Materials Characterization For Lithium-Selective Sorbents Under High Temperature
SEP 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Sorbent Technology Background and Objectives
Lithium extraction technology has evolved significantly over the past decades, transitioning from traditional mining operations to more advanced and environmentally conscious methods. The initial extraction techniques primarily focused on hard rock mining and brine evaporation, which dominated the industry until the early 2000s. As global demand for lithium has surged due to the rapid expansion of electric vehicle markets and energy storage systems, these conventional methods have proven insufficient to meet future requirements, prompting extensive research into alternative extraction technologies.
Selective sorbent technology represents a revolutionary approach in lithium extraction, offering potential advantages in efficiency, environmental impact, and economic viability. This technology utilizes specially designed materials that can selectively capture lithium ions from various sources, including geothermal brines, seawater, and industrial waste streams. The development of these sorbents has accelerated in the past decade, with significant breakthroughs in material science enabling higher selectivity and capacity.
The technical evolution trajectory indicates a clear shift toward more sophisticated sorbent materials, including ion-exchange resins, inorganic adsorbents, and membrane-based systems. Recent innovations have focused on enhancing the temperature stability of these materials, as many lithium-rich brines naturally occur at elevated temperatures, particularly in geothermal reservoirs where temperatures can exceed 100°C.
The primary objective of current research is to characterize and develop lithium-selective sorbents capable of operating efficiently under high-temperature conditions without degradation or significant reduction in performance. This involves understanding the fundamental mechanisms of lithium adsorption at the molecular level, the structural changes that occur under thermal stress, and the long-term stability of these materials in realistic operational environments.
Additional technical goals include improving selectivity for lithium over competing ions (particularly sodium, potassium, and magnesium), increasing adsorption capacity, enhancing kinetics for faster extraction cycles, and developing regeneration processes that minimize energy consumption and chemical usage. These improvements are essential for making sorbent-based extraction economically competitive with traditional methods.
The development of high-temperature resistant lithium sorbents also aligns with broader sustainability objectives in the mining sector, as these technologies potentially offer reduced water consumption, smaller land footprint, and lower carbon emissions compared to conventional extraction methods. This technological direction supports the growing emphasis on environmentally responsible resource extraction practices within the global transition to renewable energy systems.
Selective sorbent technology represents a revolutionary approach in lithium extraction, offering potential advantages in efficiency, environmental impact, and economic viability. This technology utilizes specially designed materials that can selectively capture lithium ions from various sources, including geothermal brines, seawater, and industrial waste streams. The development of these sorbents has accelerated in the past decade, with significant breakthroughs in material science enabling higher selectivity and capacity.
The technical evolution trajectory indicates a clear shift toward more sophisticated sorbent materials, including ion-exchange resins, inorganic adsorbents, and membrane-based systems. Recent innovations have focused on enhancing the temperature stability of these materials, as many lithium-rich brines naturally occur at elevated temperatures, particularly in geothermal reservoirs where temperatures can exceed 100°C.
The primary objective of current research is to characterize and develop lithium-selective sorbents capable of operating efficiently under high-temperature conditions without degradation or significant reduction in performance. This involves understanding the fundamental mechanisms of lithium adsorption at the molecular level, the structural changes that occur under thermal stress, and the long-term stability of these materials in realistic operational environments.
Additional technical goals include improving selectivity for lithium over competing ions (particularly sodium, potassium, and magnesium), increasing adsorption capacity, enhancing kinetics for faster extraction cycles, and developing regeneration processes that minimize energy consumption and chemical usage. These improvements are essential for making sorbent-based extraction economically competitive with traditional methods.
The development of high-temperature resistant lithium sorbents also aligns with broader sustainability objectives in the mining sector, as these technologies potentially offer reduced water consumption, smaller land footprint, and lower carbon emissions compared to conventional extraction methods. This technological direction supports the growing emphasis on environmentally responsible resource extraction practices within the global transition to renewable energy systems.
Market Analysis for High-Temperature Lithium Extraction
The global market for lithium extraction technologies is experiencing unprecedented growth, driven primarily by the surging demand for lithium-ion batteries in electric vehicles, renewable energy storage systems, and portable electronics. The high-temperature lithium extraction segment represents a specialized but increasingly important niche within this broader market landscape, with particular relevance to geothermal brines and certain industrial processes where elevated temperatures are inherent to the extraction environment.
Current market valuations indicate that the global lithium extraction market exceeded $4 billion in 2022, with projections suggesting a compound annual growth rate of 8-12% through 2030. Within this, high-temperature extraction methodologies are gaining traction, particularly as companies seek more efficient and environmentally sustainable approaches to lithium recovery from unconventional sources.
The demand dynamics for high-temperature lithium-selective sorbents are shaped by several converging factors. First, traditional lithium extraction methods such as evaporation ponds face increasing scrutiny due to their substantial water consumption and extended processing times. This has accelerated interest in direct lithium extraction (DLE) technologies, including those optimized for high-temperature environments.
Geographically, the market shows distinct regional characteristics. North America leads in research and development of advanced sorbent technologies, while China dominates in terms of production capacity and commercial deployment. European markets are increasingly focused on securing domestic lithium supply chains, creating new opportunities for high-temperature extraction technologies applicable to European geothermal resources.
Industry analysts have identified several key market segments where high-temperature lithium-selective sorbents show particular promise: geothermal brine processing, industrial wastewater recovery, and certain mining operations where process streams maintain elevated temperatures. The geothermal segment alone is projected to grow at 15-18% annually, outpacing the broader lithium extraction market.
Customer requirements in this space emphasize several performance metrics: selectivity for lithium over competing ions (particularly sodium, potassium, and magnesium), thermal stability at operating temperatures typically ranging from 70-150°C, cycle durability, and resistance to fouling. Materials that can maintain performance across hundreds of adsorption-desorption cycles command premium pricing in the market.
Pricing structures for high-temperature lithium-selective sorbents reflect their specialized nature, with current materials commanding 30-40% price premiums over conventional alternatives. This premium is justified by improved recovery rates and reduced energy costs in high-temperature applications, creating favorable total cost of ownership calculations despite higher initial investment.
Current market valuations indicate that the global lithium extraction market exceeded $4 billion in 2022, with projections suggesting a compound annual growth rate of 8-12% through 2030. Within this, high-temperature extraction methodologies are gaining traction, particularly as companies seek more efficient and environmentally sustainable approaches to lithium recovery from unconventional sources.
The demand dynamics for high-temperature lithium-selective sorbents are shaped by several converging factors. First, traditional lithium extraction methods such as evaporation ponds face increasing scrutiny due to their substantial water consumption and extended processing times. This has accelerated interest in direct lithium extraction (DLE) technologies, including those optimized for high-temperature environments.
Geographically, the market shows distinct regional characteristics. North America leads in research and development of advanced sorbent technologies, while China dominates in terms of production capacity and commercial deployment. European markets are increasingly focused on securing domestic lithium supply chains, creating new opportunities for high-temperature extraction technologies applicable to European geothermal resources.
Industry analysts have identified several key market segments where high-temperature lithium-selective sorbents show particular promise: geothermal brine processing, industrial wastewater recovery, and certain mining operations where process streams maintain elevated temperatures. The geothermal segment alone is projected to grow at 15-18% annually, outpacing the broader lithium extraction market.
Customer requirements in this space emphasize several performance metrics: selectivity for lithium over competing ions (particularly sodium, potassium, and magnesium), thermal stability at operating temperatures typically ranging from 70-150°C, cycle durability, and resistance to fouling. Materials that can maintain performance across hundreds of adsorption-desorption cycles command premium pricing in the market.
Pricing structures for high-temperature lithium-selective sorbents reflect their specialized nature, with current materials commanding 30-40% price premiums over conventional alternatives. This premium is justified by improved recovery rates and reduced energy costs in high-temperature applications, creating favorable total cost of ownership calculations despite higher initial investment.
Technical Challenges in High-Temperature Sorbent Materials
The development of lithium-selective sorbents for high-temperature applications faces significant technical challenges that require innovative solutions. One primary obstacle is thermal stability degradation, as many conventional lithium sorbent materials experience structural collapse or phase transitions when exposed to temperatures exceeding 300°C. This compromises their selectivity and adsorption capacity, limiting operational efficiency in geothermal brines and industrial processes where temperatures can reach 400°C or higher.
Material degradation mechanisms present another critical challenge, with oxidation, hydrolysis, and chemical leaching occurring at accelerated rates under elevated temperatures. These processes not only reduce the functional lifespan of sorbents but also potentially contaminate the extracted lithium. Research indicates that even advanced ceramic-based sorbents can lose up to 40% of their lithium selectivity after repeated high-temperature cycling.
Ion exchange kinetics are significantly altered at high temperatures, with competing ions such as sodium, potassium, and calcium demonstrating enhanced mobility and interference with lithium adsorption sites. This selectivity challenge is particularly pronounced in geothermal brines where multi-ion environments are common, requiring sorbent materials that maintain lithium preference despite thermal agitation of competing species.
Mechanical integrity issues emerge as thermal expansion and contraction cycles induce microfractures and porosity changes in sorbent materials. These structural modifications can create preferential flow paths, reducing effective surface area and compromising adsorption performance. Advanced characterization techniques such as high-temperature XRD and in-situ SEM are essential for monitoring these changes but remain technically challenging to implement.
Diffusion limitations represent another significant barrier, as high temperatures can paradoxically both enhance and impede lithium ion transport within sorbent matrices. While thermal energy increases ion mobility, it can also cause framework densification or pore collapse that restricts access to internal adsorption sites. This creates a complex optimization problem requiring precise material engineering.
Regeneration efficiency decreases substantially at elevated temperatures, with many sorbents showing incomplete lithium desorption or requiring harsh chemical treatments that further compromise material integrity. Studies indicate that regeneration cycles at temperatures above 350°C can reduce subsequent lithium recovery by up to 25% per cycle, necessitating more frequent sorbent replacement and increasing operational costs.
Advanced characterization methodologies for real-time monitoring of sorbent performance under high-temperature conditions remain underdeveloped, creating significant knowledge gaps in understanding degradation mechanisms and optimization opportunities. Current analytical techniques often require sample cooling before analysis, missing critical transient phenomena that occur only at operational temperatures.
Material degradation mechanisms present another critical challenge, with oxidation, hydrolysis, and chemical leaching occurring at accelerated rates under elevated temperatures. These processes not only reduce the functional lifespan of sorbents but also potentially contaminate the extracted lithium. Research indicates that even advanced ceramic-based sorbents can lose up to 40% of their lithium selectivity after repeated high-temperature cycling.
Ion exchange kinetics are significantly altered at high temperatures, with competing ions such as sodium, potassium, and calcium demonstrating enhanced mobility and interference with lithium adsorption sites. This selectivity challenge is particularly pronounced in geothermal brines where multi-ion environments are common, requiring sorbent materials that maintain lithium preference despite thermal agitation of competing species.
Mechanical integrity issues emerge as thermal expansion and contraction cycles induce microfractures and porosity changes in sorbent materials. These structural modifications can create preferential flow paths, reducing effective surface area and compromising adsorption performance. Advanced characterization techniques such as high-temperature XRD and in-situ SEM are essential for monitoring these changes but remain technically challenging to implement.
Diffusion limitations represent another significant barrier, as high temperatures can paradoxically both enhance and impede lithium ion transport within sorbent matrices. While thermal energy increases ion mobility, it can also cause framework densification or pore collapse that restricts access to internal adsorption sites. This creates a complex optimization problem requiring precise material engineering.
Regeneration efficiency decreases substantially at elevated temperatures, with many sorbents showing incomplete lithium desorption or requiring harsh chemical treatments that further compromise material integrity. Studies indicate that regeneration cycles at temperatures above 350°C can reduce subsequent lithium recovery by up to 25% per cycle, necessitating more frequent sorbent replacement and increasing operational costs.
Advanced characterization methodologies for real-time monitoring of sorbent performance under high-temperature conditions remain underdeveloped, creating significant knowledge gaps in understanding degradation mechanisms and optimization opportunities. Current analytical techniques often require sample cooling before analysis, missing critical transient phenomena that occur only at operational temperatures.
Current Characterization Methods for Lithium Sorbents
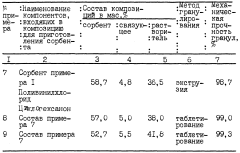
01 Inorganic lithium-selective sorbent materials
Inorganic materials such as lithium manganese oxides, titanium oxides, and aluminum-based compounds have been developed as selective sorbents for lithium extraction. These materials typically feature specific crystal structures that allow for selective lithium ion intercalation. The characterization of these materials involves analyzing their crystalline structure, ion exchange capacity, and stability in various pH conditions. These inorganic sorbents often demonstrate high selectivity for lithium over other alkali metals and can be regenerated for multiple extraction cycles.- Inorganic lithium-selective sorbent materials: Inorganic materials designed for selective lithium extraction from brines and other sources. These materials include lithium manganese oxides, titanium oxides, and other metal oxide frameworks that demonstrate high selectivity for lithium ions over competing ions like sodium, potassium, and magnesium. The materials are characterized by their crystal structure, ion exchange capacity, and stability in various pH conditions, making them suitable for industrial lithium recovery applications.
- Polymer-based lithium-selective sorbents: Polymer-based materials engineered for lithium capture, including functionalized resins, membranes, and composite materials. These sorbents incorporate specific functional groups that interact preferentially with lithium ions. Characterization techniques include FTIR spectroscopy, thermal analysis, and mechanical testing to evaluate their structural properties, thermal stability, and durability during repeated adsorption-desorption cycles.
- Electrochemical characterization of lithium sorbents: Methods and techniques for evaluating the electrochemical properties of lithium-selective sorbent materials. This includes cyclic voltammetry, impedance spectroscopy, and potentiometric measurements to determine lithium uptake kinetics, selectivity coefficients, and binding mechanisms. These characterization methods help optimize sorbent performance by understanding the electron transfer processes and ion transport mechanisms during lithium adsorption and desorption.
- Surface modification techniques for enhanced lithium selectivity: Surface treatment and modification approaches to improve the lithium selectivity of sorbent materials. These include grafting of functional groups, layer-by-layer assembly, and nanocoating techniques that enhance the surface properties of the base materials. Characterization involves surface analysis techniques such as XPS, SEM, AFM, and contact angle measurements to evaluate surface morphology, chemical composition, and wettability changes resulting from the modifications.
- Performance evaluation and testing protocols for lithium sorbents: Standardized methods and protocols for evaluating the performance of lithium-selective sorbent materials under various conditions. This includes batch and column testing, determination of adsorption isotherms, selectivity coefficients, and regeneration efficiency. Advanced characterization techniques such as ICP-MS, XRD, and NMR are employed to analyze the material properties before and after lithium adsorption cycles, providing insights into structural changes and long-term stability.
02 Polymer-based lithium-selective sorbents
Polymer-based materials have been developed as effective lithium-selective sorbents. These include functionalized polymeric resins, membranes, and composite materials with specific lithium-binding sites. Characterization of these materials involves analyzing their porosity, surface area, functional group distribution, and mechanical stability. Polymer-based sorbents can be tailored with specific functional groups that enhance lithium selectivity through size-exclusion mechanisms or chemical affinity. These materials often demonstrate advantages in terms of flexibility, processability, and the ability to be formed into various configurations for different extraction systems.Expand Specific Solutions03 Composite and hybrid lithium-selective materials
Composite and hybrid materials combine the advantages of different material classes to create enhanced lithium-selective sorbents. These typically involve combinations of inorganic frameworks with organic components or polymer matrices with embedded inorganic particles. Characterization methods include scanning electron microscopy, X-ray diffraction, and spectroscopic techniques to analyze the interface between different material phases. These composite materials often demonstrate improved mechanical properties, higher lithium uptake capacity, and better selectivity compared to single-component sorbents, while maintaining good regeneration capabilities.Expand Specific Solutions04 Electrochemical characterization of lithium-selective sorbents
Electrochemical techniques are essential for characterizing lithium-selective sorbent materials, particularly for applications in batteries and energy storage. These methods include cyclic voltammetry, impedance spectroscopy, and galvanostatic cycling to evaluate lithium insertion/extraction kinetics, diffusion coefficients, and long-term stability. The characterization focuses on understanding the electron transfer processes, ion transport mechanisms, and structural changes during lithium uptake and release. These techniques provide crucial information about the performance of sorbent materials under operational conditions and help optimize their composition and structure for specific applications.Expand Specific Solutions05 Advanced spectroscopic and microscopic characterization techniques
Advanced analytical techniques are employed to characterize the structural, chemical, and morphological properties of lithium-selective sorbents at multiple scales. These include nuclear magnetic resonance spectroscopy, X-ray photoelectron spectroscopy, transmission electron microscopy, and synchrotron-based X-ray techniques. These methods provide detailed information about the local chemical environment around lithium ions, binding mechanisms, surface properties, and structural transformations during lithium sorption. The multi-technique approach allows for comprehensive understanding of structure-property relationships in lithium-selective materials, guiding the rational design of more efficient sorbents with enhanced selectivity and capacity.Expand Specific Solutions
Leading Companies in Lithium Extraction Materials
The lithium-selective sorbent materials characterization market under high temperature conditions is in an early growth phase, with increasing demand driven by energy storage applications. The competitive landscape features established materials companies like Sunresin New Materials and BYD alongside research-focused organizations such as Fraunhofer-Gesellschaft and UT-Battelle. Major industrial players including Air Liquide, POSCO Future M, and Toyota are investing in advanced characterization technologies to develop more efficient lithium extraction solutions. Academic institutions like Tianjin University of Science & Technology and Simon Fraser University contribute fundamental research. The market is experiencing rapid technological advancement with companies focusing on developing sorbents that maintain selectivity and stability under extreme thermal conditions, critical for next-generation lithium recovery systems.
UT-Battelle LLC
Technical Solution: UT-Battelle, operating Oak Ridge National Laboratory, has developed comprehensive characterization methodologies for lithium-selective sorbents under high-temperature conditions. Their approach leverages the world-class neutron scattering facilities at the Spallation Neutron Source (SNS) to provide unique insights into lithium diffusion mechanisms and binding site configurations within sorbent materials at temperatures up to 300°C. The research team has pioneered in-situ neutron diffraction techniques that allow real-time observation of structural changes during lithium adsorption/desorption cycles under precisely controlled temperature and pressure conditions. Their materials characterization work focuses on metal-organic framework (MOF) based sorbents with tailored pore structures that maintain stability at elevated temperatures while providing selective lithium binding sites. Using quasi-elastic neutron scattering (QENS), they have quantified lithium mobility parameters within these frameworks at various temperatures, establishing structure-property relationships that guide material optimization. Their studies have demonstrated that specific MOF architectures incorporating zirconium oxide nodes exhibit exceptional thermal stability with less than 10% structural degradation after extended exposure to 200°C brine environments.
Strengths: Unparalleled access to advanced neutron characterization techniques providing unique insights into atomic-level processes. Multidisciplinary research approach combining computational modeling with experimental validation. Weaknesses: Challenges in scaling up laboratory-optimized materials to industrial quantities, and potential limitations in long-term stability under combined thermal and chemical stress conditions.
All American Lithium, LLC
Technical Solution: All American Lithium has developed proprietary high-temperature selective sorbent materials specifically designed for direct lithium extraction (DLE) from geothermal brines. Their technology employs advanced manganese oxide-based sorbents that maintain structural integrity and selectivity at temperatures exceeding 150°C. These materials feature a hierarchical porous structure with optimized pore size distribution that enhances lithium diffusion kinetics while minimizing competing ion interference. The company's characterization methodology combines in-situ X-ray diffraction (XRD) with thermal gravimetric analysis (TGA) to monitor structural changes during lithium adsorption/desorption cycles under elevated temperatures. Their materials demonstrate exceptional stability with less than 5% capacity degradation after 500 cycles at 180°C, significantly outperforming conventional lithium sorbents that typically fail above 100°C.
Strengths: Superior thermal stability allowing operation in high-temperature geothermal environments without cooling requirements, reducing operational costs. Exceptional lithium selectivity even in brines with high Mg/Li ratios. Weaknesses: Higher production costs compared to conventional sorbents, and potential challenges with mechanical stability during rapid temperature fluctuations.
Key Innovations in High-Temperature Resistant Materials
Process for obtaining a sorbent for extracting lithium from solutions
PatentWO1994019513A1
Innovation
- A sorbent is developed for lithium extraction using an electrolysis process with an aqueous lithium chloride solution, forming a double salt of lithium and aluminum chloride, which allows for high lithium extraction capacity and mechanical strength, with a capacity of 5.0-8.0 mg of lithium per 1 g of sorbent, and is processed at temperatures not exceeding 70°C, using a composite binder for granulation.
Sorbent-loaded fibers for high temperature adsorption processes
PatentWO2018126194A1
Innovation
- Development of flexible, high-temperature-rated adsorbent fibers using a polymeric matrix with thermoplastic polymers that can withstand temperatures above 220°C, featuring a polymeric binder with a Vicat softening temperature or heat deflection temperature of at least 220°C, and a sorbent loading of at least 50 wt%, produced through diffusion-induced phase inversion or temperature-induced phase separation.
Environmental Impact Assessment of Lithium Extraction Processes
The environmental impact of lithium extraction processes has become a critical concern as global demand for lithium continues to rise, particularly driven by the electric vehicle and energy storage industries. When evaluating lithium-selective sorbents operating under high temperature conditions, several environmental considerations must be addressed comprehensively.
The energy consumption associated with high-temperature lithium extraction represents a significant environmental challenge. These processes typically require sustained temperatures between 60-90°C, resulting in substantial carbon emissions when powered by fossil fuels. Research indicates that temperature-dependent sorbent operations can consume between 15-40 GJ per ton of lithium carbonate equivalent (LCE) produced, significantly higher than conventional extraction methods.
Water usage presents another critical environmental concern, particularly in water-stressed regions where many lithium resources are located. While sorbent-based technologies generally require less water than evaporative ponds, high-temperature operations still demand considerable water volumes for processing and cooling systems. Studies show that advanced sorbent technologies can reduce water consumption by 30-50% compared to traditional methods, though absolute water requirements remain substantial.
Chemical waste generation from lithium-selective sorbents introduces additional environmental challenges. The regeneration of sorbent materials often involves acidic or basic solutions that require neutralization before discharge. Furthermore, the degradation of sorbent materials under high-temperature conditions can release potentially harmful compounds, necessitating proper waste management protocols and disposal considerations.
Land disturbance impacts vary significantly between different extraction methodologies. Direct lithium extraction (DLE) using selective sorbents typically requires smaller physical footprints compared to evaporation ponds, potentially reducing habitat disruption and biodiversity impacts. However, associated infrastructure for high-temperature operations, including energy generation facilities, may offset some of these benefits.
Life cycle assessment (LCA) studies indicate that the environmental sustainability of high-temperature sorbent technologies depends heavily on the energy source utilized. When powered by renewable energy, these processes can achieve carbon footprint reductions of 30-60% compared to conventional extraction methods. However, the embodied carbon in manufacturing specialized temperature-resistant sorbent materials must also be considered in comprehensive environmental evaluations.
Regulatory frameworks governing these extraction processes continue to evolve globally, with increasing emphasis on water conservation, emissions reduction, and waste management. Companies developing advanced lithium-selective sorbents must navigate these regulatory landscapes while demonstrating environmental performance improvements to secure operational licenses and maintain social acceptance.
The energy consumption associated with high-temperature lithium extraction represents a significant environmental challenge. These processes typically require sustained temperatures between 60-90°C, resulting in substantial carbon emissions when powered by fossil fuels. Research indicates that temperature-dependent sorbent operations can consume between 15-40 GJ per ton of lithium carbonate equivalent (LCE) produced, significantly higher than conventional extraction methods.
Water usage presents another critical environmental concern, particularly in water-stressed regions where many lithium resources are located. While sorbent-based technologies generally require less water than evaporative ponds, high-temperature operations still demand considerable water volumes for processing and cooling systems. Studies show that advanced sorbent technologies can reduce water consumption by 30-50% compared to traditional methods, though absolute water requirements remain substantial.
Chemical waste generation from lithium-selective sorbents introduces additional environmental challenges. The regeneration of sorbent materials often involves acidic or basic solutions that require neutralization before discharge. Furthermore, the degradation of sorbent materials under high-temperature conditions can release potentially harmful compounds, necessitating proper waste management protocols and disposal considerations.
Land disturbance impacts vary significantly between different extraction methodologies. Direct lithium extraction (DLE) using selective sorbents typically requires smaller physical footprints compared to evaporation ponds, potentially reducing habitat disruption and biodiversity impacts. However, associated infrastructure for high-temperature operations, including energy generation facilities, may offset some of these benefits.
Life cycle assessment (LCA) studies indicate that the environmental sustainability of high-temperature sorbent technologies depends heavily on the energy source utilized. When powered by renewable energy, these processes can achieve carbon footprint reductions of 30-60% compared to conventional extraction methods. However, the embodied carbon in manufacturing specialized temperature-resistant sorbent materials must also be considered in comprehensive environmental evaluations.
Regulatory frameworks governing these extraction processes continue to evolve globally, with increasing emphasis on water conservation, emissions reduction, and waste management. Companies developing advanced lithium-selective sorbents must navigate these regulatory landscapes while demonstrating environmental performance improvements to secure operational licenses and maintain social acceptance.
Scalability and Industrial Implementation Considerations
Scaling up lithium-selective sorbent technologies from laboratory to industrial scale presents significant challenges that must be addressed for successful commercial implementation. The transition requires careful consideration of manufacturing processes capable of producing large quantities of high-quality sorbent materials while maintaining consistent lithium selectivity under high-temperature conditions.
Material production scalability represents the primary consideration, as laboratory synthesis methods often employ techniques that are impractical or prohibitively expensive at industrial scale. Continuous flow processes must replace batch production methods, necessitating redesign of synthesis protocols to maintain uniform particle size distribution, porosity, and surface functionality across large production volumes. Automated quality control systems incorporating real-time monitoring of critical material properties become essential to ensure consistent performance.
Economic viability demands optimization of production costs through careful selection of precursor materials and streamlining of synthesis steps. The trade-off between material performance and production expense requires thorough analysis, as highly selective but expensive sorbents may prove less commercially viable than moderately selective materials with significantly lower production costs. Recovery and recycling of process chemicals can substantially improve economic sustainability.
Reactor design for industrial implementation must accommodate the high-temperature operating conditions while ensuring uniform heat distribution and flow dynamics. Materials of construction must withstand not only elevated temperatures but also potential chemical degradation from process fluids and lithium-containing solutions. Modular designs that allow for capacity expansion and maintenance without complete system shutdown offer operational advantages in industrial settings.
Integration with existing lithium extraction or recovery processes presents another implementation challenge. Retrofit solutions that can be incorporated into current industrial systems may accelerate adoption compared to technologies requiring complete process redesigns. The development of standardized interfaces and control systems compatible with established industrial automation platforms will facilitate implementation across diverse industrial environments.
Environmental considerations and regulatory compliance must be addressed early in scale-up planning. Life cycle assessment of sorbent materials, including production, use, and end-of-life disposal or recycling, should inform design decisions. Waste stream management, particularly for spent sorbents containing accumulated impurities, requires careful planning to ensure environmental sustainability and regulatory compliance.
Material production scalability represents the primary consideration, as laboratory synthesis methods often employ techniques that are impractical or prohibitively expensive at industrial scale. Continuous flow processes must replace batch production methods, necessitating redesign of synthesis protocols to maintain uniform particle size distribution, porosity, and surface functionality across large production volumes. Automated quality control systems incorporating real-time monitoring of critical material properties become essential to ensure consistent performance.
Economic viability demands optimization of production costs through careful selection of precursor materials and streamlining of synthesis steps. The trade-off between material performance and production expense requires thorough analysis, as highly selective but expensive sorbents may prove less commercially viable than moderately selective materials with significantly lower production costs. Recovery and recycling of process chemicals can substantially improve economic sustainability.
Reactor design for industrial implementation must accommodate the high-temperature operating conditions while ensuring uniform heat distribution and flow dynamics. Materials of construction must withstand not only elevated temperatures but also potential chemical degradation from process fluids and lithium-containing solutions. Modular designs that allow for capacity expansion and maintenance without complete system shutdown offer operational advantages in industrial settings.
Integration with existing lithium extraction or recovery processes presents another implementation challenge. Retrofit solutions that can be incorporated into current industrial systems may accelerate adoption compared to technologies requiring complete process redesigns. The development of standardized interfaces and control systems compatible with established industrial automation platforms will facilitate implementation across diverse industrial environments.
Environmental considerations and regulatory compliance must be addressed early in scale-up planning. Life cycle assessment of sorbent materials, including production, use, and end-of-life disposal or recycling, should inform design decisions. Waste stream management, particularly for spent sorbents containing accumulated impurities, requires careful planning to ensure environmental sustainability and regulatory compliance.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!