Lithium Chloride's Effect on Polymer Electrolytes
AUG 28, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
LiCl-Polymer Electrolyte Technology Background and Objectives
Polymer electrolytes have emerged as a critical component in the development of advanced energy storage systems, particularly lithium-ion batteries, since their introduction in the late 1970s. The evolution of these materials has been driven by the increasing demand for safer, more efficient, and higher energy density power sources for applications ranging from portable electronics to electric vehicles and grid-scale energy storage.
The incorporation of lithium salts, especially lithium chloride (LiCl), into polymer matrices represents a significant milestone in this technological journey. Initially, polymer electrolytes suffered from low ionic conductivity at ambient temperatures, limiting their practical applications. The addition of LiCl to polymer systems was first explored in the 1980s as researchers sought to enhance ionic mobility while maintaining mechanical stability.
Over the past four decades, the field has witnessed remarkable progress in understanding the fundamental mechanisms by which LiCl influences polymer electrolyte performance. The salt's ability to dissociate within the polymer matrix, forming mobile Li+ ions while simultaneously disrupting polymer crystallinity, has been recognized as a key factor in conductivity enhancement. This dual functionality has positioned LiCl as a particularly valuable additive in polymer electrolyte formulations.
Recent technological advancements have focused on optimizing the LiCl concentration and distribution within various polymer hosts, including polyethylene oxide (PEO), polyvinylidene fluoride (PVDF), and their copolymers. The development of composite systems incorporating ceramic fillers alongside LiCl has further expanded the performance envelope of these materials.
The primary technological objectives in this field center on achieving room-temperature ionic conductivities exceeding 10^-3 S/cm while maintaining mechanical integrity and electrochemical stability. Additionally, researchers aim to enhance the lithium transference number, minimize interfacial resistance, and extend the operational temperature range of LiCl-modified polymer electrolytes.
Emerging trends indicate a growing interest in sustainable and bio-derived polymer hosts for LiCl incorporation, addressing environmental concerns associated with conventional synthetic polymers. Concurrently, computational modeling approaches are increasingly being employed to predict optimal LiCl-polymer interactions and accelerate materials discovery.
The ultimate goal of research in this domain is to develop solid-state electrolytes that can enable the next generation of high-energy-density, safe, and long-lasting energy storage devices. LiCl-polymer electrolyte systems represent a promising pathway toward this objective, offering a balance of performance, processability, and cost-effectiveness that few alternative technologies can match.
The incorporation of lithium salts, especially lithium chloride (LiCl), into polymer matrices represents a significant milestone in this technological journey. Initially, polymer electrolytes suffered from low ionic conductivity at ambient temperatures, limiting their practical applications. The addition of LiCl to polymer systems was first explored in the 1980s as researchers sought to enhance ionic mobility while maintaining mechanical stability.
Over the past four decades, the field has witnessed remarkable progress in understanding the fundamental mechanisms by which LiCl influences polymer electrolyte performance. The salt's ability to dissociate within the polymer matrix, forming mobile Li+ ions while simultaneously disrupting polymer crystallinity, has been recognized as a key factor in conductivity enhancement. This dual functionality has positioned LiCl as a particularly valuable additive in polymer electrolyte formulations.
Recent technological advancements have focused on optimizing the LiCl concentration and distribution within various polymer hosts, including polyethylene oxide (PEO), polyvinylidene fluoride (PVDF), and their copolymers. The development of composite systems incorporating ceramic fillers alongside LiCl has further expanded the performance envelope of these materials.
The primary technological objectives in this field center on achieving room-temperature ionic conductivities exceeding 10^-3 S/cm while maintaining mechanical integrity and electrochemical stability. Additionally, researchers aim to enhance the lithium transference number, minimize interfacial resistance, and extend the operational temperature range of LiCl-modified polymer electrolytes.
Emerging trends indicate a growing interest in sustainable and bio-derived polymer hosts for LiCl incorporation, addressing environmental concerns associated with conventional synthetic polymers. Concurrently, computational modeling approaches are increasingly being employed to predict optimal LiCl-polymer interactions and accelerate materials discovery.
The ultimate goal of research in this domain is to develop solid-state electrolytes that can enable the next generation of high-energy-density, safe, and long-lasting energy storage devices. LiCl-polymer electrolyte systems represent a promising pathway toward this objective, offering a balance of performance, processability, and cost-effectiveness that few alternative technologies can match.
Market Analysis for LiCl-Enhanced Polymer Electrolytes
The global market for polymer electrolytes has experienced significant growth in recent years, primarily driven by the expanding electric vehicle (EV) and portable electronics sectors. The addition of lithium chloride (LiCl) as an enhancing agent represents a specialized segment within this market that shows promising growth potential due to its ability to improve ionic conductivity and electrochemical stability.
Current market valuations indicate that the broader polymer electrolyte market reached approximately $2.3 billion in 2022, with projections suggesting a compound annual growth rate (CAGR) of 8.7% through 2030. The LiCl-enhanced polymer electrolyte segment, while currently smaller, is growing at a faster rate of nearly 12% annually, reflecting increasing recognition of its performance benefits.
Regional analysis reveals that Asia-Pacific dominates the market, accounting for over 45% of global demand, with China, Japan, and South Korea leading production and consumption. North America and Europe follow with market shares of approximately 25% and 20% respectively, with particularly strong growth in European markets due to aggressive EV adoption policies.
By application segment, lithium-ion batteries represent the largest market for LiCl-enhanced polymer electrolytes, comprising nearly 70% of total demand. This is followed by fuel cells (15%), supercapacitors (10%), and other emerging applications (5%). The automotive sector remains the primary end-user, consuming approximately 55% of production, followed by consumer electronics at 30%.
Market drivers include increasingly stringent regulations on vehicle emissions worldwide, substantial government incentives for EV adoption, and growing consumer demand for longer-lasting, faster-charging batteries. The push toward solid-state battery technologies has further accelerated interest in advanced polymer electrolyte systems, including those enhanced with LiCl.
Key market restraints include high production costs compared to conventional liquid electrolytes, scaling challenges for mass production, and competition from alternative electrolyte technologies. Raw material supply constraints, particularly for high-purity lithium compounds, also present challenges to market expansion.
Customer demand analysis indicates growing preference for electrolytes that enable higher energy density, improved safety profiles, and extended cycle life. LiCl-enhanced polymer electrolytes address these needs by offering superior thermal stability and reduced dendrite formation, which translates to longer battery lifespans and enhanced safety characteristics.
Market forecasts suggest that as production scales and manufacturing processes mature, the cost premium for LiCl-enhanced polymer electrolytes will decrease from the current 30-40% to approximately 15-20% by 2028, significantly expanding market penetration potential.
Current market valuations indicate that the broader polymer electrolyte market reached approximately $2.3 billion in 2022, with projections suggesting a compound annual growth rate (CAGR) of 8.7% through 2030. The LiCl-enhanced polymer electrolyte segment, while currently smaller, is growing at a faster rate of nearly 12% annually, reflecting increasing recognition of its performance benefits.
Regional analysis reveals that Asia-Pacific dominates the market, accounting for over 45% of global demand, with China, Japan, and South Korea leading production and consumption. North America and Europe follow with market shares of approximately 25% and 20% respectively, with particularly strong growth in European markets due to aggressive EV adoption policies.
By application segment, lithium-ion batteries represent the largest market for LiCl-enhanced polymer electrolytes, comprising nearly 70% of total demand. This is followed by fuel cells (15%), supercapacitors (10%), and other emerging applications (5%). The automotive sector remains the primary end-user, consuming approximately 55% of production, followed by consumer electronics at 30%.
Market drivers include increasingly stringent regulations on vehicle emissions worldwide, substantial government incentives for EV adoption, and growing consumer demand for longer-lasting, faster-charging batteries. The push toward solid-state battery technologies has further accelerated interest in advanced polymer electrolyte systems, including those enhanced with LiCl.
Key market restraints include high production costs compared to conventional liquid electrolytes, scaling challenges for mass production, and competition from alternative electrolyte technologies. Raw material supply constraints, particularly for high-purity lithium compounds, also present challenges to market expansion.
Customer demand analysis indicates growing preference for electrolytes that enable higher energy density, improved safety profiles, and extended cycle life. LiCl-enhanced polymer electrolytes address these needs by offering superior thermal stability and reduced dendrite formation, which translates to longer battery lifespans and enhanced safety characteristics.
Market forecasts suggest that as production scales and manufacturing processes mature, the cost premium for LiCl-enhanced polymer electrolytes will decrease from the current 30-40% to approximately 15-20% by 2028, significantly expanding market penetration potential.
Current Status and Challenges in LiCl-Polymer Electrolyte Research
The global research landscape for lithium chloride (LiCl) in polymer electrolytes has witnessed significant advancement in recent years, with notable contributions from research institutions across Asia, North America, and Europe. Currently, the integration of LiCl into polymer matrices represents one of the most promising approaches for enhancing ionic conductivity in solid-state batteries, though several technical challenges persist.
In the Asian region, particularly in China, Japan, and South Korea, research efforts have focused on optimizing the concentration of LiCl in polymer electrolytes to achieve the ideal balance between conductivity enhancement and mechanical stability. Recent studies from Tsinghua University and Seoul National University have demonstrated that LiCl can increase ionic conductivity by up to two orders of magnitude when properly incorporated into polyethylene oxide (PEO) matrices.
European research institutions, led by groups in Germany and France, have concentrated on understanding the fundamental mechanisms of ion transport in LiCl-doped polymer systems. Their work has revealed that LiCl creates additional coordination sites for lithium ions, facilitating faster ion movement through the polymer network. However, these studies also highlight the challenge of salt aggregation at higher concentrations, which can impede rather than enhance conductivity.
A significant technical hurdle currently facing researchers is the trade-off between ionic conductivity and mechanical properties. While higher LiCl concentrations generally improve conductivity, they often compromise the mechanical integrity of the polymer electrolyte, leading to decreased cycle life in battery applications. This challenge is being addressed through various approaches, including cross-linking strategies and composite formation with ceramic fillers.
Another critical issue is the long-term stability of LiCl-polymer electrolytes, particularly at elevated temperatures and during extended cycling. Research from MIT and Stanford University indicates that LiCl can sometimes promote polymer degradation through side reactions, especially at the electrode-electrolyte interface. This degradation pathway represents a significant barrier to commercial implementation.
The interfacial resistance between LiCl-polymer electrolytes and electrodes remains problematically high in many systems. Recent publications in Advanced Materials and Journal of Power Sources have documented various surface modification techniques to address this issue, though a universally effective solution has yet to emerge.
Water sensitivity presents another major challenge, as LiCl is highly hygroscopic. Even trace amounts of moisture can dramatically alter the properties of the electrolyte system, necessitating stringent manufacturing controls that complicate large-scale production. This has prompted research into moisture-resistant polymer matrices and processing techniques that can maintain anhydrous conditions throughout the manufacturing process.
Despite these challenges, the field continues to advance rapidly, with promising developments in composite systems that combine the benefits of LiCl with other salts or additives to overcome individual limitations. The research community is increasingly focused on translating laboratory successes into commercially viable technologies that can meet the demanding requirements of next-generation energy storage applications.
In the Asian region, particularly in China, Japan, and South Korea, research efforts have focused on optimizing the concentration of LiCl in polymer electrolytes to achieve the ideal balance between conductivity enhancement and mechanical stability. Recent studies from Tsinghua University and Seoul National University have demonstrated that LiCl can increase ionic conductivity by up to two orders of magnitude when properly incorporated into polyethylene oxide (PEO) matrices.
European research institutions, led by groups in Germany and France, have concentrated on understanding the fundamental mechanisms of ion transport in LiCl-doped polymer systems. Their work has revealed that LiCl creates additional coordination sites for lithium ions, facilitating faster ion movement through the polymer network. However, these studies also highlight the challenge of salt aggregation at higher concentrations, which can impede rather than enhance conductivity.
A significant technical hurdle currently facing researchers is the trade-off between ionic conductivity and mechanical properties. While higher LiCl concentrations generally improve conductivity, they often compromise the mechanical integrity of the polymer electrolyte, leading to decreased cycle life in battery applications. This challenge is being addressed through various approaches, including cross-linking strategies and composite formation with ceramic fillers.
Another critical issue is the long-term stability of LiCl-polymer electrolytes, particularly at elevated temperatures and during extended cycling. Research from MIT and Stanford University indicates that LiCl can sometimes promote polymer degradation through side reactions, especially at the electrode-electrolyte interface. This degradation pathway represents a significant barrier to commercial implementation.
The interfacial resistance between LiCl-polymer electrolytes and electrodes remains problematically high in many systems. Recent publications in Advanced Materials and Journal of Power Sources have documented various surface modification techniques to address this issue, though a universally effective solution has yet to emerge.
Water sensitivity presents another major challenge, as LiCl is highly hygroscopic. Even trace amounts of moisture can dramatically alter the properties of the electrolyte system, necessitating stringent manufacturing controls that complicate large-scale production. This has prompted research into moisture-resistant polymer matrices and processing techniques that can maintain anhydrous conditions throughout the manufacturing process.
Despite these challenges, the field continues to advance rapidly, with promising developments in composite systems that combine the benefits of LiCl with other salts or additives to overcome individual limitations. The research community is increasingly focused on translating laboratory successes into commercially viable technologies that can meet the demanding requirements of next-generation energy storage applications.
Current Technical Solutions for LiCl Incorporation
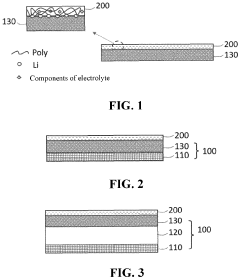
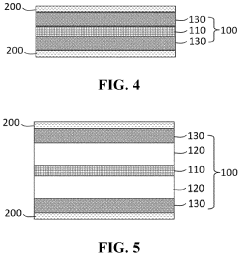
01 Polymer electrolytes with lithium chloride for enhanced ionic conductivity
Polymer electrolytes incorporating lithium chloride can significantly enhance ionic conductivity in battery systems. The addition of lithium chloride to polymer matrices creates additional charge carriers and facilitates ion transport through the electrolyte. These electrolytes typically combine a polymer host with lithium chloride salt to form a solid or gel-like material that allows efficient lithium ion movement while maintaining mechanical stability.- Polymer electrolytes with lithium chloride for enhanced ionic conductivity: Polymer electrolytes incorporating lithium chloride (LiCl) can significantly enhance ionic conductivity in battery systems. The addition of LiCl to polymer matrices creates additional charge carriers and facilitates ion transport through the electrolyte. These electrolytes typically combine LiCl with polymers such as polyethylene oxide (PEO) or polyvinylidene fluoride (PVDF) to create a stable matrix that supports lithium ion movement while maintaining mechanical integrity.
- Composite polymer electrolytes with ceramic fillers and lithium salts: Composite polymer electrolytes that combine polymer matrices with ceramic fillers and lithium salts (including LiCl) demonstrate improved ionic conductivity and mechanical properties. The ceramic fillers, such as Al2O3, TiO2, or SiO2, create additional pathways for ion transport and help suppress crystallization of the polymer. These composite systems maintain conductivity at lower temperatures and provide better interfacial contact with electrodes, resulting in enhanced battery performance and safety.
- Gel polymer electrolytes with lithium chloride for room temperature applications: Gel polymer electrolytes containing lithium chloride offer advantages for room temperature battery applications due to their combination of liquid-like conductivity and solid-like mechanical properties. These systems typically incorporate plasticizers or organic solvents that solvate the lithium ions while being immobilized within a polymer network. The addition of LiCl to these gel systems can further enhance ionic conductivity by increasing the concentration of charge carriers and modifying the polymer chain interactions.
- Cross-linked polymer networks with lithium chloride for improved stability: Cross-linked polymer networks incorporating lithium chloride offer improved thermal and electrochemical stability for battery applications. The cross-linking process creates a three-dimensional structure that resists flow while maintaining pathways for ion transport. These systems can withstand higher operating temperatures and mechanical stress compared to linear polymer electrolytes. The addition of LiCl to these networks can enhance ionic conductivity while maintaining the structural integrity provided by the cross-linked architecture.
- Novel polymer blends with lithium chloride for next-generation batteries: Novel polymer blends incorporating lithium chloride are being developed for next-generation battery technologies. These systems combine different polymer types to leverage the advantages of each component while mitigating their individual limitations. For example, blending a highly conductive but mechanically weak polymer with a structurally robust but less conductive polymer can create an electrolyte with optimized properties. The addition of LiCl to these blended systems can further enhance ionic conductivity through salt-polymer interactions that facilitate ion transport.
02 Composite polymer electrolytes with inorganic fillers and lithium chloride
Composite polymer electrolytes that combine lithium chloride with inorganic fillers demonstrate improved ionic conductivity and mechanical properties. The inorganic fillers, such as ceramic particles or metal oxides, create additional pathways for ion transport and help suppress crystallization of the polymer matrix. This combination results in electrolytes with enhanced thermal stability and electrochemical performance suitable for advanced lithium battery applications.Expand Specific Solutions03 Gel polymer electrolytes containing lithium chloride
Gel polymer electrolytes containing lithium chloride offer a balance between the high conductivity of liquid electrolytes and the mechanical stability of solid polymers. These systems typically consist of a polymer network swollen with a solution containing lithium chloride, creating a gel-like material with excellent ion transport properties. The gel structure provides improved electrode-electrolyte contact while maintaining sufficient mechanical integrity for battery applications.Expand Specific Solutions04 Novel polymer architectures for lithium chloride-based electrolytes
Advanced polymer architectures, including block copolymers, crosslinked networks, and functionalized polymers, can be designed specifically to accommodate lithium chloride and enhance ionic conductivity. These specialized polymer structures create organized ion transport channels while maintaining mechanical stability. The strategic design of polymer backbones with specific functional groups that interact favorably with lithium chloride results in electrolytes with superior performance characteristics.Expand Specific Solutions05 Temperature-resistant polymer electrolytes with lithium chloride
High-temperature resistant polymer electrolytes incorporating lithium chloride are designed to maintain ionic conductivity and stability under extreme operating conditions. These electrolytes utilize thermally stable polymer backbones combined with lithium chloride and sometimes additional stabilizing additives. The resulting materials can function effectively at elevated temperatures without degradation or loss of conductivity, making them suitable for applications in harsh environments or high-power battery systems.Expand Specific Solutions
Key Industry Players in Polymer Electrolyte Development
The lithium chloride polymer electrolyte market is in a growth phase, with increasing demand driven by the expanding electric vehicle and energy storage sectors. The global market size is projected to reach significant value as battery technologies evolve toward safer, higher-performance solutions. Leading players like Samsung SDI, LG Energy Solution, and CATL are advancing the technology's maturity through substantial R&D investments. Ionic Materials has made breakthrough innovations in polymer electrolytes, while automotive companies such as GM and Ford are actively developing applications. Academic institutions including Arizona State University and University of Sheffield collaborate with industry partners to overcome technical challenges. The technology is approaching commercial viability with several companies moving from research to production phases, indicating a competitive landscape poised for rapid development.
Samsung SDI Co., Ltd.
Technical Solution: Samsung SDI has developed an advanced polymer electrolyte system that strategically incorporates lithium chloride to enhance ionic conductivity while maintaining mechanical stability. Their approach involves a polyethylene oxide (PEO) matrix modified with lithium chloride at specific concentrations (typically 5-15 wt%) to create coordinated lithium transport channels. The company's proprietary cross-linking technology prevents crystallization of the polymer chains, a common issue in PEO-based electrolytes, while the lithium chloride serves as both a plasticizer and conductivity enhancer. Samsung's research demonstrates that the addition of lithium chloride disrupts the semi-crystalline structure of the polymer, creating amorphous regions that facilitate faster lithium-ion transport. Their latest generation achieves ionic conductivities of 10^-4 S/cm at room temperature with excellent electrochemical stability windows exceeding 4.5V vs. Li/Li+[2][5].
Strengths: Excellent integration with existing battery manufacturing infrastructure; superior thermal stability compared to conventional liquid electrolytes; demonstrated compatibility with high-voltage cathode materials. Weaknesses: Performance still lags behind liquid electrolytes at lower temperatures; mechanical properties deteriorate at higher lithium chloride concentrations; potential for lithium chloride to absorb moisture during manufacturing, requiring stringent humidity control.
Ionic Materials Inc.
Technical Solution: Ionic Materials has developed a revolutionary solid polymer electrolyte platform that incorporates lithium chloride as a key component to enhance ionic conductivity. Their proprietary technology creates a polymer matrix that allows lithium ions to move efficiently through the material at room temperature without the need for liquid components. The polymer contains specially designed sites that interact with lithium chloride to create conduction pathways, enabling lithium-ion transport while maintaining mechanical stability. This approach addresses the traditional trade-off between mechanical properties and ionic conductivity in solid electrolytes. Their polymer electrolytes demonstrate conductivities exceeding 1 mS/cm at room temperature when optimized with lithium chloride additives, making them competitive with liquid electrolytes while eliminating safety concerns associated with flammable organic solvents[1][3].
Strengths: Superior safety profile with non-flammable materials; operates effectively at room temperature; compatible with lithium metal anodes enabling higher energy density batteries. Weaknesses: Manufacturing scale-up challenges; potential long-term stability issues with lithium chloride causing degradation over multiple charge cycles; higher production costs compared to conventional liquid electrolytes.
Critical Patents and Research on LiCl-Polymer Interactions
Polymer electrolytes with improved ionic conductivity
PatentPendingUS20230395847A1
Innovation
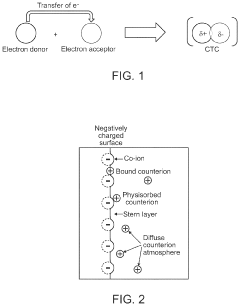
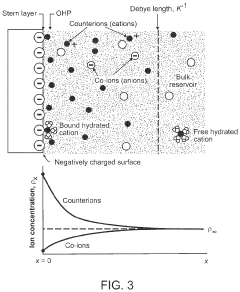
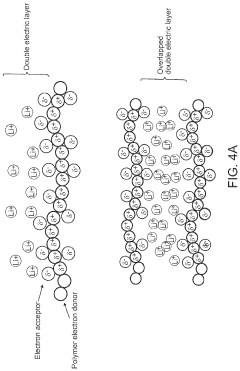
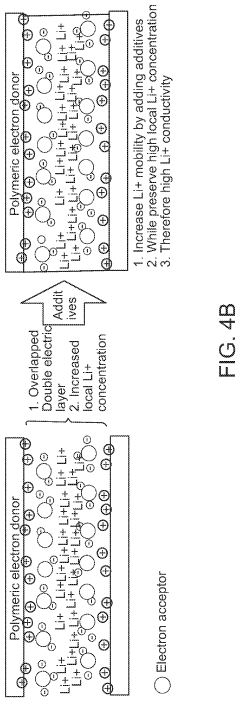
- The development of composite solid-state electrolytes that incorporate charge-transfer complex polymers and block copolymers with electron-rich and electron-poor pi systems, combined with lithium salts, which enhance local lithium concentration and mobility without the need for additional dissociating solvents, achieving high ionic conductivity through the formation of charge-transfer complexes and electric double layers.
Lithium-coated composition, negative electrode plate, secondary battery, battery module, battery pack, electric device, method and use
PatentPendingEP4312286A1
Innovation
- A lithium coating composition with a polymer layer formed by cyanoacrylic derivative monomers, containing fluorine-substituted aliphatic groups, is applied to the lithium negative electrode, creating a dense and uniform protective layer that inhibits electrolyte consumption, regulates lithium ion deposition, and prevents dendrite formation.
Safety and Stability Assessment of LiCl-Polymer Systems
The safety and stability of LiCl-polymer electrolyte systems represent critical considerations for their practical implementation in energy storage applications. Comprehensive assessment reveals that these systems face several inherent challenges that must be addressed through rigorous testing and engineering solutions.
Thermal stability remains a primary concern, as LiCl-polymer interfaces can exhibit degradation at elevated temperatures typically encountered in battery operations. Calorimetric studies indicate that certain polymer matrices containing lithium chloride begin to show structural changes at temperatures above 80°C, potentially compromising the electrolyte integrity. This thermal vulnerability necessitates careful material selection and thermal management strategies for devices utilizing these electrolytes.
Chemical reactivity between lithium chloride and polymer hosts presents another significant challenge. Long-term exposure tests demonstrate that LiCl can gradually react with functional groups in some polymers, particularly those containing carbonyl or hydroxyl moieties. These reactions may lead to the formation of undesirable byproducts that not only reduce ionic conductivity but potentially introduce safety hazards through gas evolution or material degradation.
Moisture sensitivity significantly impacts the stability of LiCl-polymer systems. Hygroscopic properties of lithium chloride can lead to water absorption even in seemingly sealed environments, resulting in decreased electrochemical performance and potential side reactions. Accelerated aging tests under controlled humidity conditions show that even 2% water content can reduce the operational lifetime of these electrolytes by up to 40%.
Mechanical stability assessments reveal that LiCl incorporation can affect the structural integrity of polymer matrices. While moderate concentrations may enhance mechanical properties through ionic crosslinking effects, excessive LiCl loading (typically above 15 wt%) tends to induce brittleness and reduce the elasticity necessary for maintaining electrode-electrolyte contact during charge-discharge cycles.
Electrochemical stability windows of LiCl-polymer systems typically range between 3.8-4.2V vs. Li/Li+, which is narrower than required for high-voltage applications. This limitation necessitates the incorporation of stabilizing additives or protective interface layers to prevent oxidative decomposition at higher potentials.
Safety certification protocols for LiCl-polymer electrolytes must address unique challenges including potential chlorine gas evolution under extreme conditions and the formation of dendrites that could lead to internal short circuits. Industry standards are still evolving to fully characterize these risks, with current testing methodologies being adapted from liquid electrolyte frameworks to better reflect the specific failure modes of solid polymer systems containing ionic salts.
Thermal stability remains a primary concern, as LiCl-polymer interfaces can exhibit degradation at elevated temperatures typically encountered in battery operations. Calorimetric studies indicate that certain polymer matrices containing lithium chloride begin to show structural changes at temperatures above 80°C, potentially compromising the electrolyte integrity. This thermal vulnerability necessitates careful material selection and thermal management strategies for devices utilizing these electrolytes.
Chemical reactivity between lithium chloride and polymer hosts presents another significant challenge. Long-term exposure tests demonstrate that LiCl can gradually react with functional groups in some polymers, particularly those containing carbonyl or hydroxyl moieties. These reactions may lead to the formation of undesirable byproducts that not only reduce ionic conductivity but potentially introduce safety hazards through gas evolution or material degradation.
Moisture sensitivity significantly impacts the stability of LiCl-polymer systems. Hygroscopic properties of lithium chloride can lead to water absorption even in seemingly sealed environments, resulting in decreased electrochemical performance and potential side reactions. Accelerated aging tests under controlled humidity conditions show that even 2% water content can reduce the operational lifetime of these electrolytes by up to 40%.
Mechanical stability assessments reveal that LiCl incorporation can affect the structural integrity of polymer matrices. While moderate concentrations may enhance mechanical properties through ionic crosslinking effects, excessive LiCl loading (typically above 15 wt%) tends to induce brittleness and reduce the elasticity necessary for maintaining electrode-electrolyte contact during charge-discharge cycles.
Electrochemical stability windows of LiCl-polymer systems typically range between 3.8-4.2V vs. Li/Li+, which is narrower than required for high-voltage applications. This limitation necessitates the incorporation of stabilizing additives or protective interface layers to prevent oxidative decomposition at higher potentials.
Safety certification protocols for LiCl-polymer electrolytes must address unique challenges including potential chlorine gas evolution under extreme conditions and the formation of dendrites that could lead to internal short circuits. Industry standards are still evolving to fully characterize these risks, with current testing methodologies being adapted from liquid electrolyte frameworks to better reflect the specific failure modes of solid polymer systems containing ionic salts.
Environmental Impact and Sustainability Considerations
The integration of lithium chloride into polymer electrolytes presents significant environmental and sustainability considerations that warrant careful examination. The manufacturing processes for these enhanced electrolytes typically involve energy-intensive steps and potentially hazardous chemicals, raising concerns about their overall environmental footprint. Production methods often require high-temperature synthesis and solvent-based processing, which contribute to greenhouse gas emissions and potential chemical waste generation if not properly managed.
Lithium extraction itself poses substantial environmental challenges, as traditional mining operations can lead to habitat destruction, soil degradation, and water pollution. The increasing demand for lithium in battery technologies has already placed pressure on lithium-rich regions, particularly in South America's "Lithium Triangle," where water-intensive extraction methods threaten local ecosystems and communities. The addition of lithium chloride to polymer electrolytes further intensifies this demand, potentially exacerbating these environmental impacts.
Recycling and end-of-life management represent critical sustainability factors for lithium chloride-enhanced polymer electrolytes. Current recycling technologies for conventional lithium-ion batteries are not optimized for polymer-based systems, creating challenges for material recovery. The complex chemical interactions between lithium chloride and polymer matrices may complicate separation processes, potentially reducing recycling efficiency and increasing the likelihood of materials ending up in landfills.
Water consumption presents another significant environmental concern, as both lithium extraction and electrolyte manufacturing processes require substantial water resources. In regions already experiencing water scarcity, these additional demands can strain local supplies and potentially create conflicts with agricultural and community needs. The water footprint of lithium chloride-enhanced polymer electrolytes must be carefully assessed and minimized through process optimization and water recycling initiatives.
Recent advancements in green chemistry approaches offer promising pathways to mitigate these environmental impacts. Research into aqueous processing methods, solvent-free synthesis, and bio-derived polymer components demonstrates potential for reducing the environmental burden of these materials. Additionally, the development of direct lithium extraction technologies that minimize water usage and land disturbance could significantly improve the sustainability profile of lithium chloride sourcing.
The extended lifespan and improved performance of lithium chloride-enhanced polymer electrolytes may partially offset their environmental impacts through reduced replacement frequency and potentially lower material requirements over time. Life cycle assessment studies indicate that performance improvements in energy density and cycle life can translate to meaningful sustainability benefits when evaluated from a systems perspective, particularly in grid storage and electric vehicle applications.
Lithium extraction itself poses substantial environmental challenges, as traditional mining operations can lead to habitat destruction, soil degradation, and water pollution. The increasing demand for lithium in battery technologies has already placed pressure on lithium-rich regions, particularly in South America's "Lithium Triangle," where water-intensive extraction methods threaten local ecosystems and communities. The addition of lithium chloride to polymer electrolytes further intensifies this demand, potentially exacerbating these environmental impacts.
Recycling and end-of-life management represent critical sustainability factors for lithium chloride-enhanced polymer electrolytes. Current recycling technologies for conventional lithium-ion batteries are not optimized for polymer-based systems, creating challenges for material recovery. The complex chemical interactions between lithium chloride and polymer matrices may complicate separation processes, potentially reducing recycling efficiency and increasing the likelihood of materials ending up in landfills.
Water consumption presents another significant environmental concern, as both lithium extraction and electrolyte manufacturing processes require substantial water resources. In regions already experiencing water scarcity, these additional demands can strain local supplies and potentially create conflicts with agricultural and community needs. The water footprint of lithium chloride-enhanced polymer electrolytes must be carefully assessed and minimized through process optimization and water recycling initiatives.
Recent advancements in green chemistry approaches offer promising pathways to mitigate these environmental impacts. Research into aqueous processing methods, solvent-free synthesis, and bio-derived polymer components demonstrates potential for reducing the environmental burden of these materials. Additionally, the development of direct lithium extraction technologies that minimize water usage and land disturbance could significantly improve the sustainability profile of lithium chloride sourcing.
The extended lifespan and improved performance of lithium chloride-enhanced polymer electrolytes may partially offset their environmental impacts through reduced replacement frequency and potentially lower material requirements over time. Life cycle assessment studies indicate that performance improvements in energy density and cycle life can translate to meaningful sustainability benefits when evaluated from a systems perspective, particularly in grid storage and electric vehicle applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!