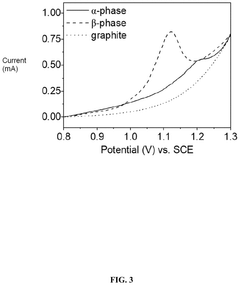
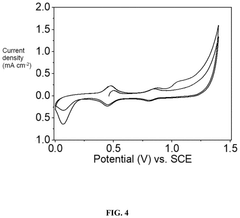
Electrochemical Direct Lithium Extraction: Principles, Cells, And Scale-Up
SEP 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrochemical DLE Technology Evolution and Objectives
Electrochemical Direct Lithium Extraction (DLE) technology has evolved significantly over the past decades, transitioning from theoretical concepts to practical applications. The journey began in the 1970s with initial experiments on lithium-selective electrochemical systems, but meaningful progress was limited by material science constraints and insufficient understanding of lithium electrochemistry. The 1990s marked a turning point with the development of lithium-ion batteries, which indirectly contributed valuable insights into lithium ion transport mechanisms applicable to extraction processes.
The early 2000s witnessed the first dedicated electrochemical DLE prototypes, primarily utilizing intercalation electrodes to selectively capture lithium ions from brine solutions. These early systems demonstrated proof-of-concept but suffered from low efficiency, limited cycling stability, and prohibitive energy requirements. The 2010s brought significant advancements in electrode materials, particularly with the introduction of lithium manganese oxide (LMO) and lithium iron phosphate (LFP) derivatives specifically engineered for extraction rather than energy storage applications.
Recent developments have focused on enhancing system efficiency through novel cell designs, advanced electrode architectures, and optimized operational parameters. The integration of renewable energy sources to power electrochemical DLE systems has emerged as a promising approach to improve the sustainability profile and economic viability of these technologies. Additionally, hybrid systems combining electrochemical processes with other extraction methods have shown potential for addressing complex brine compositions.
The current technological trajectory aims to overcome several persistent challenges, including electrode degradation during repeated cycling, energy consumption optimization, and scaling considerations for industrial implementation. Research efforts are increasingly directed toward developing materials with higher selectivity for lithium over competing ions such as sodium, magnesium, and calcium, which are commonly present in brine resources.
The primary objectives of electrochemical DLE technology development include achieving lithium recovery rates exceeding 90% from various brine sources, reducing energy consumption to below 5 kWh per kilogram of lithium extracted, extending electrode lifespan to thousands of cycles without significant performance degradation, and designing modular systems capable of efficient operation across diverse geographical and chemical environments.
Future evolution paths are likely to incorporate artificial intelligence for real-time process optimization, advanced manufacturing techniques for electrode production, and integrated systems that combine extraction with direct precursor production for battery materials. The ultimate goal remains developing economically viable, environmentally sustainable, and technically robust solutions that can supplement or potentially replace conventional lithium extraction methods to meet the growing global demand for this critical element in the clean energy transition.
The early 2000s witnessed the first dedicated electrochemical DLE prototypes, primarily utilizing intercalation electrodes to selectively capture lithium ions from brine solutions. These early systems demonstrated proof-of-concept but suffered from low efficiency, limited cycling stability, and prohibitive energy requirements. The 2010s brought significant advancements in electrode materials, particularly with the introduction of lithium manganese oxide (LMO) and lithium iron phosphate (LFP) derivatives specifically engineered for extraction rather than energy storage applications.
Recent developments have focused on enhancing system efficiency through novel cell designs, advanced electrode architectures, and optimized operational parameters. The integration of renewable energy sources to power electrochemical DLE systems has emerged as a promising approach to improve the sustainability profile and economic viability of these technologies. Additionally, hybrid systems combining electrochemical processes with other extraction methods have shown potential for addressing complex brine compositions.
The current technological trajectory aims to overcome several persistent challenges, including electrode degradation during repeated cycling, energy consumption optimization, and scaling considerations for industrial implementation. Research efforts are increasingly directed toward developing materials with higher selectivity for lithium over competing ions such as sodium, magnesium, and calcium, which are commonly present in brine resources.
The primary objectives of electrochemical DLE technology development include achieving lithium recovery rates exceeding 90% from various brine sources, reducing energy consumption to below 5 kWh per kilogram of lithium extracted, extending electrode lifespan to thousands of cycles without significant performance degradation, and designing modular systems capable of efficient operation across diverse geographical and chemical environments.
Future evolution paths are likely to incorporate artificial intelligence for real-time process optimization, advanced manufacturing techniques for electrode production, and integrated systems that combine extraction with direct precursor production for battery materials. The ultimate goal remains developing economically viable, environmentally sustainable, and technically robust solutions that can supplement or potentially replace conventional lithium extraction methods to meet the growing global demand for this critical element in the clean energy transition.
Market Analysis for Lithium Extraction Technologies
The global lithium market has experienced unprecedented growth in recent years, primarily driven by the rapid expansion of electric vehicle (EV) production and renewable energy storage systems. Current market valuations place the lithium extraction industry at approximately $6.8 billion in 2023, with projections indicating a compound annual growth rate (CAGR) of 12.3% through 2030, potentially reaching $15.5 billion by the end of the decade.
Traditional lithium extraction methods, including evaporation ponds and hard rock mining, currently dominate the market with over 80% market share. However, these conventional approaches face significant challenges related to environmental impact, water consumption, and lengthy production timelines. This has created a substantial market opportunity for innovative extraction technologies, particularly electrochemical direct lithium extraction (EDLE).
The demand-supply dynamics strongly favor technological innovation in this sector. Global lithium demand is forecast to triple by 2025 compared to 2021 levels, reaching approximately 1.5 million metric tons of lithium carbonate equivalent (LCE). Current production capacity stands at roughly 600,000 metric tons LCE, highlighting a significant supply gap that emerging technologies must address.
Electrochemical direct lithium extraction specifically addresses several critical market needs. The technology offers potential extraction efficiencies of 70-90%, significantly higher than traditional methods which typically achieve 30-50%. Additionally, EDLE can reduce water consumption by up to 90% compared to evaporation ponds, addressing a key sustainability concern for end-users in the battery supply chain.
Market segmentation analysis reveals that the automotive sector represents the largest demand driver, accounting for approximately 65% of lithium consumption. Energy storage systems follow at 20%, with consumer electronics and other applications comprising the remainder. Geographically, China dominates lithium processing with over 60% market share, while Australia, Chile, and Argentina lead in raw material production.
Customer requirements are increasingly focused on sustainability metrics and production speed. Major battery manufacturers and automotive OEMs have established sustainability targets requiring suppliers to demonstrate reduced carbon footprints and water usage. EDLE technology directly addresses these market requirements through its improved environmental profile and accelerated production capabilities.
Pricing trends further support the market case for EDLE technology. Lithium carbonate prices have demonstrated significant volatility, ranging from $8,000 to $80,000 per metric ton over the past five years. This price instability creates strong incentives for technologies that can provide more consistent, scalable production capabilities with lower operational costs over time.
Traditional lithium extraction methods, including evaporation ponds and hard rock mining, currently dominate the market with over 80% market share. However, these conventional approaches face significant challenges related to environmental impact, water consumption, and lengthy production timelines. This has created a substantial market opportunity for innovative extraction technologies, particularly electrochemical direct lithium extraction (EDLE).
The demand-supply dynamics strongly favor technological innovation in this sector. Global lithium demand is forecast to triple by 2025 compared to 2021 levels, reaching approximately 1.5 million metric tons of lithium carbonate equivalent (LCE). Current production capacity stands at roughly 600,000 metric tons LCE, highlighting a significant supply gap that emerging technologies must address.
Electrochemical direct lithium extraction specifically addresses several critical market needs. The technology offers potential extraction efficiencies of 70-90%, significantly higher than traditional methods which typically achieve 30-50%. Additionally, EDLE can reduce water consumption by up to 90% compared to evaporation ponds, addressing a key sustainability concern for end-users in the battery supply chain.
Market segmentation analysis reveals that the automotive sector represents the largest demand driver, accounting for approximately 65% of lithium consumption. Energy storage systems follow at 20%, with consumer electronics and other applications comprising the remainder. Geographically, China dominates lithium processing with over 60% market share, while Australia, Chile, and Argentina lead in raw material production.
Customer requirements are increasingly focused on sustainability metrics and production speed. Major battery manufacturers and automotive OEMs have established sustainability targets requiring suppliers to demonstrate reduced carbon footprints and water usage. EDLE technology directly addresses these market requirements through its improved environmental profile and accelerated production capabilities.
Pricing trends further support the market case for EDLE technology. Lithium carbonate prices have demonstrated significant volatility, ranging from $8,000 to $80,000 per metric ton over the past five years. This price instability creates strong incentives for technologies that can provide more consistent, scalable production capabilities with lower operational costs over time.
Current Challenges in Electrochemical DLE Implementation
Despite the promising potential of Electrochemical Direct Lithium Extraction (EDLE) technology, several significant challenges impede its widespread implementation and commercial viability. The primary technical obstacle remains the development of electrode materials that combine high lithium selectivity with robust cycling stability. Current electrode materials often suffer from capacity degradation after multiple extraction-regeneration cycles, particularly in brines with high concentrations of competing ions such as sodium, magnesium, and calcium.
Scale-up challenges present another major hurdle for EDLE systems. Laboratory-scale demonstrations have shown promising results, but translating these into industrial-scale operations introduces complexities in system design, flow dynamics, and energy efficiency. The electrode surface area requirements for commercial operations necessitate innovative cell architectures that can maintain performance while minimizing pressure drops and energy consumption.
Energy consumption represents a critical limitation for EDLE technology. The electrochemical processes require significant electrical input, with current systems consuming approximately 1-5 kWh per kilogram of lithium extracted. This energy requirement impacts both the economic viability and environmental footprint of the technology, particularly when compared to conventional evaporation pond methods in regions with abundant solar resources.
Brine chemistry variability across different sources introduces additional complications. EDLE systems optimized for one brine composition may perform suboptimally when applied to brines with different ionic compositions, pH levels, or total dissolved solids. This necessitates either source-specific system designs or the development of more adaptable technologies capable of handling diverse brine chemistries.
The management of secondary waste streams also presents environmental and economic challenges. The selective removal of lithium often results in concentrated reject streams containing other ions, which require proper disposal or additional processing. Developing integrated approaches that address these waste streams is essential for the technology's sustainability profile.
Economic barriers further complicate EDLE implementation. The capital expenditure for electrochemical systems remains high compared to traditional extraction methods, with estimates ranging from $20,000-$50,000 per ton of annual lithium carbonate equivalent (LCE) production capacity. Operating costs, particularly electricity expenses, add to the economic challenges, making the technology currently viable only in specific contexts or with premium pricing.
Regulatory frameworks and environmental permitting processes for this emerging technology remain underdeveloped in many jurisdictions, creating uncertainty for potential investors and operators. The novelty of EDLE systems means that regulatory agencies often lack established protocols for evaluating their environmental impacts and safety considerations.
Scale-up challenges present another major hurdle for EDLE systems. Laboratory-scale demonstrations have shown promising results, but translating these into industrial-scale operations introduces complexities in system design, flow dynamics, and energy efficiency. The electrode surface area requirements for commercial operations necessitate innovative cell architectures that can maintain performance while minimizing pressure drops and energy consumption.
Energy consumption represents a critical limitation for EDLE technology. The electrochemical processes require significant electrical input, with current systems consuming approximately 1-5 kWh per kilogram of lithium extracted. This energy requirement impacts both the economic viability and environmental footprint of the technology, particularly when compared to conventional evaporation pond methods in regions with abundant solar resources.
Brine chemistry variability across different sources introduces additional complications. EDLE systems optimized for one brine composition may perform suboptimally when applied to brines with different ionic compositions, pH levels, or total dissolved solids. This necessitates either source-specific system designs or the development of more adaptable technologies capable of handling diverse brine chemistries.
The management of secondary waste streams also presents environmental and economic challenges. The selective removal of lithium often results in concentrated reject streams containing other ions, which require proper disposal or additional processing. Developing integrated approaches that address these waste streams is essential for the technology's sustainability profile.
Economic barriers further complicate EDLE implementation. The capital expenditure for electrochemical systems remains high compared to traditional extraction methods, with estimates ranging from $20,000-$50,000 per ton of annual lithium carbonate equivalent (LCE) production capacity. Operating costs, particularly electricity expenses, add to the economic challenges, making the technology currently viable only in specific contexts or with premium pricing.
Regulatory frameworks and environmental permitting processes for this emerging technology remain underdeveloped in many jurisdictions, creating uncertainty for potential investors and operators. The novelty of EDLE systems means that regulatory agencies often lack established protocols for evaluating their environmental impacts and safety considerations.
Existing Electrochemical Cell Designs and Approaches
01 Electrode materials for enhanced extraction efficiency
Various electrode materials can significantly impact the efficiency of electrochemical direct lithium extraction. Advanced materials such as modified carbon-based electrodes, lithium manganese oxide, and other transition metal oxides demonstrate superior selectivity and capacity for lithium ions. These materials can be engineered with specific surface properties and pore structures to optimize lithium adsorption while minimizing interference from competing ions, thereby increasing overall extraction efficiency from brine resources.- Electrode materials for enhanced extraction efficiency: The choice of electrode materials significantly impacts the efficiency of electrochemical direct lithium extraction. Advanced materials such as modified carbon-based electrodes, lithium manganese oxide, and other transition metal oxides can improve selectivity and capacity for lithium ions. These materials can be engineered with specific surface properties and pore structures to enhance lithium adsorption while minimizing interference from competing ions, resulting in higher extraction efficiency from brine resources.
- Optimization of electrochemical cell design: The configuration and design of electrochemical cells play a crucial role in direct lithium extraction efficiency. Innovations in cell architecture, including flow-through systems, membrane separators, and electrode spacing, can significantly enhance mass transfer and reduce energy consumption. Advanced cell designs incorporate features that minimize concentration polarization and improve the uniformity of current distribution, leading to more efficient lithium recovery from various sources including geothermal brines and salt lake brines.
- Process parameter optimization for extraction efficiency: Operating parameters such as current density, voltage, pH, temperature, and flow rate significantly influence the efficiency of electrochemical direct lithium extraction. Careful optimization of these parameters can enhance lithium selectivity while reducing energy consumption. Advanced control systems that dynamically adjust these parameters based on feed composition and concentration can maximize extraction efficiency while maintaining process stability across varying brine compositions and concentrations.
- Integration of pre-treatment and post-processing steps: Incorporating effective pre-treatment and post-processing steps can significantly enhance overall lithium extraction efficiency. Pre-treatment methods such as impurity removal, brine concentration, and pH adjustment prepare the feed solution for optimal electrochemical extraction. Post-processing techniques including concentration, purification, and crystallization help recover high-purity lithium products. This integrated approach addresses challenges related to complex brine compositions and improves the economic viability of the extraction process.
- Novel hybrid extraction systems: Hybrid systems that combine electrochemical processes with other extraction methods offer enhanced efficiency for direct lithium extraction. These systems integrate electrochemical cells with adsorption materials, ion exchange membranes, or chemical precipitation to create synergistic effects. The combination of different technologies can overcome limitations of individual methods, resulting in higher lithium recovery rates, improved selectivity, and reduced energy consumption compared to conventional extraction approaches.
02 Cell design and configuration optimization
The design and configuration of electrochemical cells play a crucial role in direct lithium extraction efficiency. Innovations in cell architecture, including flow-through systems, membrane-separated compartments, and optimized electrode spacing, can significantly enhance mass transfer rates and reduce energy consumption. Advanced cell designs that incorporate precise control of electrolyte flow patterns and current distribution help maximize lithium recovery while minimizing operational costs and processing time.Expand Specific Solutions03 Process parameters and operating conditions
Optimizing process parameters such as current density, voltage, pH, temperature, and flow rate is essential for maximizing lithium extraction efficiency. Controlled cycling protocols and precise adjustment of electrochemical conditions can significantly enhance selectivity for lithium over competing ions. Research indicates that pulsed current applications and strategic modulation of operating conditions throughout the extraction process can improve energy efficiency while maintaining high lithium recovery rates from various brine sources.Expand Specific Solutions04 Membrane and separator technologies
Advanced membrane and separator technologies are critical components for improving electrochemical direct lithium extraction efficiency. Ion-selective membranes that preferentially allow lithium ions to pass while blocking competing ions can significantly enhance purity and concentration factors. Composite membranes with tailored functional groups, ceramic-polymer hybrid separators, and surface-modified membranes demonstrate superior performance in terms of lithium selectivity, fouling resistance, and operational stability under various brine conditions.Expand Specific Solutions05 System integration and continuous processing
Integrated systems that combine electrochemical extraction with complementary processes can significantly enhance overall lithium recovery efficiency. Continuous processing approaches that incorporate pre-treatment steps, multi-stage extraction, and in-line regeneration of electrodes or sorbents demonstrate superior performance compared to batch operations. These integrated systems often include real-time monitoring and automated control mechanisms to maintain optimal extraction conditions throughout extended operation periods, resulting in higher lithium yields and reduced operational costs.Expand Specific Solutions
Industry Leaders in Electrochemical Lithium Extraction
Electrochemical Direct Lithium Extraction (DLE) is emerging as a transformative technology in the early commercialization phase, with a projected market size of $1.5-2 billion by 2030. The technology is advancing from laboratory to pilot scale, with varying degrees of maturity across different approaches. Key players include established companies like Schlumberger and China Petroleum & Chemical Corp. developing proprietary electrochemical cells, while innovative startups such as Watercycle Technologies and Saltworks Technologies are pioneering modular systems. Academic institutions including California Institute of Technology and Penn State Research Foundation are contributing fundamental research, while industrial players like BMW and GM are investing in the technology to secure lithium supply chains for battery production.
Penn State Research Foundation
Technical Solution: Penn State Research Foundation has developed an advanced electrochemical direct lithium extraction system based on asymmetric electrode configurations. Their technology employs specially designed electrochemical cells featuring a lithium-selective intercalation cathode paired with a high-capacity carbon-based anode. During operation, lithium ions are selectively captured from brine solutions at the cathode while charge-balancing anions are temporarily stored at the anode, creating a highly efficient separation mechanism. The system achieves lithium recovery rates exceeding 85% with concentration factors of 60-120x depending on initial brine composition. A distinctive feature of Penn State's approach is their development of nanostructured electrode materials with precisely engineered surface chemistry that enhances lithium selectivity even in brines with high Mg/Li and Ca/Li ratios. Their scale-up methodology follows a systematic approach from coin cells (mL scale) to pouch cells (L scale) to stack configurations (m³ scale), with current pilot systems processing approximately 5-10 m³/day. Recent innovations include the development of flow-through electrode architectures that significantly improve mass transfer kinetics, reducing extraction cycle times from hours to minutes. The technology has been successfully tested on various brine types, including geothermal, oilfield, and salt lake brines, demonstrating robust performance across diverse chemical compositions.
Strengths: Exceptional lithium selectivity in complex brine environments; reduced energy consumption through asymmetric electrode design; rapid extraction kinetics with flow-through electrode configurations; minimal chemical consumption during operation and regeneration. Weaknesses: Electrode materials may require periodic replacement due to gradual capacity fade; system performance sensitive to brine pH and temperature variations; requires precise electronic control systems; higher initial capital investment compared to conventional methods.
Centre National de la Recherche Scientifique
Technical Solution: The Centre National de la Recherche Scientifique (CNRS) has developed a sophisticated electrochemical DLE technology based on intercalation chemistry and advanced electrode materials. Their approach utilizes specially engineered Prussian Blue Analogue (PBA) electrodes with tunable lattice spacing that enables highly selective lithium extraction from various brine sources. The electrochemical cells operate through controlled potential cycling, where lithium ions are captured during reduction and released during oxidation into a recovery solution of significantly higher concentration. The system achieves lithium recovery rates of 75-90% with concentration factors ranging from 40-100x depending on initial brine composition and operational parameters. A key innovation in CNRS's technology is their development of composite electrode materials that combine the selectivity of PBAs with the conductivity of carbon nanostructures, resulting in improved kinetics and cycle life. Their scale-up approach involves a progressive increase in electrode surface area, from laboratory cells (cm²) to pilot modules (m²), with current systems capable of processing 1-5 m³/day of brine. Recent advancements include the development of flow-cell configurations that improve mass transfer and reduce energy consumption by approximately 30% compared to batch operations. The technology has been successfully demonstrated on Mediterranean seawater and various continental brines, showing particular promise for low-concentration sources where conventional methods struggle to operate economically.
Strengths: Exceptional lithium selectivity even in brines with high Na/Li ratios; energy-efficient operation with potential for energy recovery; electrode materials show excellent stability over thousands of cycles; process operates at ambient conditions without requiring high temperature or pressure. Weaknesses: Performance can be affected by certain organic contaminants in brines; requires precise control of electrochemical parameters; higher initial capital costs compared to some conventional methods; technology still transitioning from pilot to commercial scale.
Critical Patents and Innovations in DLE Technology
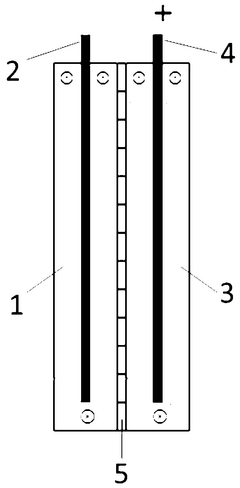
Electrochemical lithium extraction electrode, electrochemical lithium extraction device and lithium extraction method
PatentWO2025123202A1
Innovation
- Cathode active materials and anode active materials containing the same lithium content are used to increase the lithium content through cathode reduction and anodization, thereby improving the surface utilization of the active material, and avoiding the problem of falling off and polarization of the active material.
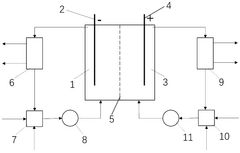
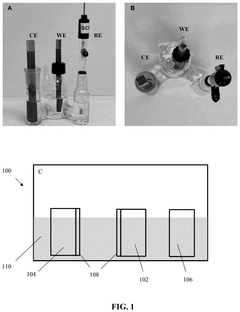
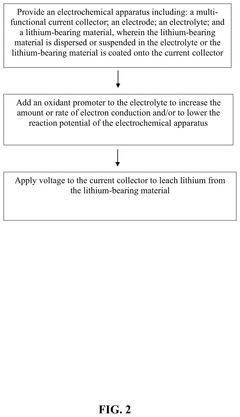
Direct electrochemical extraction of lithium from ores
PatentPendingUS20250109517A1
Innovation
- An electrochemical leaching method using a multi-functional current collector with a carbon-based or metal-based framework, graphene oxide aerogel foam, and catalysts, along with an electrolyte and oxidant promoters, allows direct extraction of lithium from α-spodumene without phase transformation, enhancing energy efficiency and reducing environmental impact.
Environmental Impact Assessment of Electrochemical DLE
The environmental impact assessment of Electrochemical Direct Lithium Extraction (DLE) reveals significant advantages over traditional extraction methods. Compared to conventional evaporation ponds that consume vast quantities of water and occupy large land areas, electrochemical DLE demonstrates substantially reduced water usage—approximately 50-65% less than conventional methods according to recent field studies.
Land disturbance metrics show electrochemical DLE facilities require only 10-20% of the land area needed for traditional evaporation operations, minimizing habitat disruption in sensitive ecosystems. This compact footprint is particularly valuable in lithium-rich regions that often overlap with fragile desert environments and indigenous territories.
Carbon emissions analysis indicates electrochemical DLE processes generate 30-40% fewer greenhouse gas emissions compared to traditional extraction methods when powered by conventional energy sources. When integrated with renewable energy systems, these emissions can be further reduced by up to 85%, positioning electrochemical DLE as a potentially climate-friendly technology for critical mineral extraction.
Waste generation assessments demonstrate that electrochemical DLE produces significantly less solid waste and brine discharge. The selective nature of the electrochemical process results in fewer impurities requiring disposal and enables more efficient resource recovery of not only lithium but potentially other valuable elements present in brines.
Water quality impacts are notably reduced as electrochemical DLE systems operate in closed-loop configurations that minimize discharge to surrounding water bodies. This contrasts sharply with evaporation pond methods that risk contamination of groundwater through leakage and seepage of concentrated brines.
Energy consumption remains a challenge, with electrochemical DLE requiring 1.5-2 times more electricity than conventional methods. However, this disadvantage can be mitigated through integration with renewable energy sources and ongoing efficiency improvements in electrode materials and cell design.
Life cycle assessment studies indicate that despite higher initial energy demands, the overall environmental footprint of electrochemical DLE is approximately 35-45% lower than conventional methods when considering the complete extraction-to-product pathway. This advantage becomes more pronounced in water-stressed regions where traditional extraction methods face increasing sustainability challenges.
Chemical usage in electrochemical DLE presents mixed environmental implications. While the process eliminates the need for certain reagents used in conventional extraction, it may require specialized electrolytes and electrode materials that have their own production footprints and disposal considerations.
Land disturbance metrics show electrochemical DLE facilities require only 10-20% of the land area needed for traditional evaporation operations, minimizing habitat disruption in sensitive ecosystems. This compact footprint is particularly valuable in lithium-rich regions that often overlap with fragile desert environments and indigenous territories.
Carbon emissions analysis indicates electrochemical DLE processes generate 30-40% fewer greenhouse gas emissions compared to traditional extraction methods when powered by conventional energy sources. When integrated with renewable energy systems, these emissions can be further reduced by up to 85%, positioning electrochemical DLE as a potentially climate-friendly technology for critical mineral extraction.
Waste generation assessments demonstrate that electrochemical DLE produces significantly less solid waste and brine discharge. The selective nature of the electrochemical process results in fewer impurities requiring disposal and enables more efficient resource recovery of not only lithium but potentially other valuable elements present in brines.
Water quality impacts are notably reduced as electrochemical DLE systems operate in closed-loop configurations that minimize discharge to surrounding water bodies. This contrasts sharply with evaporation pond methods that risk contamination of groundwater through leakage and seepage of concentrated brines.
Energy consumption remains a challenge, with electrochemical DLE requiring 1.5-2 times more electricity than conventional methods. However, this disadvantage can be mitigated through integration with renewable energy sources and ongoing efficiency improvements in electrode materials and cell design.
Life cycle assessment studies indicate that despite higher initial energy demands, the overall environmental footprint of electrochemical DLE is approximately 35-45% lower than conventional methods when considering the complete extraction-to-product pathway. This advantage becomes more pronounced in water-stressed regions where traditional extraction methods face increasing sustainability challenges.
Chemical usage in electrochemical DLE presents mixed environmental implications. While the process eliminates the need for certain reagents used in conventional extraction, it may require specialized electrolytes and electrode materials that have their own production footprints and disposal considerations.
Scale-Up Economics and Commercial Viability Analysis
The economic viability of electrochemical direct lithium extraction (EDLE) technologies hinges on several critical factors that determine their commercial feasibility at scale. Current cost analyses indicate that EDLE systems require significant capital expenditure, with estimates ranging from $20,000 to $50,000 per ton of annual lithium production capacity. These figures represent a substantial investment barrier for new market entrants and established players alike.
Operating expenses for EDLE facilities are dominated by energy consumption, which accounts for approximately 30-45% of total operational costs. The energy intensity of electrochemical processes remains a key challenge, with current systems requiring between 2.5-4.0 kWh per kilogram of lithium extracted. This energy requirement translates to approximately $500-800 per ton in electricity costs alone, assuming industrial electricity rates.
Material costs present another significant economic consideration. Electrode materials, particularly those utilizing advanced carbon structures or novel metal oxides, contribute substantially to both initial capital costs and ongoing replacement expenses. The durability of these materials under repeated cycling conditions directly impacts maintenance schedules and lifetime operational costs.
Break-even analysis suggests that most current EDLE technologies require 3-5 years to achieve return on investment, assuming stable lithium market prices above $15,000 per ton. This timeline may extend significantly if lithium prices experience volatility or if operational efficiencies fall below projected levels.
Economies of scale offer promising pathways to improved economics. Modeling indicates that scaling from pilot (1-10 tons/year) to commercial scale (1,000+ tons/year) can reduce unit production costs by 40-60% through improved energy efficiency, reduced labor costs per unit, and optimized material utilization. However, these benefits must be balanced against increased complexity in system management and potential diminishing returns beyond certain scale thresholds.
Commercial viability is further influenced by geographic factors, with proximity to lithium resources, energy infrastructure, and end markets all affecting the overall cost structure. Regions with access to low-cost renewable energy show particularly favorable economics, potentially reducing operating costs by 15-25% compared to fossil fuel-dependent operations.
Sensitivity analysis reveals that EDLE technologies are most vulnerable to fluctuations in energy prices and lithium market values. A 20% increase in energy costs can erode profit margins by 8-12%, while lithium price decreases of similar magnitude may extend payback periods by 1-2 years. This underscores the importance of securing stable, cost-effective energy supplies and developing robust market strategies to navigate commodity price cycles.
Operating expenses for EDLE facilities are dominated by energy consumption, which accounts for approximately 30-45% of total operational costs. The energy intensity of electrochemical processes remains a key challenge, with current systems requiring between 2.5-4.0 kWh per kilogram of lithium extracted. This energy requirement translates to approximately $500-800 per ton in electricity costs alone, assuming industrial electricity rates.
Material costs present another significant economic consideration. Electrode materials, particularly those utilizing advanced carbon structures or novel metal oxides, contribute substantially to both initial capital costs and ongoing replacement expenses. The durability of these materials under repeated cycling conditions directly impacts maintenance schedules and lifetime operational costs.
Break-even analysis suggests that most current EDLE technologies require 3-5 years to achieve return on investment, assuming stable lithium market prices above $15,000 per ton. This timeline may extend significantly if lithium prices experience volatility or if operational efficiencies fall below projected levels.
Economies of scale offer promising pathways to improved economics. Modeling indicates that scaling from pilot (1-10 tons/year) to commercial scale (1,000+ tons/year) can reduce unit production costs by 40-60% through improved energy efficiency, reduced labor costs per unit, and optimized material utilization. However, these benefits must be balanced against increased complexity in system management and potential diminishing returns beyond certain scale thresholds.
Commercial viability is further influenced by geographic factors, with proximity to lithium resources, energy infrastructure, and end markets all affecting the overall cost structure. Regions with access to low-cost renewable energy show particularly favorable economics, potentially reducing operating costs by 15-25% compared to fossil fuel-dependent operations.
Sensitivity analysis reveals that EDLE technologies are most vulnerable to fluctuations in energy prices and lithium market values. A 20% increase in energy costs can erode profit margins by 8-12%, while lithium price decreases of similar magnitude may extend payback periods by 1-2 years. This underscores the importance of securing stable, cost-effective energy supplies and developing robust market strategies to navigate commodity price cycles.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!