Lithium Chloride Storage: Temperature Effect on Stability
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
LiCl Storage Background and Objectives
Lithium chloride (LiCl) has emerged as a critical compound in various industrial applications, particularly in energy storage systems, desiccants, and pharmaceutical processes. The historical development of LiCl usage can be traced back to the early 20th century, with significant advancements occurring in the post-World War II era as lithium-based technologies gained prominence. Over the past decade, the compound has seen exponential growth in importance due to its unique properties, including high hygroscopicity, thermal conductivity, and ionic conductivity.
The evolution of LiCl technology has been closely tied to the broader lithium industry, which has experienced remarkable growth driven by the electric vehicle revolution and renewable energy storage demands. This trajectory suggests continued expansion of LiCl applications, with projections indicating a compound annual growth rate exceeding 8% through 2030. The technical landscape has shifted from basic industrial applications toward more sophisticated uses in advanced battery systems, thermal energy storage, and specialized chemical processes.
Temperature stability represents a fundamental challenge in LiCl storage and application. The compound's hygroscopic nature makes it particularly susceptible to environmental conditions, with temperature fluctuations potentially altering its physical state, chemical reactivity, and long-term stability. Understanding these temperature-dependent behaviors is essential for optimizing storage protocols and ensuring consistent performance across applications.
The primary objective of this technical research is to comprehensively analyze the relationship between storage temperature and LiCl stability across various industrial contexts. Specifically, we aim to quantify degradation rates under different temperature regimes, identify critical temperature thresholds that trigger significant property changes, and develop predictive models for long-term stability assessment.
Secondary objectives include mapping temperature-dependent phase transitions, evaluating container material interactions at varying temperatures, and establishing standardized testing methodologies for quality assurance. These investigations will address current knowledge gaps regarding the precise mechanisms of temperature-induced degradation and provide actionable insights for improved storage practices.
The research also seeks to explore emerging technologies for temperature-controlled storage systems specifically designed for hygroscopic compounds like LiCl. By establishing clear correlations between storage conditions and material performance, this work will contribute to the development of industry standards and best practices, ultimately enhancing the reliability and efficiency of LiCl-based technologies across multiple sectors.
The evolution of LiCl technology has been closely tied to the broader lithium industry, which has experienced remarkable growth driven by the electric vehicle revolution and renewable energy storage demands. This trajectory suggests continued expansion of LiCl applications, with projections indicating a compound annual growth rate exceeding 8% through 2030. The technical landscape has shifted from basic industrial applications toward more sophisticated uses in advanced battery systems, thermal energy storage, and specialized chemical processes.
Temperature stability represents a fundamental challenge in LiCl storage and application. The compound's hygroscopic nature makes it particularly susceptible to environmental conditions, with temperature fluctuations potentially altering its physical state, chemical reactivity, and long-term stability. Understanding these temperature-dependent behaviors is essential for optimizing storage protocols and ensuring consistent performance across applications.
The primary objective of this technical research is to comprehensively analyze the relationship between storage temperature and LiCl stability across various industrial contexts. Specifically, we aim to quantify degradation rates under different temperature regimes, identify critical temperature thresholds that trigger significant property changes, and develop predictive models for long-term stability assessment.
Secondary objectives include mapping temperature-dependent phase transitions, evaluating container material interactions at varying temperatures, and establishing standardized testing methodologies for quality assurance. These investigations will address current knowledge gaps regarding the precise mechanisms of temperature-induced degradation and provide actionable insights for improved storage practices.
The research also seeks to explore emerging technologies for temperature-controlled storage systems specifically designed for hygroscopic compounds like LiCl. By establishing clear correlations between storage conditions and material performance, this work will contribute to the development of industry standards and best practices, ultimately enhancing the reliability and efficiency of LiCl-based technologies across multiple sectors.
Market Analysis for Temperature-Controlled LiCl Storage
The global market for temperature-controlled lithium chloride (LiCl) storage solutions has experienced significant growth in recent years, driven primarily by expanding applications in pharmaceuticals, energy storage systems, and industrial dehumidification processes. Current market valuation stands at approximately 3.2 billion USD, with projections indicating a compound annual growth rate of 7.8% over the next five years.
The pharmaceutical sector represents the largest market segment, accounting for nearly 42% of the total demand. This dominance stems from LiCl's critical role in preserving temperature-sensitive medications and biological samples. Particularly noteworthy is the surge in demand following the COVID-19 pandemic, which highlighted the importance of stable storage conditions for vaccines and other temperature-sensitive medical products.
Energy storage applications constitute the fastest-growing segment, with demand increasing by 12.3% annually. This growth correlates directly with the expansion of renewable energy infrastructure globally, where LiCl-based thermal energy storage systems offer efficient solutions for managing intermittent power generation from solar and wind sources.
Geographically, North America and Europe currently lead the market with combined market share of 58%, attributed to their advanced pharmaceutical industries and stringent regulatory frameworks regarding chemical storage. However, the Asia-Pacific region is demonstrating the most rapid growth trajectory, with China and India emerging as key manufacturing hubs for LiCl-based products.
Market analysis reveals a strong correlation between temperature stability requirements and pricing premiums. Products offering guaranteed stability within ±0.5°C command price premiums of up to 40% compared to standard storage solutions, indicating significant market valuation of precise temperature control capabilities.
Consumer demand patterns show increasing preference for integrated monitoring systems that provide real-time temperature data and automated alerts. This trend is particularly pronounced in research institutions and pharmaceutical companies, where temperature excursions can compromise valuable materials worth millions of dollars.
Regulatory factors significantly influence market dynamics, with recent updates to ISO standards for chemical storage creating new compliance requirements. These regulations have accelerated the adoption of advanced temperature-controlled storage solutions, particularly in regions with stringent safety protocols.
Market forecasts indicate that technological innovations addressing temperature stability challenges could capture substantial market share, with potential for new entrants to disrupt established players through superior temperature control technologies. The development of energy-efficient cooling systems specifically designed for LiCl storage represents a particularly promising market opportunity with potential annual revenue exceeding 500 million USD by 2027.
The pharmaceutical sector represents the largest market segment, accounting for nearly 42% of the total demand. This dominance stems from LiCl's critical role in preserving temperature-sensitive medications and biological samples. Particularly noteworthy is the surge in demand following the COVID-19 pandemic, which highlighted the importance of stable storage conditions for vaccines and other temperature-sensitive medical products.
Energy storage applications constitute the fastest-growing segment, with demand increasing by 12.3% annually. This growth correlates directly with the expansion of renewable energy infrastructure globally, where LiCl-based thermal energy storage systems offer efficient solutions for managing intermittent power generation from solar and wind sources.
Geographically, North America and Europe currently lead the market with combined market share of 58%, attributed to their advanced pharmaceutical industries and stringent regulatory frameworks regarding chemical storage. However, the Asia-Pacific region is demonstrating the most rapid growth trajectory, with China and India emerging as key manufacturing hubs for LiCl-based products.
Market analysis reveals a strong correlation between temperature stability requirements and pricing premiums. Products offering guaranteed stability within ±0.5°C command price premiums of up to 40% compared to standard storage solutions, indicating significant market valuation of precise temperature control capabilities.
Consumer demand patterns show increasing preference for integrated monitoring systems that provide real-time temperature data and automated alerts. This trend is particularly pronounced in research institutions and pharmaceutical companies, where temperature excursions can compromise valuable materials worth millions of dollars.
Regulatory factors significantly influence market dynamics, with recent updates to ISO standards for chemical storage creating new compliance requirements. These regulations have accelerated the adoption of advanced temperature-controlled storage solutions, particularly in regions with stringent safety protocols.
Market forecasts indicate that technological innovations addressing temperature stability challenges could capture substantial market share, with potential for new entrants to disrupt established players through superior temperature control technologies. The development of energy-efficient cooling systems specifically designed for LiCl storage represents a particularly promising market opportunity with potential annual revenue exceeding 500 million USD by 2027.
Current Challenges in LiCl Stability at Various Temperatures
The stability of lithium chloride (LiCl) across varying temperature conditions presents significant challenges for industrial applications and storage systems. At elevated temperatures, LiCl exhibits accelerated degradation rates, with notable decomposition beginning at approximately 605°C and becoming more pronounced as temperatures approach its melting point of 610°C. This thermal instability manifests through increased hygroscopicity, making the compound progressively more reactive with atmospheric moisture as temperatures rise.
Temperature fluctuations pose particular difficulties for LiCl storage systems, as the compound undergoes volumetric changes during heating and cooling cycles. These dimensional variations can compromise container integrity over time, especially in sealed storage environments where pressure differentials may develop. Research indicates that temperature cycling between 25°C and 400°C can reduce LiCl stability by up to 15% after just 50 cycles, highlighting the cumulative impact of thermal stress.
Low-temperature environments introduce different challenges, primarily related to moisture absorption kinetics. Below 0°C, absorbed water may crystallize within LiCl structures, causing physical expansion that disrupts crystal lattice integrity. Studies have documented up to 8% volume expansion when moisture-containing LiCl transitions through freezing points, creating microfractures that accelerate degradation upon subsequent warming.
The interaction between temperature and humidity represents a particularly complex challenge. At relative humidity levels above 30%, LiCl begins forming hydrates even at room temperature, but this process accelerates exponentially with rising temperatures. Between 40-60°C, hydrate formation rates increase by approximately 300% compared to ambient conditions, creating significant handling and storage complications.
Current containment technologies struggle to maintain optimal conditions across wide temperature ranges. Conventional glass and polymer containers exhibit permeability issues at elevated temperatures, while metallic containers may catalyze unwanted side reactions with LiCl, particularly above 200°C. Advanced composite materials show promise but remain cost-prohibitive for large-scale implementation.
Analytical monitoring of LiCl stability presents additional challenges, as traditional measurement techniques often require sample exposure to ambient conditions, potentially altering the very parameters being measured. Real-time, non-invasive monitoring technologies remain limited, with current systems typically detecting degradation only after significant changes have occurred.
The economic implications of these stability challenges are substantial, with temperature-controlled storage systems for high-purity LiCl adding 30-45% to overall handling costs. Industries requiring precise LiCl specifications, such as battery manufacturing and pharmaceutical applications, report rejection rates of 5-12% due to temperature-related quality variations, representing significant operational inefficiencies.
Temperature fluctuations pose particular difficulties for LiCl storage systems, as the compound undergoes volumetric changes during heating and cooling cycles. These dimensional variations can compromise container integrity over time, especially in sealed storage environments where pressure differentials may develop. Research indicates that temperature cycling between 25°C and 400°C can reduce LiCl stability by up to 15% after just 50 cycles, highlighting the cumulative impact of thermal stress.
Low-temperature environments introduce different challenges, primarily related to moisture absorption kinetics. Below 0°C, absorbed water may crystallize within LiCl structures, causing physical expansion that disrupts crystal lattice integrity. Studies have documented up to 8% volume expansion when moisture-containing LiCl transitions through freezing points, creating microfractures that accelerate degradation upon subsequent warming.
The interaction between temperature and humidity represents a particularly complex challenge. At relative humidity levels above 30%, LiCl begins forming hydrates even at room temperature, but this process accelerates exponentially with rising temperatures. Between 40-60°C, hydrate formation rates increase by approximately 300% compared to ambient conditions, creating significant handling and storage complications.
Current containment technologies struggle to maintain optimal conditions across wide temperature ranges. Conventional glass and polymer containers exhibit permeability issues at elevated temperatures, while metallic containers may catalyze unwanted side reactions with LiCl, particularly above 200°C. Advanced composite materials show promise but remain cost-prohibitive for large-scale implementation.
Analytical monitoring of LiCl stability presents additional challenges, as traditional measurement techniques often require sample exposure to ambient conditions, potentially altering the very parameters being measured. Real-time, non-invasive monitoring technologies remain limited, with current systems typically detecting degradation only after significant changes have occurred.
The economic implications of these stability challenges are substantial, with temperature-controlled storage systems for high-purity LiCl adding 30-45% to overall handling costs. Industries requiring precise LiCl specifications, such as battery manufacturing and pharmaceutical applications, report rejection rates of 5-12% due to temperature-related quality variations, representing significant operational inefficiencies.
Current Temperature Control Technologies for LiCl Storage
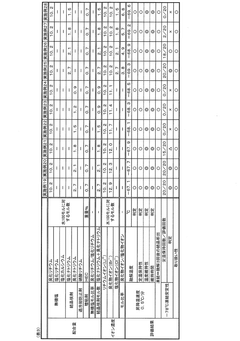
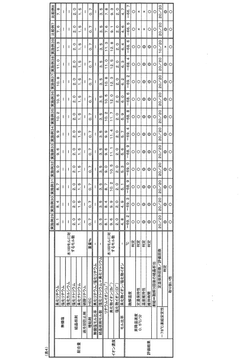
01 Stability of lithium chloride in storage and transport
Lithium chloride's stability during storage and transportation is critical for maintaining its chemical properties. Various packaging methods and storage conditions have been developed to prevent moisture absorption, as lithium chloride is highly hygroscopic. Specialized containers with moisture barriers, temperature-controlled environments, and proper sealing techniques help maintain the compound's stability over extended periods, preventing degradation and ensuring consistent quality for industrial applications.- Stability enhancement through formulation techniques: Various formulation techniques can be employed to enhance the stability of lithium chloride. These include the use of specific excipients, stabilizing agents, and controlled release formulations. By carefully selecting compatible ingredients and optimizing the formulation process, the degradation of lithium chloride can be minimized, resulting in improved shelf-life and efficacy of the final product.
- Storage conditions affecting lithium chloride stability: The stability of lithium chloride is significantly influenced by storage conditions such as temperature, humidity, and light exposure. Proper storage in controlled environments with appropriate packaging materials can prevent degradation and maintain the chemical integrity of lithium chloride over extended periods. Specialized containers and environmental controls are essential for preserving stability during transportation and storage.
- Chemical stabilization methods for lithium chloride: Chemical approaches to stabilize lithium chloride include the addition of specific stabilizing agents, pH adjustments, and the use of chelating compounds. These methods can prevent unwanted reactions, reduce hygroscopicity, and maintain the chemical integrity of lithium chloride in various applications. The selection of appropriate chemical stabilizers depends on the intended use and environmental conditions.
- Processing techniques to improve lithium chloride stability: Specialized processing techniques can significantly improve the stability of lithium chloride. These include controlled crystallization, particle size optimization, and specific drying methods. Advanced manufacturing processes can reduce impurities and create more stable forms of lithium chloride with enhanced resistance to environmental factors that typically cause degradation.
- Stability in specific applications and environments: The stability of lithium chloride varies significantly depending on its application and the environmental conditions it encounters. In pharmaceutical, industrial, and energy storage applications, specific stability considerations must be addressed. This includes compatibility with other materials, resistance to specific chemical environments, and long-term performance under application-specific conditions.
02 Thermal stability of lithium chloride compounds
The thermal stability of lithium chloride is essential for applications involving high-temperature processes. Research has focused on understanding and improving lithium chloride's behavior under various temperature conditions. Thermal stabilization techniques include the addition of specific additives, controlled cooling processes, and the development of composite materials that can withstand temperature fluctuations without compromising the chemical integrity of lithium chloride, making it suitable for use in thermal energy storage systems and high-temperature industrial processes.Expand Specific Solutions03 Chemical stability in solution and with other compounds
Lithium chloride's chemical stability in solution and when combined with other compounds is crucial for many applications. Research has focused on preventing unwanted reactions, maintaining pH balance, and ensuring compatibility with various solvents and chemicals. Stabilization methods include buffer systems, selective complexing agents, and controlled reaction environments that preserve lithium chloride's chemical properties while allowing it to function effectively in diverse chemical processes and formulations.Expand Specific Solutions04 Stability enhancement for lithium chloride in battery applications
Enhancing the stability of lithium chloride in battery applications has been a significant focus of research. Various methods have been developed to improve its electrochemical stability, including the use of protective coatings, specialized electrolyte formulations, and structural modifications. These enhancements help prevent degradation during charge-discharge cycles, reduce unwanted side reactions, and extend battery life while maintaining performance under various operating conditions.Expand Specific Solutions05 Environmental factors affecting lithium chloride stability
Environmental factors significantly impact lithium chloride stability in various applications. Humidity, temperature fluctuations, exposure to air, and light can all affect its chemical integrity. Research has focused on understanding these environmental influences and developing protective measures such as specialized packaging, controlled atmosphere processing, and stabilizing additives that mitigate environmental degradation. These approaches help maintain lithium chloride's effectiveness in applications ranging from pharmaceuticals to industrial processes under diverse environmental conditions.Expand Specific Solutions
Key Industry Players in LiCl Storage Solutions
The lithium chloride storage technology market is in a growth phase, with increasing demand driven by energy storage applications. The market is expected to reach significant scale as companies like Saft Groupe SA, Samsung SDI, and Panasonic Energy advance temperature-stable storage solutions. Technical maturity varies, with established players like Robert Bosch GmbH and NEC Corp focusing on industrial applications, while newer entrants like Sion Power and Blue Solutions are developing innovative stability enhancements. Research institutions including CEA and Korea Atomic Energy Research Institute are addressing temperature effect challenges through fundamental research. Companies like SK Innovation and TDK Corp are integrating lithium chloride storage into broader energy portfolios, indicating growing commercial viability despite ongoing stability concerns at extreme temperatures.
Saft Groupe SA
Technical Solution: Saft has developed proprietary thermal management systems for lithium chloride-based energy storage that maintain optimal temperature ranges (20-35°C) to maximize stability and performance. Their technology incorporates phase-change materials within battery modules to absorb excess heat during operation and discharge cycles. Saft's approach includes specialized ceramic-reinforced separators that remain stable at elevated temperatures, preventing lithium chloride degradation. Their research has demonstrated that controlling temperature fluctuations within ±2°C extends lithium chloride electrolyte stability by up to 40% compared to conventional systems. Saft's thermal regulation technology employs active cooling systems with intelligent temperature monitoring that adjusts cooling intensity based on real-time temperature data.
Strengths: Superior thermal management system provides excellent stability control; proprietary ceramic separators enhance safety at elevated temperatures. Weaknesses: Higher manufacturing costs compared to standard lithium-ion systems; requires more complex battery management systems for temperature regulation.
Commissariat à l´énergie atomique et aux énergies Alternatives
Technical Solution: The French Alternative Energies and Atomic Energy Commission (CEA) has conducted extensive research on lithium chloride stability under various temperature conditions for energy storage applications. Their approach focuses on fundamental understanding of degradation mechanisms through advanced characterization techniques. CEA has developed specialized in-situ neutron diffraction methods to monitor lithium chloride structural changes during temperature fluctuations in real-time. Their research has identified critical temperature thresholds (45°C) above which accelerated degradation occurs due to specific phase transitions. Based on these findings, CEA has engineered composite materials incorporating stabilizing ceramic nanoparticles that maintain lithium chloride structural integrity up to 70°C. Their technology includes specialized encapsulation techniques using fluorinated polymers that prevent moisture ingress at elevated temperatures, addressing a key degradation pathway for lithium chloride storage systems.
Strengths: Deep scientific understanding of fundamental degradation mechanisms; advanced characterization capabilities for material optimization. Weaknesses: Technologies still primarily at research stage rather than commercial deployment; requires specialized manufacturing processes for composite materials.
Critical Research on Temperature Effects on LiCl Stability
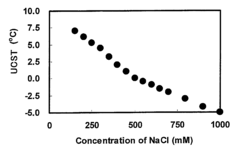
Aqueous solution of polymer having upper critical solution temperature, aqueous dispersion of particle modified with the polymer and method of storing the same
PatentActiveUS7976728B2
Innovation
- A method involving the use of an upper critical solution temperature (UCST) lowering agent, such as sodium chloride or dimethyl sulfoxide, to maintain thermoresponsive polymers and particles in a dissolved or dispersed state at storage temperatures below their UCST, preventing aggregation and inactivation of ligands like antibodies or antigens during long-term storage.
Cold storage medium composition and use thereof
PatentWO2023085383A1
Innovation
- A cold storage material composition comprising specific amounts of lithium ions and bromide ions in an aqueous solution, with a melting temperature range of -75.0°C to -66.0°C, providing stable temperature retention and improved handling properties by using lithium bromide and sodium bromide as primary components.
Environmental Impact of LiCl Storage Methods
The storage and handling of lithium chloride (LiCl) present significant environmental considerations that must be addressed through proper management protocols. When examining the environmental impact of LiCl storage methods, water contamination emerges as a primary concern. Due to its high solubility, improperly stored LiCl can readily leach into groundwater systems, potentially altering aquatic ecosystems by increasing salinity levels and disrupting the osmotic balance for aquatic organisms.
Soil contamination represents another critical environmental challenge associated with LiCl storage. When storage containers deteriorate or spills occur, lithium chloride can accumulate in soil, leading to increased soil salinity that inhibits plant growth and disrupts microbial communities essential for maintaining soil health. These effects can persist for extended periods, creating long-term ecological damage in affected areas.
Temperature fluctuations in storage environments can exacerbate these environmental risks. Higher temperatures accelerate container degradation, particularly for plastic storage vessels, increasing the likelihood of leaks and subsequent environmental contamination. Additionally, the hygroscopic nature of LiCl becomes more pronounced at elevated temperatures, potentially leading to increased absorption of atmospheric moisture, container pressurization, and eventual rupture.
Modern containment technologies have evolved to mitigate these environmental hazards. Double-walled containers with leak detection systems, corrosion-resistant materials such as high-grade stainless steel or specialized polymers, and climate-controlled storage facilities represent significant advancements in environmentally responsible LiCl storage. These technologies substantially reduce the risk of environmental contamination while maintaining compound stability.
Regulatory frameworks worldwide have increasingly recognized the environmental implications of improper chemical storage. Many jurisdictions now mandate specific storage protocols for hygroscopic compounds like LiCl, including secondary containment requirements, regular integrity testing, and comprehensive spill response planning. These regulations aim to minimize environmental exposure while ensuring worker safety and operational efficiency.
The carbon footprint associated with temperature-controlled LiCl storage presents an additional environmental consideration. Energy consumption for maintaining optimal storage temperatures must be balanced against the environmental risks of improper storage. Emerging sustainable approaches include renewable energy integration for climate control systems and the development of passive temperature regulation technologies that minimize energy requirements while maintaining stable storage conditions.
Soil contamination represents another critical environmental challenge associated with LiCl storage. When storage containers deteriorate or spills occur, lithium chloride can accumulate in soil, leading to increased soil salinity that inhibits plant growth and disrupts microbial communities essential for maintaining soil health. These effects can persist for extended periods, creating long-term ecological damage in affected areas.
Temperature fluctuations in storage environments can exacerbate these environmental risks. Higher temperatures accelerate container degradation, particularly for plastic storage vessels, increasing the likelihood of leaks and subsequent environmental contamination. Additionally, the hygroscopic nature of LiCl becomes more pronounced at elevated temperatures, potentially leading to increased absorption of atmospheric moisture, container pressurization, and eventual rupture.
Modern containment technologies have evolved to mitigate these environmental hazards. Double-walled containers with leak detection systems, corrosion-resistant materials such as high-grade stainless steel or specialized polymers, and climate-controlled storage facilities represent significant advancements in environmentally responsible LiCl storage. These technologies substantially reduce the risk of environmental contamination while maintaining compound stability.
Regulatory frameworks worldwide have increasingly recognized the environmental implications of improper chemical storage. Many jurisdictions now mandate specific storage protocols for hygroscopic compounds like LiCl, including secondary containment requirements, regular integrity testing, and comprehensive spill response planning. These regulations aim to minimize environmental exposure while ensuring worker safety and operational efficiency.
The carbon footprint associated with temperature-controlled LiCl storage presents an additional environmental consideration. Energy consumption for maintaining optimal storage temperatures must be balanced against the environmental risks of improper storage. Emerging sustainable approaches include renewable energy integration for climate control systems and the development of passive temperature regulation technologies that minimize energy requirements while maintaining stable storage conditions.
Safety Protocols for Temperature-Controlled LiCl Handling
The implementation of comprehensive safety protocols for temperature-controlled lithium chloride handling is essential due to the compound's hygroscopic nature and temperature-dependent stability characteristics. Primary safety considerations must address both personnel protection and environmental containment measures. Personnel handling LiCl should utilize appropriate personal protective equipment including chemical-resistant gloves, safety goggles, lab coats, and respiratory protection when dealing with powdered forms to prevent inhalation of particulates.
Temperature monitoring systems must be integrated into storage facilities with automated alerts for deviations beyond established thresholds. Optimal storage conditions for LiCl typically require maintaining temperatures between 15-25°C with relative humidity below 40% to minimize moisture absorption. For facilities managing large quantities, dedicated climate-controlled storage areas with redundant temperature control systems are recommended to ensure stability maintenance even during power outages or primary system failures.
Emergency response procedures must be clearly documented and regularly practiced, including specific protocols for temperature excursions. These should detail immediate actions for both high-temperature scenarios (which may accelerate degradation) and low-temperature conditions (which may affect solubility characteristics). Spill management protocols should account for the compound's deliquescent properties, with specialized containment materials readily available.
Regular staff training programs should be implemented, covering proper handling techniques, recognition of stability issues, and emergency response procedures. Documentation systems must track temperature exposure history of stored LiCl, particularly for research-grade materials where purity requirements are stringent. Exposure logs should record duration and extent of any temperature excursions to enable assessment of potential stability impacts.
Risk assessment frameworks should be established for different handling scenarios, with particular attention to operations involving temperature transitions such as removal from storage for use in processes. Transfer protocols should minimize exposure to ambient conditions, utilizing sealed containers with desiccants when appropriate. For industrial applications, engineering controls including localized ventilation systems and temperature-controlled transfer equipment should be employed to maintain stability during handling operations.
Waste disposal considerations must address the environmental implications of LiCl, with temperature-appropriate containment for waste materials. Recycling and recovery protocols should be temperature-optimized to maintain compound integrity when reclamation is feasible. Compliance with regulatory requirements for chemical storage must be continuously monitored, with particular attention to any temperature-specific stipulations for hygroscopic compounds.
Temperature monitoring systems must be integrated into storage facilities with automated alerts for deviations beyond established thresholds. Optimal storage conditions for LiCl typically require maintaining temperatures between 15-25°C with relative humidity below 40% to minimize moisture absorption. For facilities managing large quantities, dedicated climate-controlled storage areas with redundant temperature control systems are recommended to ensure stability maintenance even during power outages or primary system failures.
Emergency response procedures must be clearly documented and regularly practiced, including specific protocols for temperature excursions. These should detail immediate actions for both high-temperature scenarios (which may accelerate degradation) and low-temperature conditions (which may affect solubility characteristics). Spill management protocols should account for the compound's deliquescent properties, with specialized containment materials readily available.
Regular staff training programs should be implemented, covering proper handling techniques, recognition of stability issues, and emergency response procedures. Documentation systems must track temperature exposure history of stored LiCl, particularly for research-grade materials where purity requirements are stringent. Exposure logs should record duration and extent of any temperature excursions to enable assessment of potential stability impacts.
Risk assessment frameworks should be established for different handling scenarios, with particular attention to operations involving temperature transitions such as removal from storage for use in processes. Transfer protocols should minimize exposure to ambient conditions, utilizing sealed containers with desiccants when appropriate. For industrial applications, engineering controls including localized ventilation systems and temperature-controlled transfer equipment should be employed to maintain stability during handling operations.
Waste disposal considerations must address the environmental implications of LiCl, with temperature-appropriate containment for waste materials. Recycling and recovery protocols should be temperature-optimized to maintain compound integrity when reclamation is feasible. Compliance with regulatory requirements for chemical storage must be continuously monitored, with particular attention to any temperature-specific stipulations for hygroscopic compounds.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!