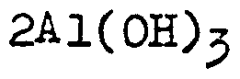How to Test Lithium Chloride in Surfactant Production
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
LiCl Testing Background and Objectives
Lithium chloride (LiCl) testing in surfactant production has evolved significantly over the past decades, transitioning from rudimentary analytical methods to sophisticated instrumental techniques. The historical development of LiCl testing methodologies reflects broader trends in analytical chemistry, with early approaches relying primarily on gravimetric and titrimetric analyses that offered limited precision and required substantial sample quantities. As surfactant formulations became more complex and performance requirements more stringent, the need for more accurate, sensitive, and efficient testing methods became increasingly apparent.
The presence of lithium chloride in surfactant production processes serves multiple critical functions, including acting as a catalyst in certain reactions, modifying rheological properties, and enhancing product stability. Precise monitoring of LiCl concentrations throughout the production cycle is essential for maintaining product quality, ensuring batch-to-batch consistency, and optimizing manufacturing efficiency. Deviations in LiCl content can significantly impact surfactant performance characteristics such as foaming properties, viscosity, and cleaning efficacy.
Current technological objectives in LiCl testing focus on developing methods that offer real-time monitoring capabilities, reduced sample preparation requirements, and enhanced sensitivity at lower detection limits. The industry is increasingly moving toward non-destructive testing approaches that can be integrated directly into production lines, allowing for continuous process monitoring rather than relying solely on discrete sampling and laboratory analysis. This shift aligns with broader Industry 4.0 trends emphasizing process automation and data-driven manufacturing optimization.
Another key objective is the development of more environmentally sustainable testing methodologies that reduce chemical waste generation and energy consumption while maintaining or improving analytical performance. This includes exploring green chemistry principles in test design and implementing miniaturized analytical systems that require smaller reagent volumes and generate less waste.
The global regulatory landscape also shapes testing objectives, with increasing requirements for traceability, validation, and documentation of analytical methods used in quality control processes. Testing protocols must now demonstrate compliance with various international standards and guidelines, including those established by organizations such as ISO, ASTM, and industry-specific regulatory bodies.
Technological convergence represents another important trend, with the integration of multiple analytical techniques into unified testing platforms that can simultaneously evaluate multiple parameters beyond just LiCl concentration. These comprehensive analytical approaches aim to provide a more complete characterization of surfactant properties and composition, enabling more informed process control decisions and product development strategies.
The presence of lithium chloride in surfactant production processes serves multiple critical functions, including acting as a catalyst in certain reactions, modifying rheological properties, and enhancing product stability. Precise monitoring of LiCl concentrations throughout the production cycle is essential for maintaining product quality, ensuring batch-to-batch consistency, and optimizing manufacturing efficiency. Deviations in LiCl content can significantly impact surfactant performance characteristics such as foaming properties, viscosity, and cleaning efficacy.
Current technological objectives in LiCl testing focus on developing methods that offer real-time monitoring capabilities, reduced sample preparation requirements, and enhanced sensitivity at lower detection limits. The industry is increasingly moving toward non-destructive testing approaches that can be integrated directly into production lines, allowing for continuous process monitoring rather than relying solely on discrete sampling and laboratory analysis. This shift aligns with broader Industry 4.0 trends emphasizing process automation and data-driven manufacturing optimization.
Another key objective is the development of more environmentally sustainable testing methodologies that reduce chemical waste generation and energy consumption while maintaining or improving analytical performance. This includes exploring green chemistry principles in test design and implementing miniaturized analytical systems that require smaller reagent volumes and generate less waste.
The global regulatory landscape also shapes testing objectives, with increasing requirements for traceability, validation, and documentation of analytical methods used in quality control processes. Testing protocols must now demonstrate compliance with various international standards and guidelines, including those established by organizations such as ISO, ASTM, and industry-specific regulatory bodies.
Technological convergence represents another important trend, with the integration of multiple analytical techniques into unified testing platforms that can simultaneously evaluate multiple parameters beyond just LiCl concentration. These comprehensive analytical approaches aim to provide a more complete characterization of surfactant properties and composition, enabling more informed process control decisions and product development strategies.
Surfactant Market Analysis and Testing Requirements
The global surfactant market has been experiencing steady growth, valued at approximately $43.6 billion in 2022 and projected to reach $58.5 billion by 2030, growing at a CAGR of 3.7%. This growth is primarily driven by increasing demand across various end-use industries including personal care, household detergents, industrial cleaning, and agricultural chemicals. The Asia-Pacific region dominates the market share, accounting for over 35% of global consumption, followed by North America and Europe.
Within the surfactant industry, lithium-based compounds, particularly lithium chloride, have gained significant attention due to their unique properties and applications in specialty surfactant formulations. The market for lithium-containing surfactants is relatively niche but growing at an accelerated rate of 5.2% annually, driven by their superior performance characteristics in specific applications.
Testing requirements for lithium chloride in surfactant production have become increasingly stringent due to quality control concerns and regulatory compliance needs. The primary testing parameters include concentration verification, purity assessment, moisture content, and potential contaminants detection. Industry standards typically require lithium chloride purity levels of at least 98.5% for surfactant applications, with moisture content below 0.5%.
The testing methodologies commonly employed in the industry include atomic absorption spectroscopy (AAS), inductively coupled plasma mass spectrometry (ICP-MS), and ion chromatography. These methods provide detection limits in the parts per million (ppm) range, which meets the industry requirements for precise quantification of lithium content and impurities.
Market analysis indicates that surfactant manufacturers are increasingly demanding rapid, accurate, and cost-effective testing solutions that can be integrated into production processes. This has led to the development of specialized testing kits and automated analytical systems specifically designed for lithium compound analysis in surfactant matrices.
Regulatory requirements for lithium-containing products vary significantly across regions, with the European Union implementing the most stringent standards through REACH regulations. In the United States, the FDA and EPA provide guidelines for lithium compounds in consumer products, while Asian markets generally follow international standards with some country-specific variations.
The cost implications of lithium chloride testing in surfactant production are substantial, with quality control processes accounting for approximately 3-4% of total production costs. However, the economic impact of inadequate testing can be far greater, including product recalls, regulatory penalties, and brand reputation damage.
Within the surfactant industry, lithium-based compounds, particularly lithium chloride, have gained significant attention due to their unique properties and applications in specialty surfactant formulations. The market for lithium-containing surfactants is relatively niche but growing at an accelerated rate of 5.2% annually, driven by their superior performance characteristics in specific applications.
Testing requirements for lithium chloride in surfactant production have become increasingly stringent due to quality control concerns and regulatory compliance needs. The primary testing parameters include concentration verification, purity assessment, moisture content, and potential contaminants detection. Industry standards typically require lithium chloride purity levels of at least 98.5% for surfactant applications, with moisture content below 0.5%.
The testing methodologies commonly employed in the industry include atomic absorption spectroscopy (AAS), inductively coupled plasma mass spectrometry (ICP-MS), and ion chromatography. These methods provide detection limits in the parts per million (ppm) range, which meets the industry requirements for precise quantification of lithium content and impurities.
Market analysis indicates that surfactant manufacturers are increasingly demanding rapid, accurate, and cost-effective testing solutions that can be integrated into production processes. This has led to the development of specialized testing kits and automated analytical systems specifically designed for lithium compound analysis in surfactant matrices.
Regulatory requirements for lithium-containing products vary significantly across regions, with the European Union implementing the most stringent standards through REACH regulations. In the United States, the FDA and EPA provide guidelines for lithium compounds in consumer products, while Asian markets generally follow international standards with some country-specific variations.
The cost implications of lithium chloride testing in surfactant production are substantial, with quality control processes accounting for approximately 3-4% of total production costs. However, the economic impact of inadequate testing can be far greater, including product recalls, regulatory penalties, and brand reputation damage.
Current LiCl Detection Methods and Limitations
The detection of lithium chloride in surfactant production processes currently employs several analytical methods, each with specific advantages and limitations. Atomic Absorption Spectroscopy (AAS) represents one of the most established techniques, offering detection limits in the parts per million (ppm) range. While AAS provides reliable quantitative analysis, it requires sample preparation that can be time-consuming and involves destructive testing, making it unsuitable for real-time monitoring during production.
Ion Chromatography (IC) offers excellent selectivity for lithium ions in complex surfactant matrices, with detection limits approaching 0.1 ppm. However, the equipment is costly, requires specialized training, and analysis times typically range from 15-30 minutes, creating bottlenecks in high-throughput production environments. Additionally, surfactant components can interfere with column performance, necessitating frequent maintenance.
Inductively Coupled Plasma Mass Spectrometry (ICP-MS) delivers superior sensitivity with detection limits in the parts per billion (ppb) range, making it ideal for trace lithium analysis. Despite its analytical power, ICP-MS systems are prohibitively expensive for many manufacturers, require highly trained operators, and involve complex sample preparation protocols that limit throughput.
Flame photometry represents a more accessible alternative with moderate sensitivity (1-5 ppm) and relatively simple operation. However, this technique suffers from matrix effects when analyzing surfactant samples, leading to potential inaccuracies without careful calibration. The method also lacks the precision of more advanced techniques when measuring lithium at concentrations below 1 ppm.
Potentiometric methods using lithium-selective electrodes offer the advantage of potential in-line monitoring capabilities but demonstrate limited selectivity in the presence of interfering ions commonly found in surfactant formulations. These electrodes typically exhibit drift over time and require frequent recalibration, limiting their reliability in production settings.
Colorimetric test kits provide rapid qualitative or semi-quantitative results but lack the precision required for quality control in surfactant manufacturing. These kits often suffer from interference from surfactant components and have relatively high detection limits (typically 5-10 ppm), making them suitable only for preliminary screening.
A significant limitation across all current methods is the challenge of analyzing lithium chloride directly in surfactant matrices without extensive sample preparation. Surfactants create emulsions, foaming, and other interferences that complicate accurate analysis. Additionally, most techniques require taking samples offline, creating delays between production and analytical results that can lead to manufacturing inefficiencies and quality control issues.
Ion Chromatography (IC) offers excellent selectivity for lithium ions in complex surfactant matrices, with detection limits approaching 0.1 ppm. However, the equipment is costly, requires specialized training, and analysis times typically range from 15-30 minutes, creating bottlenecks in high-throughput production environments. Additionally, surfactant components can interfere with column performance, necessitating frequent maintenance.
Inductively Coupled Plasma Mass Spectrometry (ICP-MS) delivers superior sensitivity with detection limits in the parts per billion (ppb) range, making it ideal for trace lithium analysis. Despite its analytical power, ICP-MS systems are prohibitively expensive for many manufacturers, require highly trained operators, and involve complex sample preparation protocols that limit throughput.
Flame photometry represents a more accessible alternative with moderate sensitivity (1-5 ppm) and relatively simple operation. However, this technique suffers from matrix effects when analyzing surfactant samples, leading to potential inaccuracies without careful calibration. The method also lacks the precision of more advanced techniques when measuring lithium at concentrations below 1 ppm.
Potentiometric methods using lithium-selective electrodes offer the advantage of potential in-line monitoring capabilities but demonstrate limited selectivity in the presence of interfering ions commonly found in surfactant formulations. These electrodes typically exhibit drift over time and require frequent recalibration, limiting their reliability in production settings.
Colorimetric test kits provide rapid qualitative or semi-quantitative results but lack the precision required for quality control in surfactant manufacturing. These kits often suffer from interference from surfactant components and have relatively high detection limits (typically 5-10 ppm), making them suitable only for preliminary screening.
A significant limitation across all current methods is the challenge of analyzing lithium chloride directly in surfactant matrices without extensive sample preparation. Surfactants create emulsions, foaming, and other interferences that complicate accurate analysis. Additionally, most techniques require taking samples offline, creating delays between production and analytical results that can lead to manufacturing inefficiencies and quality control issues.
Established LiCl Testing Protocols in Surfactant Industry
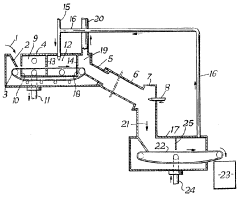
01 Lithium chloride extraction and production methods
Various methods for extracting and producing lithium chloride from different sources, including brine solutions and lithium-containing minerals. These processes involve techniques such as evaporation, crystallization, and chemical treatment to obtain high-purity lithium chloride. The methods aim to improve efficiency, reduce environmental impact, and increase yield of lithium chloride production for industrial applications.- Lithium chloride extraction and production methods: Various methods for extracting and producing lithium chloride from natural sources such as brines and minerals. These processes typically involve concentration, purification steps, and crystallization techniques to obtain high-purity lithium chloride. The methods aim to improve efficiency, reduce environmental impact, and increase yield of lithium chloride production for industrial applications.
- Lithium chloride in pharmaceutical applications: Lithium chloride is utilized in pharmaceutical formulations for treating various conditions, particularly psychiatric disorders. The compound serves as an active pharmaceutical ingredient or precursor in drug manufacturing processes. Pharmaceutical applications focus on controlled delivery systems, stability enhancement, and bioavailability improvement of lithium-based medications.
- Lithium chloride in battery technology: Applications of lithium chloride in battery technology, including its use in electrolytes, electrode materials, and battery manufacturing processes. Lithium chloride serves as a precursor for lithium-ion battery components or as an additive to improve battery performance characteristics such as capacity, cycle life, and safety. These innovations contribute to advancements in energy storage solutions.
- Lithium chloride in industrial processes and materials: Utilization of lithium chloride in various industrial applications including as a desiccant, flux for welding and soldering, catalyst in chemical reactions, and additive in ceramics and glass manufacturing. The compound's hygroscopic properties and chemical characteristics make it valuable for humidity control, material processing, and as a component in specialized industrial materials.
- Lithium chloride recovery and recycling methods: Techniques for recovering and recycling lithium chloride from waste streams, spent batteries, and industrial byproducts. These methods focus on sustainable practices to reclaim lithium compounds, reduce environmental impact, and address the growing demand for lithium resources. The processes typically involve separation, purification, and conversion steps to obtain reusable lithium chloride.
02 Lithium chloride in battery technologies
Applications of lithium chloride in battery technologies, particularly in lithium-ion batteries and energy storage systems. Lithium chloride serves as a precursor for cathode materials or as an electrolyte component, contributing to improved battery performance, longer cycle life, and enhanced energy density. These innovations address the growing demand for efficient energy storage solutions in various sectors including electric vehicles and renewable energy systems.Expand Specific Solutions03 Lithium chloride in pharmaceutical and medical applications
Use of lithium chloride in pharmaceutical formulations and medical applications, including as a therapeutic agent for mental health conditions and as a component in drug delivery systems. Lithium chloride has been studied for its mood-stabilizing properties and potential applications in treating bipolar disorder and depression. Research also explores its use in novel drug formulations and medical devices.Expand Specific Solutions04 Lithium chloride in industrial processes and materials
Applications of lithium chloride in various industrial processes and materials, including as a desiccant, flux for soldering and welding, and as an additive in ceramics and glass manufacturing. Lithium chloride's hygroscopic properties make it valuable for humidity control in industrial settings. It is also used in heat transfer fluids, concrete additives, and as a catalyst in certain chemical reactions.Expand Specific Solutions05 Lithium chloride in environmental and mining applications
Use of lithium chloride in environmental remediation, water treatment, and sustainable mining practices. Applications include lithium recovery from waste streams, water purification systems, and environmentally friendly extraction methods. These technologies aim to reduce the environmental footprint of lithium production while addressing growing demand for lithium compounds in clean energy technologies and other applications.Expand Specific Solutions
Leading Analytical Equipment Manufacturers and Service Providers
The lithium chloride testing in surfactant production market is currently in a growth phase, with increasing demand driven by expanding applications in battery technology and specialty chemicals. The global market size is estimated to be growing at 8-10% annually, reaching approximately $300 million. From a technological maturity perspective, the landscape shows varying degrees of advancement. Major players like Ganfeng Lithium and Tianqi Lithium have established sophisticated testing protocols, while FUJIFILM and Terumo have developed precision analytical instruments. Qinghai Salt Lake Industry and POSCO Holdings leverage their mineral extraction expertise to enhance testing methodologies. Emerging companies like Metallogenics and Soulbrain are introducing innovative approaches to lithium chloride purity verification, indicating a competitive but not yet fully consolidated market.
Schlumberger Technologies, Inc.
Technical Solution: Schlumberger has developed an advanced electrochemical testing platform for lithium chloride monitoring in surfactant production. Their system utilizes ion-selective electrodes (ISEs) specifically calibrated for lithium detection in complex surfactant matrices. The technology incorporates a temperature-compensated measurement system that maintains accuracy across the wide temperature ranges encountered during industrial surfactant production. Schlumberger's approach features a flow-through cell design that allows for continuous monitoring of lithium chloride levels during production, with response times under 30 seconds and detection limits of approximately 5 ppm. The company has integrated this technology with their digital oilfield platform, enabling automated process control adjustments based on real-time lithium chloride measurements. Their testing protocol includes an automated calibration system that periodically verifies sensor performance against standard solutions, ensuring measurement reliability over extended production runs. Schlumberger has also developed specialized algorithms that compensate for common interfering ions in surfactant formulations, significantly improving measurement specificity.
Strengths: Real-time continuous monitoring capability; integration with process control systems; relatively low operational costs after initial setup; minimal consumables required. Weaknesses: Periodic sensor replacement necessary; potential for electrode fouling in certain surfactant formulations; requires regular calibration to maintain accuracy.
Henkel IP & Holding GmbH
Technical Solution: Henkel has developed a comprehensive testing protocol for lithium chloride in surfactant production that combines multiple analytical techniques. Their approach utilizes ion chromatography (IC) as the primary method, which allows for precise quantification of lithium ions at concentrations as low as 0.5 ppm. This is complemented by inductively coupled plasma mass spectrometry (ICP-MS) for ultra-trace analysis when needed. Henkel's protocol includes a proprietary sample preparation method that effectively separates lithium chloride from complex surfactant matrices through selective precipitation and filtration steps. The company has also implemented an automated quality control system that continuously monitors lithium chloride levels throughout the production process using in-line sensors calibrated against reference standards. This multi-tiered approach ensures both accuracy and efficiency in lithium chloride testing across their surfactant product lines.
Strengths: Comprehensive multi-method approach provides redundancy and verification; automated in-line monitoring enables real-time quality control; proprietary sample preparation methods overcome matrix interference issues. Weaknesses: Higher implementation cost compared to single-method approaches; requires specialized training for operators; some methods may have longer turnaround times affecting production efficiency.
Key Analytical Technologies for Lithium Chloride Detection
Lithium chloride production
PatentInactiveGB891784A
Innovation
- A process involving preheating and roasting of a lithium ore mixture with calcium chloride, where lithium chloride-containing gases are evolved and separated without recycling through the preheating zone, using separate firing means and compartments for drying and partial calcination, and a gas outlet conduit to bypass the preheating zone, facilitating continuous and economical lithium chloride recovery.
Process for obtaining lithium chloride from solutions and a device for carrying out the same
PatentWO1994019280A1
Innovation
- A method involving a stepwise-protivotchnom mode for lithium sorption and desorption using a granulated sorbent, with double contact operation in sorption and desorption zones, followed by electrodialysis for concentration, achieving maximum lithium chloride extraction and minimizing impurities.
Quality Control Standards and Regulatory Compliance
Quality control for lithium chloride testing in surfactant production must adhere to stringent international and regional standards. The American Society for Testing and Materials (ASTM) provides specific guidelines for testing inorganic salts in surfactant formulations, with ASTM D4252 and D4191 being particularly relevant for lithium compound analysis. Similarly, the International Organization for Standardization (ISO) has established ISO 17025 for laboratory testing competence, which applies directly to facilities conducting lithium chloride analysis.
Regulatory compliance varies significantly across regions. In the European Union, the REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation requires comprehensive documentation of lithium compounds used in surfactant production, including detailed testing methodologies and results. The EU's Classification, Labeling and Packaging (CLP) regulation further mandates specific hazard communication standards for lithium chloride.
In the United States, the Environmental Protection Agency (EPA) regulates lithium compounds under the Toxic Substances Control Act (TSCA), requiring manufacturers to report testing procedures and results. Additionally, the Food and Drug Administration (FDA) imposes requirements for surfactants used in personal care products, necessitating validated testing protocols for lithium chloride impurities.
Quality control laboratories must maintain detailed Standard Operating Procedures (SOPs) for lithium chloride testing, including sample preparation, instrument calibration, and result interpretation. These SOPs should be validated through interlaboratory comparison studies to ensure reproducibility and accuracy. Method validation parameters must include specificity, linearity, range, accuracy, precision, detection limit, and quantitation limit as outlined in ICH Q2(R1) guidelines.
Documentation requirements are equally rigorous, with complete traceability from raw material to finished product testing. Each batch of surfactant must have comprehensive records of lithium chloride testing, including calibration certificates for equipment used, analyst qualifications, and deviation reports if applicable. Many regulatory bodies now require electronic data management systems with audit trail capabilities to prevent unauthorized data manipulation.
Emerging global harmonization efforts, such as the Globally Harmonized System of Classification and Labeling of Chemicals (GHS), are standardizing testing requirements across borders. Companies engaged in international trade must navigate these evolving standards while maintaining compliance with local regulations. Industry associations like the American Cleaning Institute and the European Committee of Surfactants and their Organic Intermediates (CESIO) provide guidance on best practices for quality control and regulatory compliance specific to surfactant production.
Regulatory compliance varies significantly across regions. In the European Union, the REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation requires comprehensive documentation of lithium compounds used in surfactant production, including detailed testing methodologies and results. The EU's Classification, Labeling and Packaging (CLP) regulation further mandates specific hazard communication standards for lithium chloride.
In the United States, the Environmental Protection Agency (EPA) regulates lithium compounds under the Toxic Substances Control Act (TSCA), requiring manufacturers to report testing procedures and results. Additionally, the Food and Drug Administration (FDA) imposes requirements for surfactants used in personal care products, necessitating validated testing protocols for lithium chloride impurities.
Quality control laboratories must maintain detailed Standard Operating Procedures (SOPs) for lithium chloride testing, including sample preparation, instrument calibration, and result interpretation. These SOPs should be validated through interlaboratory comparison studies to ensure reproducibility and accuracy. Method validation parameters must include specificity, linearity, range, accuracy, precision, detection limit, and quantitation limit as outlined in ICH Q2(R1) guidelines.
Documentation requirements are equally rigorous, with complete traceability from raw material to finished product testing. Each batch of surfactant must have comprehensive records of lithium chloride testing, including calibration certificates for equipment used, analyst qualifications, and deviation reports if applicable. Many regulatory bodies now require electronic data management systems with audit trail capabilities to prevent unauthorized data manipulation.
Emerging global harmonization efforts, such as the Globally Harmonized System of Classification and Labeling of Chemicals (GHS), are standardizing testing requirements across borders. Companies engaged in international trade must navigate these evolving standards while maintaining compliance with local regulations. Industry associations like the American Cleaning Institute and the European Committee of Surfactants and their Organic Intermediates (CESIO) provide guidance on best practices for quality control and regulatory compliance specific to surfactant production.
Environmental Impact of LiCl Testing Methods
The testing methods for lithium chloride in surfactant production carry significant environmental implications that warrant careful consideration. Traditional analytical techniques such as atomic absorption spectroscopy (AAS) and inductively coupled plasma mass spectrometry (ICP-MS) generate hazardous waste streams containing heavy metals and organic solvents. These wastes require specialized disposal protocols to prevent contamination of soil and groundwater systems, adding substantial environmental management costs to production operations.
Water consumption represents another critical environmental concern, particularly in regions facing water scarcity. Many LiCl testing procedures require substantial volumes of ultra-pure water for sample preparation and equipment cleaning. The purification processes for this water themselves contribute to environmental stress through energy consumption and waste generation, creating a compounding environmental impact beyond the immediate testing procedure.
Chemical reagents used in lithium chloride testing, including strong acids, organic solvents, and indicator compounds, present ecological risks when improperly managed. These substances can disrupt aquatic ecosystems even at low concentrations, potentially causing long-term damage to biodiversity and water quality. The manufacturing of these reagents also carries its own environmental footprint, encompassing resource extraction, energy consumption, and transportation emissions.
Recent life cycle assessments of analytical methods have revealed that the energy requirements for operating sophisticated testing equipment contribute significantly to the carbon footprint of quality control operations. Instruments such as ICP-MS and ion chromatography systems demand continuous power supply and controlled environmental conditions, resulting in substantial greenhouse gas emissions when powered by non-renewable energy sources.
Emerging green analytical chemistry approaches offer promising alternatives with reduced environmental impact. These include solvent-free extraction techniques, miniaturized testing platforms requiring smaller sample volumes, and reagent-free methods utilizing direct physical measurements. Companies implementing these sustainable testing protocols have reported reductions in hazardous waste generation by up to 60% while maintaining analytical precision and reliability.
Regulatory frameworks increasingly recognize the environmental implications of analytical procedures, with several jurisdictions now requiring environmental impact assessments for laboratory operations. Forward-thinking surfactant manufacturers are adopting environmental management systems specifically addressing analytical waste streams, implementing closed-loop solvent recovery systems, and exploring renewable energy options to power testing facilities.
Water consumption represents another critical environmental concern, particularly in regions facing water scarcity. Many LiCl testing procedures require substantial volumes of ultra-pure water for sample preparation and equipment cleaning. The purification processes for this water themselves contribute to environmental stress through energy consumption and waste generation, creating a compounding environmental impact beyond the immediate testing procedure.
Chemical reagents used in lithium chloride testing, including strong acids, organic solvents, and indicator compounds, present ecological risks when improperly managed. These substances can disrupt aquatic ecosystems even at low concentrations, potentially causing long-term damage to biodiversity and water quality. The manufacturing of these reagents also carries its own environmental footprint, encompassing resource extraction, energy consumption, and transportation emissions.
Recent life cycle assessments of analytical methods have revealed that the energy requirements for operating sophisticated testing equipment contribute significantly to the carbon footprint of quality control operations. Instruments such as ICP-MS and ion chromatography systems demand continuous power supply and controlled environmental conditions, resulting in substantial greenhouse gas emissions when powered by non-renewable energy sources.
Emerging green analytical chemistry approaches offer promising alternatives with reduced environmental impact. These include solvent-free extraction techniques, miniaturized testing platforms requiring smaller sample volumes, and reagent-free methods utilizing direct physical measurements. Companies implementing these sustainable testing protocols have reported reductions in hazardous waste generation by up to 60% while maintaining analytical precision and reliability.
Regulatory frameworks increasingly recognize the environmental implications of analytical procedures, with several jurisdictions now requiring environmental impact assessments for laboratory operations. Forward-thinking surfactant manufacturers are adopting environmental management systems specifically addressing analytical waste streams, implementing closed-loop solvent recovery systems, and exploring renewable energy options to power testing facilities.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!