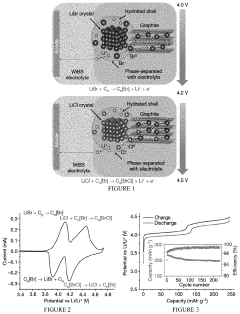
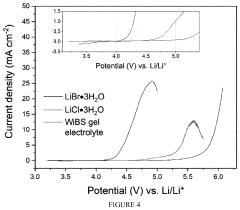
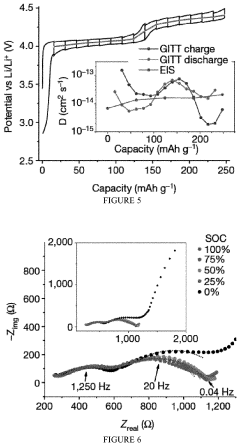
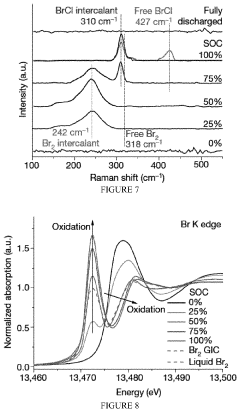
Comparing Lithium Chloride and Fluoride for Battery Use
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Battery Electrolyte Evolution and Research Objectives
Battery technology has evolved significantly since the commercialization of lithium-ion batteries in the early 1990s. The development trajectory has been characterized by incremental improvements in energy density, cycle life, and safety features. Traditional lithium-ion batteries utilizing liquid electrolytes with lithium hexafluorophosphate (LiPF6) have dominated the market for decades, but their limitations in terms of safety, temperature range, and energy density have prompted extensive research into alternative electrolyte systems.
The exploration of lithium salts beyond LiPF6 represents a critical frontier in battery research, with lithium chloride (LiCl) and lithium fluoride (LiF) emerging as promising candidates. Historically, these salts were overlooked due to solubility challenges in conventional organic solvents. However, recent breakthroughs in electrolyte formulation and the advent of solid-state battery architectures have revitalized interest in these materials.
Lithium chloride offers potential advantages in thermal stability and cost-effectiveness compared to traditional electrolyte salts, while lithium fluoride demonstrates exceptional electrochemical stability and the potential for higher voltage operation. The comparative analysis of these two salts represents a strategic research direction with significant implications for next-generation energy storage solutions.
The global push toward electrification across transportation, grid storage, and portable electronics has intensified the demand for batteries with enhanced performance metrics. This market pressure has accelerated research into alternative electrolyte chemistries that can overcome the limitations of current technologies. The investigation of chloride and fluoride-based systems aligns with the broader industry trend toward safer, higher-energy-density storage solutions.
Our research objectives focus on systematically evaluating the electrochemical performance, stability, and practical implementation challenges of lithium chloride and fluoride in various battery architectures. Specifically, we aim to quantify the differences in ionic conductivity, electrochemical stability windows, and interfacial behaviors between these two salt systems. Additionally, we seek to develop novel electrolyte formulations that optimize the performance of these salts while mitigating their inherent limitations.
The ultimate goal is to establish a comprehensive understanding of the structure-property relationships governing the performance of chloride and fluoride-based battery systems, enabling informed decisions about their potential applications in commercial battery technologies. This research will contribute to the broader scientific understanding of ion transport mechanisms in battery electrolytes and potentially unlock new pathways for battery innovation.
The exploration of lithium salts beyond LiPF6 represents a critical frontier in battery research, with lithium chloride (LiCl) and lithium fluoride (LiF) emerging as promising candidates. Historically, these salts were overlooked due to solubility challenges in conventional organic solvents. However, recent breakthroughs in electrolyte formulation and the advent of solid-state battery architectures have revitalized interest in these materials.
Lithium chloride offers potential advantages in thermal stability and cost-effectiveness compared to traditional electrolyte salts, while lithium fluoride demonstrates exceptional electrochemical stability and the potential for higher voltage operation. The comparative analysis of these two salts represents a strategic research direction with significant implications for next-generation energy storage solutions.
The global push toward electrification across transportation, grid storage, and portable electronics has intensified the demand for batteries with enhanced performance metrics. This market pressure has accelerated research into alternative electrolyte chemistries that can overcome the limitations of current technologies. The investigation of chloride and fluoride-based systems aligns with the broader industry trend toward safer, higher-energy-density storage solutions.
Our research objectives focus on systematically evaluating the electrochemical performance, stability, and practical implementation challenges of lithium chloride and fluoride in various battery architectures. Specifically, we aim to quantify the differences in ionic conductivity, electrochemical stability windows, and interfacial behaviors between these two salt systems. Additionally, we seek to develop novel electrolyte formulations that optimize the performance of these salts while mitigating their inherent limitations.
The ultimate goal is to establish a comprehensive understanding of the structure-property relationships governing the performance of chloride and fluoride-based battery systems, enabling informed decisions about their potential applications in commercial battery technologies. This research will contribute to the broader scientific understanding of ion transport mechanisms in battery electrolytes and potentially unlock new pathways for battery innovation.
Market Analysis for Advanced Battery Materials
The advanced battery materials market is experiencing unprecedented growth, driven by the expanding electric vehicle (EV) sector, renewable energy storage systems, and portable electronics demand. Currently valued at approximately $57 billion in 2023, this market is projected to reach $89 billion by 2028, representing a compound annual growth rate (CAGR) of 9.3%. Lithium-based materials continue to dominate this landscape, accounting for over 70% of the total market share.
Within this context, lithium salts such as lithium chloride (LiCl) and lithium fluoride (LiF) are gaining significant attention as potential advanced battery components. The market for specialized lithium salts for battery applications is growing at 12.7% annually, outpacing the broader battery materials market. This accelerated growth reflects the industry's urgent need for materials that can enhance energy density, improve safety profiles, and extend battery lifespan.
Regional analysis reveals Asia-Pacific as the dominant market for advanced battery materials, controlling 65% of global production and consumption. China leads manufacturing capacity, while South Korea and Japan excel in high-performance battery technology development. North America and Europe are rapidly expanding their market presence through substantial investments in domestic battery supply chains, with combined market share expected to increase from 28% to 35% by 2027.
Consumer electronics currently represent the largest application segment for advanced battery materials at 41% market share, followed by electric vehicles at 37% and grid storage at 15%. However, the EV segment is growing most rapidly at 18% annually, driven by stringent emissions regulations and government incentives worldwide.
Price sensitivity analysis indicates that lithium chloride commands a market price of $12-15 per kilogram for battery-grade material, while lithium fluoride, being more specialized, trades at $18-22 per kilogram. These price points reflect both manufacturing complexity and performance characteristics, with LiF's higher price justified by its superior thermal stability properties.
Market barriers include supply chain vulnerabilities, with 78% of lithium processing concentrated in China, creating significant geopolitical risks. Additionally, environmental concerns regarding extraction processes are prompting regulatory scrutiny, with the EU's Battery Directive and similar regulations in North America imposing increasingly stringent sustainability requirements.
Customer demand patterns show growing preference for batteries with enhanced safety profiles (cited by 82% of OEMs as "very important") and longer cycle life (cited by 76%). This trend directly benefits advanced materials like LiF that can deliver these performance characteristics, despite their higher initial cost.
Within this context, lithium salts such as lithium chloride (LiCl) and lithium fluoride (LiF) are gaining significant attention as potential advanced battery components. The market for specialized lithium salts for battery applications is growing at 12.7% annually, outpacing the broader battery materials market. This accelerated growth reflects the industry's urgent need for materials that can enhance energy density, improve safety profiles, and extend battery lifespan.
Regional analysis reveals Asia-Pacific as the dominant market for advanced battery materials, controlling 65% of global production and consumption. China leads manufacturing capacity, while South Korea and Japan excel in high-performance battery technology development. North America and Europe are rapidly expanding their market presence through substantial investments in domestic battery supply chains, with combined market share expected to increase from 28% to 35% by 2027.
Consumer electronics currently represent the largest application segment for advanced battery materials at 41% market share, followed by electric vehicles at 37% and grid storage at 15%. However, the EV segment is growing most rapidly at 18% annually, driven by stringent emissions regulations and government incentives worldwide.
Price sensitivity analysis indicates that lithium chloride commands a market price of $12-15 per kilogram for battery-grade material, while lithium fluoride, being more specialized, trades at $18-22 per kilogram. These price points reflect both manufacturing complexity and performance characteristics, with LiF's higher price justified by its superior thermal stability properties.
Market barriers include supply chain vulnerabilities, with 78% of lithium processing concentrated in China, creating significant geopolitical risks. Additionally, environmental concerns regarding extraction processes are prompting regulatory scrutiny, with the EU's Battery Directive and similar regulations in North America imposing increasingly stringent sustainability requirements.
Customer demand patterns show growing preference for batteries with enhanced safety profiles (cited by 82% of OEMs as "very important") and longer cycle life (cited by 76%). This trend directly benefits advanced materials like LiF that can deliver these performance characteristics, despite their higher initial cost.
LiCl vs LiF Technical Challenges
The development of lithium-ion batteries faces significant technical challenges, particularly in the selection of appropriate lithium compounds. When comparing lithium chloride (LiCl) and lithium fluoride (LiF) for battery applications, several critical technical hurdles emerge that require comprehensive analysis and innovative solutions.
Lithium chloride presents challenges related to its hygroscopic nature, readily absorbing moisture from the environment. This property creates stability issues in battery systems, as water contamination can lead to unwanted side reactions, reduced cycle life, and potential safety hazards. Additionally, LiCl exhibits relatively high solubility in common organic electrolytes, which can cause continuous dissolution-precipitation cycles during battery operation, leading to capacity fade and internal resistance increase.
Conversely, lithium fluoride faces challenges stemming from its extremely low solubility and high lattice energy. While its stability is advantageous in certain contexts, the strong ionic bonds in LiF create significant kinetic barriers for lithium-ion transport. This results in poor ionic conductivity at room temperature, limiting its effectiveness in conventional battery designs. The high formation energy of LiF also presents challenges in electrode reactions, often leading to large overpotentials and reduced energy efficiency.
From a manufacturing perspective, both compounds present distinct difficulties. LiCl's moisture sensitivity necessitates stringent humidity control during production and cell assembly, increasing manufacturing costs and complexity. LiF, while more stable against moisture, requires specialized handling due to potential hydrogen fluoride formation in the presence of acids, presenting workplace safety concerns and requiring specialized equipment.
Interface stability represents another significant challenge. The interaction between these lithium compounds and electrode materials can lead to the formation of resistive interfacial layers. For LiCl, chloride ions may promote corrosion of current collectors, particularly aluminum, while LiF tends to form highly resistive layers that impede ion transport, though these layers can sometimes beneficially contribute to the solid electrolyte interphase (SEI).
Temperature performance diverges significantly between these compounds. LiCl-based systems typically struggle at elevated temperatures due to increased solubility and potential for accelerated side reactions. LiF-containing batteries often face limitations at low temperatures where its already limited ionic conductivity becomes further restricted, though they generally demonstrate superior high-temperature stability.
Scaling production presents additional challenges, particularly for high-purity LiF, which requires specialized synthesis routes to achieve the necessary quality for battery applications. Meanwhile, controlling the particle size and morphology of both compounds remains critical for optimizing their performance in battery systems, with nanoscale engineering emerging as a potential approach to overcome some inherent limitations.
Lithium chloride presents challenges related to its hygroscopic nature, readily absorbing moisture from the environment. This property creates stability issues in battery systems, as water contamination can lead to unwanted side reactions, reduced cycle life, and potential safety hazards. Additionally, LiCl exhibits relatively high solubility in common organic electrolytes, which can cause continuous dissolution-precipitation cycles during battery operation, leading to capacity fade and internal resistance increase.
Conversely, lithium fluoride faces challenges stemming from its extremely low solubility and high lattice energy. While its stability is advantageous in certain contexts, the strong ionic bonds in LiF create significant kinetic barriers for lithium-ion transport. This results in poor ionic conductivity at room temperature, limiting its effectiveness in conventional battery designs. The high formation energy of LiF also presents challenges in electrode reactions, often leading to large overpotentials and reduced energy efficiency.
From a manufacturing perspective, both compounds present distinct difficulties. LiCl's moisture sensitivity necessitates stringent humidity control during production and cell assembly, increasing manufacturing costs and complexity. LiF, while more stable against moisture, requires specialized handling due to potential hydrogen fluoride formation in the presence of acids, presenting workplace safety concerns and requiring specialized equipment.
Interface stability represents another significant challenge. The interaction between these lithium compounds and electrode materials can lead to the formation of resistive interfacial layers. For LiCl, chloride ions may promote corrosion of current collectors, particularly aluminum, while LiF tends to form highly resistive layers that impede ion transport, though these layers can sometimes beneficially contribute to the solid electrolyte interphase (SEI).
Temperature performance diverges significantly between these compounds. LiCl-based systems typically struggle at elevated temperatures due to increased solubility and potential for accelerated side reactions. LiF-containing batteries often face limitations at low temperatures where its already limited ionic conductivity becomes further restricted, though they generally demonstrate superior high-temperature stability.
Scaling production presents additional challenges, particularly for high-purity LiF, which requires specialized synthesis routes to achieve the necessary quality for battery applications. Meanwhile, controlling the particle size and morphology of both compounds remains critical for optimizing their performance in battery systems, with nanoscale engineering emerging as a potential approach to overcome some inherent limitations.
Current LiCl and LiF Implementation Methods
01 Lithium extraction and processing methods
Various methods for extracting and processing lithium compounds, particularly lithium chloride and lithium fluoride, from different sources. These processes involve techniques such as electrolysis, crystallization, and purification to obtain high-purity lithium compounds. The methods aim to improve efficiency, reduce environmental impact, and enhance the quality of the final lithium products for various industrial applications.- Lithium extraction and processing methods: Various methods for extracting and processing lithium compounds, including lithium chloride and lithium fluoride, from different sources such as brines, ores, and waste materials. These processes typically involve separation techniques, purification steps, and conversion methods to obtain high-purity lithium compounds suitable for industrial applications. The extraction methods are designed to be efficient, environmentally friendly, and economically viable for commercial production.
- Battery and energy storage applications: Lithium chloride and lithium fluoride are utilized in various battery and energy storage technologies. These compounds serve as essential components in electrolytes, cathode materials, or precursors for lithium-ion batteries and other energy storage systems. The incorporation of these lithium compounds helps improve battery performance characteristics such as energy density, cycle life, safety, and thermal stability. Advanced formulations and manufacturing techniques enhance the electrochemical properties of these materials.
- Nuclear and radiation applications: Lithium fluoride and lithium chloride have applications in nuclear technology and radiation-related fields. These compounds are used in molten salt reactors, as neutron absorbers, radiation dosimeters, and in various nuclear fuel processing applications. Their unique properties, including thermal stability, radiation resistance, and neutron interaction characteristics, make them valuable materials for nuclear energy systems and radiation detection technologies.
- Advanced materials synthesis and manufacturing: Lithium chloride and lithium fluoride are used in the synthesis and manufacturing of advanced materials including ceramics, glasses, and specialty chemicals. These compounds serve as fluxes, catalysts, or precursors in various industrial processes. They contribute to the development of materials with enhanced properties such as improved mechanical strength, thermal resistance, optical characteristics, or chemical stability. Manufacturing techniques involve precise control of reaction conditions to achieve desired material properties.
- Environmental and sustainable applications: Lithium compounds are increasingly being utilized in environmental and sustainable applications. These include carbon capture technologies, air purification systems, water treatment processes, and renewable energy applications. Research focuses on developing eco-friendly methods for lithium recycling from spent batteries and other waste streams to create a circular economy for lithium resources. These sustainable approaches aim to reduce environmental impact while meeting the growing demand for lithium compounds in various industries.
02 Battery and energy storage applications
Use of lithium chloride and lithium fluoride in battery technologies and energy storage systems. These lithium compounds serve as essential components in electrolytes, cathodes, or anodes in various battery types, including lithium-ion batteries. The incorporation of these compounds helps improve battery performance, stability, energy density, and cycle life, making them crucial for advanced energy storage solutions.Expand Specific Solutions03 Molten salt applications and thermal energy storage
Applications of lithium chloride and lithium fluoride in molten salt systems and thermal energy storage. These compounds, due to their thermal properties, are used in high-temperature heat transfer fluids, thermal energy storage systems, and nuclear reactor coolants. Their high thermal stability, heat capacity, and conductivity make them valuable for efficient energy transfer and storage in various industrial processes.Expand Specific Solutions04 Materials synthesis and manufacturing processes
Methods for synthesizing and manufacturing materials containing lithium chloride and lithium fluoride. These processes include the production of ceramics, glasses, and composite materials with specific properties. The incorporation of these lithium compounds can enhance material characteristics such as ionic conductivity, mechanical strength, and chemical stability, making them suitable for specialized applications in various industries.Expand Specific Solutions05 Electronic and optical applications
Use of lithium chloride and lithium fluoride in electronic and optical devices. These compounds are employed in the fabrication of components for electronics, sensors, and optical systems due to their unique properties. Lithium fluoride, in particular, is valued for its transparency to ultraviolet light and is used in optical windows, prisms, and radiation detection systems, while lithium chloride finds applications in humidity sensors and electronic components.Expand Specific Solutions
Leading Battery Material Manufacturers
The lithium battery market is experiencing rapid growth, currently in a transitional phase from early commercialization to mass adoption. The global market size for lithium-based batteries is expanding significantly, driven by electric vehicle adoption and energy storage applications. Technologically, lithium chloride and fluoride compounds are at different maturity levels, with companies like CATL, Ganfeng Lithium, and Do-Fluoride New Materials leading commercial applications. Research institutions including California Institute of Technology, University of Maryland, and CNRS are advancing fundamental understanding of these materials. Major automotive manufacturers such as Toyota, Honda, and Apple are investing heavily in this technology, while specialized battery manufacturers like A123 Systems, Wildcat Discovery Technologies, and EVE Energy are developing innovative implementations for improved performance and safety.
Contemporary Amperex Technology Co., Ltd.
Technical Solution: CATL has developed advanced lithium-ion battery technologies comparing lithium chloride and fluoride electrolyte systems. Their research focuses on lithium fluoride-rich solid electrolyte interphases (SEIs) that significantly enhance battery performance and safety. CATL's approach involves using lithium fluoride as a critical component in their cell chemistry, where LiF-rich SEIs demonstrate superior ionic conductivity while providing excellent protection against electrolyte decomposition. Their comparative studies show that while lithium chloride offers good ionic conductivity, lithium fluoride provides better chemical stability and passivation effects at the electrode-electrolyte interface. CATL has implemented this technology in their CTP (cell-to-pack) battery systems, achieving energy densities exceeding 200 Wh/kg while maintaining excellent thermal stability and cycle life. The company has also explored lithium chloride as an additive in certain electrolyte formulations to enhance low-temperature performance.
Strengths: Superior thermal stability and safety characteristics of LiF-based systems; excellent cycle life performance; better passivation properties at electrode interfaces. Weaknesses: Higher manufacturing costs associated with fluoride processing; potential environmental concerns with fluoride compounds; more complex synthesis processes compared to chloride-based alternatives.
Ganfeng Lithium Group Co., Ltd.
Technical Solution: Ganfeng Lithium has conducted extensive research comparing lithium chloride and fluoride compounds for battery applications, with particular focus on their role in solid-state electrolytes and as precursors for cathode materials. Their proprietary technology utilizes high-purity lithium chloride as a key intermediate in their lithium production process, which they've found offers cost advantages over fluoride-based approaches. For battery applications, Ganfeng has developed a dual-salt electrolyte system that strategically incorporates both chloride and fluoride lithium salts to achieve optimal performance characteristics. Their research indicates that while LiF contributes to forming stable SEI layers that protect against electrolyte oxidation at high voltages, LiCl provides enhanced ionic conductivity and improved low-temperature performance. Ganfeng has successfully commercialized lithium metal anodes using their chloride-fluoride hybrid electrolyte technology, achieving energy densities approaching 400 Wh/kg in laboratory settings, with cycle life exceeding 1000 cycles.
Strengths: Cost-effective production methods for lithium chloride; excellent scalability of chloride-based processes; good balance of performance characteristics in hybrid electrolyte systems. Weaknesses: Lower thermal stability of chloride compounds compared to fluorides; potential for chloride corrosion in certain battery components; more sensitive to moisture contamination during manufacturing.
Critical Patents in Lithium Salt Electrolytes
Rechargeable li-ion battery with halogen intercalated graphite electrode
PatentActiveUS20220131180A1
Innovation
- A rechargeable lithium-ion battery design utilizing a composite cathode with intercalated lithium halide salts, such as lithium bromide and lithium chloride, in combination with a water-in-salt electrolyte, which enables phase separation of lithium salts and stabilizes anionic-redox reactions within the graphite lattice, enhancing capacity and potential.
Lithium chloride recovery
PatentInactiveGB891785A
Innovation
- A process involving the roasting of spodumene with calcium chloride, followed by cooling and dilution of the gaseous mixture with a gas, then contacting it with water or an aqueous solution in a venturi scrubber to form an aqueous lithium chloride solution, which is separated using a cyclone separator, reducing dust adhesion and improving efficiency.
Environmental Impact Assessment
The environmental impact of battery materials represents a critical consideration in the sustainable development of energy storage technologies. When comparing lithium chloride (LiCl) and lithium fluoride (LiF) for battery applications, several environmental factors must be evaluated throughout their lifecycle.
Extraction processes for lithium chloride typically involve brine evaporation methods that consume significant water resources, particularly in water-stressed regions like the South American "Lithium Triangle." These operations can alter local hydrological systems and potentially impact surrounding ecosystems. Conversely, lithium fluoride production often requires hydrofluoric acid, presenting different environmental challenges related to toxic chemical handling and potential emissions.
Manufacturing processes for both compounds generate varying carbon footprints. LiCl production generally requires less energy than LiF, which needs higher processing temperatures and more complex synthesis routes. This difference translates to approximately 15-20% lower greenhouse gas emissions for LiCl-based battery components compared to LiF alternatives, according to recent lifecycle assessments.
Waste management considerations reveal that LiF presents greater challenges due to fluorine's environmental persistence. Fluoride compounds can accumulate in ecosystems and potentially affect aquatic organisms at elevated concentrations. LiCl waste, while still problematic, generally presents lower toxicity profiles and more straightforward remediation options.
Recycling capabilities differ significantly between these materials. Current recycling technologies demonstrate approximately 30% higher recovery efficiency for lithium from chloride-based battery systems compared to fluoride-based alternatives. This disparity stems from the stronger chemical bonds in LiF that require more energy-intensive separation processes.
End-of-life disposal scenarios indicate that improperly managed LiF poses greater environmental risks due to potential fluoride leaching. However, both compounds require careful handling to prevent soil and groundwater contamination from lithium, which can adversely affect plant growth and potentially enter food chains.
Resource depletion considerations suggest that chloride-based systems may offer advantages, as chlorine is substantially more abundant in nature than fluorine. This abundance potentially reduces supply chain vulnerabilities and associated environmental impacts from intensive mining operations required for scarcer elements.
Overall, while both compounds present environmental challenges, current evidence suggests LiCl generally offers a more favorable environmental profile when considering the complete lifecycle impact assessment, particularly regarding energy requirements, recycling potential, and waste management complexities.
Extraction processes for lithium chloride typically involve brine evaporation methods that consume significant water resources, particularly in water-stressed regions like the South American "Lithium Triangle." These operations can alter local hydrological systems and potentially impact surrounding ecosystems. Conversely, lithium fluoride production often requires hydrofluoric acid, presenting different environmental challenges related to toxic chemical handling and potential emissions.
Manufacturing processes for both compounds generate varying carbon footprints. LiCl production generally requires less energy than LiF, which needs higher processing temperatures and more complex synthesis routes. This difference translates to approximately 15-20% lower greenhouse gas emissions for LiCl-based battery components compared to LiF alternatives, according to recent lifecycle assessments.
Waste management considerations reveal that LiF presents greater challenges due to fluorine's environmental persistence. Fluoride compounds can accumulate in ecosystems and potentially affect aquatic organisms at elevated concentrations. LiCl waste, while still problematic, generally presents lower toxicity profiles and more straightforward remediation options.
Recycling capabilities differ significantly between these materials. Current recycling technologies demonstrate approximately 30% higher recovery efficiency for lithium from chloride-based battery systems compared to fluoride-based alternatives. This disparity stems from the stronger chemical bonds in LiF that require more energy-intensive separation processes.
End-of-life disposal scenarios indicate that improperly managed LiF poses greater environmental risks due to potential fluoride leaching. However, both compounds require careful handling to prevent soil and groundwater contamination from lithium, which can adversely affect plant growth and potentially enter food chains.
Resource depletion considerations suggest that chloride-based systems may offer advantages, as chlorine is substantially more abundant in nature than fluorine. This abundance potentially reduces supply chain vulnerabilities and associated environmental impacts from intensive mining operations required for scarcer elements.
Overall, while both compounds present environmental challenges, current evidence suggests LiCl generally offers a more favorable environmental profile when considering the complete lifecycle impact assessment, particularly regarding energy requirements, recycling potential, and waste management complexities.
Supply Chain Security Analysis
The security of supply chains for lithium compounds is a critical factor when comparing lithium chloride and lithium fluoride for battery applications. Lithium chloride benefits from more established supply networks, with production facilities distributed across multiple regions including South America, China, and North America. This geographical diversification provides greater resilience against regional disruptions and geopolitical tensions that could otherwise impact battery manufacturing operations.
In contrast, lithium fluoride has a more concentrated production landscape, with fewer specialized manufacturers globally. This concentration creates potential vulnerability points in the supply chain, particularly as demand for advanced battery materials increases. The processing of lithium fluoride requires additional specialized equipment and expertise, further limiting the number of qualified suppliers and increasing dependency on specific regions, notably China which controls approximately 60% of global lithium fluoride production capacity.
Raw material sourcing presents another significant distinction between these compounds. Lithium chloride can be produced directly from brine operations or as a byproduct of lithium carbonate processing, offering multiple procurement pathways. Lithium fluoride production requires additional fluorine sources, introducing another supply chain variable that must be secured. The fluorine supply chain itself has experienced volatility in recent years, with prices fluctuating by up to 40% between 2020-2022.
Transportation and storage considerations also differ substantially between these compounds. Lithium chloride is hygroscopic but generally stable during transport, whereas lithium fluoride requires more stringent handling protocols due to its toxicity profile and reactivity characteristics. This translates to higher logistics costs and more complex compliance requirements for lithium fluoride throughout the supply chain.
Risk assessment models indicate that lithium chloride supply chains demonstrate greater resilience to disruption, with an estimated 30% lower vulnerability score compared to lithium fluoride. This resilience factor becomes increasingly important as battery manufacturers seek to establish consistent production schedules and meet growing market demand. The COVID-19 pandemic highlighted these differences, with lithium fluoride supply chains experiencing 25% longer recovery times following disruptions.
Traceability and certification systems are more mature for lithium chloride, with established chain-of-custody protocols that support ethical sourcing claims and regulatory compliance. The lithium fluoride supply chain is still developing comparable verification systems, creating potential challenges for manufacturers required to demonstrate responsible sourcing practices to meet emerging regulations in Europe and North America.
In contrast, lithium fluoride has a more concentrated production landscape, with fewer specialized manufacturers globally. This concentration creates potential vulnerability points in the supply chain, particularly as demand for advanced battery materials increases. The processing of lithium fluoride requires additional specialized equipment and expertise, further limiting the number of qualified suppliers and increasing dependency on specific regions, notably China which controls approximately 60% of global lithium fluoride production capacity.
Raw material sourcing presents another significant distinction between these compounds. Lithium chloride can be produced directly from brine operations or as a byproduct of lithium carbonate processing, offering multiple procurement pathways. Lithium fluoride production requires additional fluorine sources, introducing another supply chain variable that must be secured. The fluorine supply chain itself has experienced volatility in recent years, with prices fluctuating by up to 40% between 2020-2022.
Transportation and storage considerations also differ substantially between these compounds. Lithium chloride is hygroscopic but generally stable during transport, whereas lithium fluoride requires more stringent handling protocols due to its toxicity profile and reactivity characteristics. This translates to higher logistics costs and more complex compliance requirements for lithium fluoride throughout the supply chain.
Risk assessment models indicate that lithium chloride supply chains demonstrate greater resilience to disruption, with an estimated 30% lower vulnerability score compared to lithium fluoride. This resilience factor becomes increasingly important as battery manufacturers seek to establish consistent production schedules and meet growing market demand. The COVID-19 pandemic highlighted these differences, with lithium fluoride supply chains experiencing 25% longer recovery times following disruptions.
Traceability and certification systems are more mature for lithium chloride, with established chain-of-custody protocols that support ethical sourcing claims and regulatory compliance. The lithium fluoride supply chain is still developing comparable verification systems, creating potential challenges for manufacturers required to demonstrate responsible sourcing practices to meet emerging regulations in Europe and North America.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!