithium supplies. The European Battery Alliance and the U.S. Critical Minerals Initiative have both identified lithium as a strategic resource, allocating significant funding for domestic production capabilities.
Consumer trends further support market development, with end-users increasingly demanding responsibly sourced materials. Environmental, Social, and Governance (ESG) considerations are becoming decisive factors in supply chain decisions, with 73% of major battery manufacturers now publishing sustainability reports that include raw material sourcing practices. This shift creates a distinct market advantage for geothermal lithium, which typically has a carbon footprint 30-60% lower than conventional lithium production methods.
Geothermal brines represent a significant yet largely untapped lithium resource. Unlike traditional mining operations or high-concentration salt flat extractions, geothermal lithium recovery offers substantial environmental advantages, including minimal land disturbance, reduced water consumption, and lower carbon emissions. Current estimates suggest that geothermal brines in the Salton Sea region of California alone could potentially supply up to 40% of global lithium demand.
The market for low-concentration lithium recovery technologies is still in its nascent stage but demonstrates enormous potential. Early commercial implementations have attracted substantial investment, with venture capital funding exceeding $1.2 billion in 2022 for companies specializing in direct lithium extraction (DLE) technologies. Major automotive manufacturers and battery producers are increasingly securing strategic partnerships with technology developers to ensure future supply chain stability.
Demand-side analysis reveals that battery manufacturers require increasingly higher purity lithium products, with battery-grade lithium carbonate or hydroxide typically requiring 99.5% purity or higher. This quality requirement creates a premium market segment for advanced extraction technologies that can deliver high-purity lithium from low-concentration sources. The price differential between technical-grade and battery-grade lithium compounds can exceed 20%, providing strong economic incentives for technology optimization.
Regional market assessment indicates that North America and Europe are positioning themselves as key growth markets for geothermal lithium recovery, driven by governmental initiatives to reduce dependency on foreign lithium supplies. The European Battery Alliance and the U.S. Critical Minerals Initiative have both identified lithium as a strategic resource, allocating significant funding for domestic production capabilities.
Consumer trends further support market development, with end-users increasingly demanding responsibly sourced materials. Environmental, Social, and Governance (ESG) considerations are becoming decisive factors in supply chain decisions, with 73% of major battery manufacturers now publishing sustainability reports that include raw material sourcing practices. This shift creates a distinct market advantage for geothermal lithium, which typically has a carbon footprint 30-60% lower than conventional lithium production methods.
Technical Challenges in Geothermal Lithium Sorbent Technology
The extraction of lithium from geothermal brines presents significant technical challenges that must be overcome to achieve commercial viability. Current sorbent technologies face multiple obstacles when dealing with low-concentration lithium streams typical in geothermal resources. The primary challenge lies in achieving high selectivity for lithium ions in the presence of competing ions such as sodium, potassium, magnesium, and calcium, which are typically present at concentrations 1000-10000 times higher than lithium in geothermal brines.
Temperature stability represents another critical challenge, as geothermal fluids often exceed 100°C, degrading many conventional ion exchange materials and reducing their operational lifespan. Most commercial sorbents demonstrate optimal performance at ambient temperatures, with significant decreases in selectivity and capacity as temperatures rise, necessitating cooling processes that reduce overall energy efficiency.
Chemical durability poses additional complications, as geothermal brines typically exhibit high salinity, extreme pH conditions, and contain dissolved gases like H2S and CO2 that can corrode or deactivate sorbent materials. These harsh chemical environments accelerate material degradation and require frequent replacement of extraction media, substantially increasing operational costs.
Kinetic limitations further constrain extraction efficiency, with many sorbents exhibiting slow lithium uptake rates that necessitate extended contact times incompatible with industrial-scale continuous flow operations. The trade-off between selectivity and kinetics often forces compromises in system design that limit overall recovery rates.
Regeneration processes present their own set of challenges, as the desorption of captured lithium typically requires harsh chemical conditions or significant energy inputs. Incomplete regeneration leads to capacity loss over multiple cycles, while aggressive regeneration methods can damage the sorbent structure and reduce its useful life.
Scaling issues emerge when transitioning from laboratory to industrial implementation, as flow dynamics, pressure drops, and channeling effects in large-scale columns can significantly reduce real-world performance compared to bench-scale results. The physical properties of sorbents, including particle size distribution, mechanical strength, and hydraulic conductivity, become increasingly critical at commercial scales.
Economic viability remains perhaps the most significant barrier, as the cost of specialized lithium-selective sorbents must be balanced against their performance and lifespan. Current materials often require complex synthesis procedures using expensive precursors, making the capital investment for sorbent-based extraction systems prohibitively high for many potential geothermal lithium projects.
Temperature stability represents another critical challenge, as geothermal fluids often exceed 100°C, degrading many conventional ion exchange materials and reducing their operational lifespan. Most commercial sorbents demonstrate optimal performance at ambient temperatures, with significant decreases in selectivity and capacity as temperatures rise, necessitating cooling processes that reduce overall energy efficiency.
Chemical durability poses additional complications, as geothermal brines typically exhibit high salinity, extreme pH conditions, and contain dissolved gases like H2S and CO2 that can corrode or deactivate sorbent materials. These harsh chemical environments accelerate material degradation and require frequent replacement of extraction media, substantially increasing operational costs.
Kinetic limitations further constrain extraction efficiency, with many sorbents exhibiting slow lithium uptake rates that necessitate extended contact times incompatible with industrial-scale continuous flow operations. The trade-off between selectivity and kinetics often forces compromises in system design that limit overall recovery rates.
Regeneration processes present their own set of challenges, as the desorption of captured lithium typically requires harsh chemical conditions or significant energy inputs. Incomplete regeneration leads to capacity loss over multiple cycles, while aggressive regeneration methods can damage the sorbent structure and reduce its useful life.
Scaling issues emerge when transitioning from laboratory to industrial implementation, as flow dynamics, pressure drops, and channeling effects in large-scale columns can significantly reduce real-world performance compared to bench-scale results. The physical properties of sorbents, including particle size distribution, mechanical strength, and hydraulic conductivity, become increasingly critical at commercial scales.
Economic viability remains perhaps the most significant barrier, as the cost of specialized lithium-selective sorbents must be balanced against their performance and lifespan. Current materials often require complex synthesis procedures using expensive precursors, making the capital investment for sorbent-based extraction systems prohibitively high for many potential geothermal lithium projects.
Current Sorbent Technologies for Low-Concentration Lithium
01 Metal-organic frameworks for selective adsorption
Metal-organic frameworks (MOFs) are advanced sorbent materials that offer high selectivity for specific gas or liquid separations. These crystalline porous materials combine metal ions with organic linkers to create structures with tunable pore sizes and chemical functionalities. The selectivity of MOFs can be engineered by modifying their composition, structure, and surface chemistry to target specific molecules based on size, shape, or chemical affinity. These materials show particular promise for carbon dioxide capture, hydrogen storage, and separation of complex gas mixtures.- Metal-organic frameworks for selective adsorption: Metal-organic frameworks (MOFs) are advanced sorbent materials that offer high selectivity for specific gas or liquid separations. These crystalline porous materials combine metal ions with organic linkers to create structures with tunable pore sizes and chemical functionalities. The selectivity of MOFs can be engineered by modifying their composition, structure, and surface chemistry to target specific molecules based on size, shape, or chemical affinity. These materials show particular promise for carbon capture, hydrogen storage, and separation of complex gas mixtures.
- Carbon-based sorbents with enhanced selectivity: Carbon-based materials, including activated carbon, carbon molecular sieves, and graphene-based composites, can be modified to achieve high selectivity for specific contaminants or target molecules. These modifications may involve surface functionalization, pore size distribution control, or incorporation of dopants to enhance binding affinity for particular substances. The selectivity mechanisms typically involve a combination of physical adsorption, chemical interactions, and molecular sieving effects, making these materials versatile for applications in water purification, gas separation, and environmental remediation.
- Zeolite-based selective sorbents: Zeolites are crystalline aluminosilicate materials with well-defined pore structures that enable molecular sieving capabilities. Their selectivity can be tailored by adjusting the silicon-to-aluminum ratio, introducing specific cations, or post-synthesis modifications. The uniform pore size distribution and strong electrostatic fields within zeolite frameworks allow for selective adsorption based on molecular size, shape, and polarity. These properties make zeolites particularly effective for gas separations, water purification, and selective removal of contaminants in various industrial processes.
- Polymer-based selective sorbents: Polymer-based sorbents offer tunable selectivity through the incorporation of specific functional groups, controlled crosslinking, and pore structure engineering. These materials can be designed as resins, membranes, or composite structures with affinity for target molecules based on chemical interactions, size exclusion, or a combination of mechanisms. Advanced polymer sorbents may incorporate molecular imprinting techniques to create recognition sites with high specificity for particular compounds, similar to enzyme-substrate interactions. Applications include pharmaceutical purification, environmental remediation, and selective recovery of valuable resources.
- Composite and hybrid sorbents with enhanced selectivity: Composite and hybrid sorbents combine different materials to achieve superior selectivity compared to single-component systems. These may include organic-inorganic hybrids, layered materials, or core-shell structures that leverage synergistic effects between components. The selectivity mechanisms often involve multiple interaction types, such as combining size exclusion with chemical affinity or integrating different functional domains within a single material. These advanced sorbents can be designed for challenging separations where conventional materials lack sufficient selectivity, including mixed gas streams, complex industrial effluents, or biological media containing similar molecules.
02 Zeolite-based selective adsorbents
Zeolites are aluminosilicate minerals with highly ordered microporous structures that enable molecular sieving capabilities. Their selectivity stems from precise pore dimensions and cation exchange properties that can be tailored for specific separation applications. Modified zeolites can selectively adsorb target compounds based on molecular size, shape, and polarity differences. These materials are particularly effective for gas separations, water purification, and removal of specific contaminants from industrial streams. The selectivity can be enhanced through post-synthesis modifications including ion exchange, dealumination, or incorporation of specific functional groups.Expand Specific Solutions03 Carbon-based selective sorbents
Activated carbons, carbon molecular sieves, and graphene-based materials offer selective adsorption properties that can be tailored through controlled synthesis and surface modification. These carbon-based sorbents provide selectivity through a combination of pore size distribution, surface functional groups, and specific surface area characteristics. The selectivity can be enhanced by chemical treatments that introduce specific functional groups or by physical activation processes that create precise micropore structures. These materials are particularly effective for organic compound separations, water treatment applications, and selective gas adsorption.Expand Specific Solutions04 Polymer-based selective adsorbents
Polymeric sorbents offer tunable selectivity through the incorporation of specific functional groups and controlled cross-linking. These materials can be designed with precise pore structures and chemical affinities to target specific compounds. Molecularly imprinted polymers represent an advanced category that can recognize specific molecular templates with high selectivity. The selectivity mechanisms include size exclusion, hydrogen bonding, electrostatic interactions, and hydrophobic effects. These materials find applications in pharmaceutical separations, environmental remediation, and selective recovery of valuable compounds from complex mixtures.Expand Specific Solutions05 Composite and hybrid selective sorbents
Hybrid and composite sorbents combine different materials to achieve enhanced selectivity that exceeds the capabilities of individual components. These materials often integrate organic and inorganic components to create synergistic effects. Examples include polymer-inorganic composites, layered double hydroxides, and functionalized mesoporous silicas. The selectivity can be tailored by controlling the composition, morphology, and interfacial properties of the composite structure. These advanced sorbents are particularly valuable for challenging separations in complex industrial streams, including selective metal ion recovery, rare earth element separation, and removal of emerging contaminants.Expand Specific Solutions
Leading Companies in Geothermal Lithium Recovery
The geothermal lithium extraction market is in its early growth phase, characterized by increasing technological innovation and commercial pilot projects. The global market for direct lithium extraction technologies is projected to expand significantly as demand for battery-grade lithium continues to rise. Currently, the technology maturity varies across players, with specialized companies like Lilac Solutions and Vulcan Energie leading innovation with ion-exchange technologies specifically designed for low-concentration geothermal brines. Established industrial players such as Eramet, Sunresin, and Schlumberger are leveraging their expertise in material science and resource extraction to develop competitive sorbent technologies. Chinese companies including Bangpu Recycling Technology are advancing hydrometallurgical processes, while research institutions like Central South University and Commissariat à l'énergie atomique are contributing fundamental advancements in selective sorbent development for challenging low-concentration lithium streams.
Lilac Solutions, Inc.
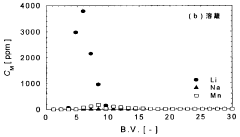
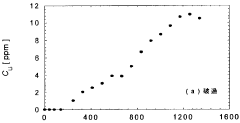
Technical Solution: Lilac Solutions has developed a proprietary ion exchange technology specifically designed for extracting lithium from low-concentration geothermal brines. Their approach uses novel ion exchange beads with high selectivity for lithium over competing ions like sodium, potassium, magnesium, and calcium. The technology employs a continuous countercurrent system where the ion exchange material is cycled between absorption and desorption stages, allowing for continuous operation. Lilac's sorbents feature engineered pore structures that enable rapid lithium uptake kinetics while maintaining mechanical durability under geothermal conditions. The company has demonstrated lithium recovery rates exceeding 90% from brines with concentrations as low as 100 ppm, significantly outperforming traditional evaporation pond methods which typically achieve 40-50% recovery rates. Their process reduces water consumption by over 99% compared to evaporation ponds and can be deployed in modular units directly at geothermal sites.
Strengths: Superior lithium selectivity in complex brine environments; rapid processing time (hours vs. months for evaporation); minimal environmental footprint; modular scalability. Weaknesses: Requires periodic replacement of ion exchange materials; higher upfront capital costs compared to traditional methods; performance may degrade in extremely high-temperature geothermal sources.
IFP Energies Nouvelles
Technical Solution: IFP Energies Nouvelles has developed an innovative sorbent technology for lithium extraction from low-concentration geothermal brines based on functionalized metal-organic frameworks (MOFs). Their approach utilizes highly porous crystalline materials with precisely engineered binding sites that demonstrate exceptional selectivity for lithium ions over competing species commonly found in geothermal fluids. The technology employs a pressure-swing adsorption process where lithium-loaded sorbents are regenerated using controlled pressure differentials rather than harsh chemicals, significantly reducing environmental impact. IFP's sorbents feature hierarchical pore structures that facilitate rapid mass transfer kinetics while maintaining mechanical and chemical stability under geothermal conditions. Their system incorporates advanced process control algorithms that continuously optimize operating parameters based on real-time monitoring of brine composition and temperature fluctuations. Laboratory and pilot-scale testing has demonstrated lithium recovery efficiencies exceeding 80% from brines containing as little as 50-100 ppm lithium, with minimal co-extraction of magnesium, calcium, and other impurities.
Strengths: Exceptional lithium/magnesium selectivity ratio; environmentally friendly regeneration process; stable performance across wide temperature ranges; low chemical consumption. Weaknesses: Higher manufacturing costs for specialized MOF materials; requires precise control of flow rates and pressure; potential for fouling in high-silica geothermal brines.
Key Innovations in Selective Lithium Sorbent Materials
Method for producing lithium adsorbent, lithium adsorbent, starting materials for lithium adsorbent, lithium concentration method, and lithium concentration device
PatentWO2011058841A1
Innovation
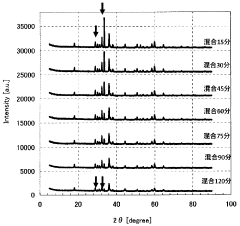
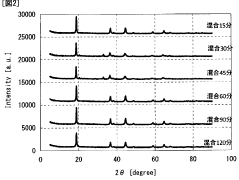
- A method involving the production of a lithium adsorbent using a molar ratio of manganese to lithium between 1:1 and 1.5, with mechanochemical pulverization and two-step calcination at 375°C to 450°C and 475°C to 550°C, followed by acid elution, which enhances the adsorbent's stability and selectivity for lithium ions.
Porous activated alumina based sorbent for lithium extraction
PatentActiveUS20180056283A1
Innovation
- A method involving the preparation of a solid sorbent composition with an activated alumina lithium intercalate, where lithium salts are infused into three-dimensionally structured porous activated alumina, creating a composition with a controlled lithium to aluminum ratio, enhancing lithium extraction capacity and stability.
Environmental Impact Assessment of Extraction Processes
The environmental impact of lithium extraction processes from geothermal brines represents a critical consideration in the development and implementation of sorbent-based technologies. Traditional lithium extraction methods, particularly from hard rock mining and salt flat evaporation, have been associated with significant environmental footprints including habitat disruption, water depletion, and chemical contamination. In contrast, geothermal lithium extraction offers potentially reduced environmental impacts, though the specific environmental profile depends heavily on the sorbent materials and process configurations employed.
Sorbent-based extraction processes for low-concentration geothermal lithium streams introduce several environmental considerations that must be systematically evaluated. The production and manufacturing of sorbent materials themselves require energy inputs and raw materials, creating an upstream environmental burden that varies significantly depending on the sorbent type. Inorganic ion exchange materials typically demand less energy-intensive production processes compared to engineered organic frameworks, though the latter may offer superior performance characteristics that reduce operational impacts.
Water usage represents another critical environmental parameter in geothermal lithium extraction. While geothermal processes generally consume less freshwater than evaporative methods, the management of process water, particularly during sorbent regeneration cycles, requires careful consideration. Closed-loop systems that minimize freshwater inputs and wastewater outputs demonstrate superior environmental performance but may increase system complexity and capital costs.
Chemical consumption during sorbent regeneration and lithium recovery stages constitutes a significant environmental concern. Acid and base requirements for pH adjustment in adsorption-desorption cycles can lead to potential chemical waste streams that require treatment or disposal. Advanced sorbent designs that enable regeneration under milder chemical conditions or with recoverable reagents offer pathways to reducing this environmental burden.
Energy efficiency across the extraction process lifecycle significantly influences the carbon footprint of lithium production. Geothermal lithium extraction benefits from integration with existing geothermal energy production, potentially utilizing waste heat for process operations. However, pumping requirements, particularly for deep geothermal resources, and energy demands for sorbent regeneration must be carefully optimized to maintain favorable energy balances.
Land use impacts from geothermal lithium extraction facilities are generally less extensive than traditional mining operations but still require consideration. Surface facilities for sorbent processing, regeneration, and lithium recovery must be designed with minimal footprint and potential for landscape restoration. The subsurface impacts of geothermal fluid extraction and reinjection, including potential induced seismicity, require monitoring and management protocols to ensure environmental protection.
Sorbent-based extraction processes for low-concentration geothermal lithium streams introduce several environmental considerations that must be systematically evaluated. The production and manufacturing of sorbent materials themselves require energy inputs and raw materials, creating an upstream environmental burden that varies significantly depending on the sorbent type. Inorganic ion exchange materials typically demand less energy-intensive production processes compared to engineered organic frameworks, though the latter may offer superior performance characteristics that reduce operational impacts.
Water usage represents another critical environmental parameter in geothermal lithium extraction. While geothermal processes generally consume less freshwater than evaporative methods, the management of process water, particularly during sorbent regeneration cycles, requires careful consideration. Closed-loop systems that minimize freshwater inputs and wastewater outputs demonstrate superior environmental performance but may increase system complexity and capital costs.
Chemical consumption during sorbent regeneration and lithium recovery stages constitutes a significant environmental concern. Acid and base requirements for pH adjustment in adsorption-desorption cycles can lead to potential chemical waste streams that require treatment or disposal. Advanced sorbent designs that enable regeneration under milder chemical conditions or with recoverable reagents offer pathways to reducing this environmental burden.
Energy efficiency across the extraction process lifecycle significantly influences the carbon footprint of lithium production. Geothermal lithium extraction benefits from integration with existing geothermal energy production, potentially utilizing waste heat for process operations. However, pumping requirements, particularly for deep geothermal resources, and energy demands for sorbent regeneration must be carefully optimized to maintain favorable energy balances.
Land use impacts from geothermal lithium extraction facilities are generally less extensive than traditional mining operations but still require consideration. Surface facilities for sorbent processing, regeneration, and lithium recovery must be designed with minimal footprint and potential for landscape restoration. The subsurface impacts of geothermal fluid extraction and reinjection, including potential induced seismicity, require monitoring and management protocols to ensure environmental protection.
Economic Viability and Scalability Analysis
The economic viability of lithium extraction from geothermal brines hinges on several critical factors, particularly when dealing with low-concentration streams. Current market analysis indicates that traditional lithium extraction methods become economically unfeasible when concentrations fall below 100 mg/L, whereas many geothermal resources contain only 10-50 mg/L of lithium. This concentration gap represents a significant economic challenge that selective sorbent technology aims to address.
Capital expenditure requirements for implementing selective sorbent systems vary considerably based on scale, with pilot plants requiring approximately $5-10 million and commercial-scale operations necessitating investments of $50-150 million. These figures must be evaluated against projected lithium market prices, which have shown volatility but maintain a strong upward trajectory due to increasing demand from electric vehicle manufacturers and energy storage systems.
Operational expenditure analysis reveals that energy consumption constitutes 30-40% of ongoing costs in sorbent-based extraction systems. The regeneration of sorbents typically requires thermal or chemical processes that impact the overall energy balance of the operation. Innovative approaches using waste heat from geothermal power generation could potentially reduce these costs by 15-25%, significantly improving economic viability.
Scalability considerations demonstrate that selective sorbent technologies offer modular implementation advantages, allowing for phased capacity expansion as operational experience grows. Laboratory tests have successfully demonstrated lithium recovery rates of 80-90% at small scales, but maintaining these efficiencies at commercial scale remains challenging. Engineering solutions involving optimized flow dynamics and sorbent column designs are being developed to address these scale-up issues.
Return on investment calculations indicate that with current lithium prices, selective sorbent systems could achieve payback periods of 4-7 years for operations extracting from brines with concentrations as low as 30 mg/L. This timeline improves significantly with higher concentration streams or through integration with existing geothermal power operations where infrastructure costs can be shared.
Supply chain resilience represents another critical economic factor. Diversifying lithium sources through geothermal extraction reduces dependence on traditional mining operations concentrated in South America and Australia. This geographic diversification offers strategic advantages for manufacturers seeking to secure stable supply chains, potentially commanding premium pricing for domestically sourced lithium in certain markets.
Capital expenditure requirements for implementing selective sorbent systems vary considerably based on scale, with pilot plants requiring approximately $5-10 million and commercial-scale operations necessitating investments of $50-150 million. These figures must be evaluated against projected lithium market prices, which have shown volatility but maintain a strong upward trajectory due to increasing demand from electric vehicle manufacturers and energy storage systems.
Operational expenditure analysis reveals that energy consumption constitutes 30-40% of ongoing costs in sorbent-based extraction systems. The regeneration of sorbents typically requires thermal or chemical processes that impact the overall energy balance of the operation. Innovative approaches using waste heat from geothermal power generation could potentially reduce these costs by 15-25%, significantly improving economic viability.
Scalability considerations demonstrate that selective sorbent technologies offer modular implementation advantages, allowing for phased capacity expansion as operational experience grows. Laboratory tests have successfully demonstrated lithium recovery rates of 80-90% at small scales, but maintaining these efficiencies at commercial scale remains challenging. Engineering solutions involving optimized flow dynamics and sorbent column designs are being developed to address these scale-up issues.
Return on investment calculations indicate that with current lithium prices, selective sorbent systems could achieve payback periods of 4-7 years for operations extracting from brines with concentrations as low as 30 mg/L. This timeline improves significantly with higher concentration streams or through integration with existing geothermal power operations where infrastructure costs can be shared.
Supply chain resilience represents another critical economic factor. Diversifying lithium sources through geothermal extraction reduces dependence on traditional mining operations concentrated in South America and Australia. This geographic diversification offers strategic advantages for manufacturers seeking to secure stable supply chains, potentially commanding premium pricing for domestically sourced lithium in certain markets.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!