How to Evaluate Battery Performance with Lithium Chloride
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Chloride Battery Technology Background and Objectives
Lithium-ion batteries have revolutionized portable electronics and electric vehicles since their commercial introduction in the early 1990s. The evolution of battery technology has been marked by continuous improvements in energy density, cycle life, and safety features. Within this landscape, lithium chloride (LiCl) has emerged as a significant component in various battery technologies, particularly in solid-state electrolytes and as an additive in conventional liquid electrolytes.
The historical trajectory of lithium chloride in battery applications can be traced back to fundamental research in the 1970s on ionic conductivity in solid materials. However, it wasn't until recent advances in materials science and electrochemistry that its potential has been more fully recognized. The current technological push toward safer, higher-capacity energy storage solutions has positioned lithium chloride as a material of considerable interest.
The primary objective of evaluating battery performance with lithium chloride is to determine its efficacy in enhancing critical battery parameters including ionic conductivity, interfacial stability, and electrochemical performance across varying temperature ranges. Specifically, researchers aim to understand how lithium chloride influences the formation and stability of the solid electrolyte interphase (SEI) layer, which is crucial for battery longevity and safety.
Another key goal is to assess lithium chloride's role in mitigating dendrite formation—a common failure mechanism in lithium-based batteries that can lead to short circuits and safety hazards. The evaluation seeks to quantify how different concentrations and formulations of lithium chloride affect this phenomenon.
From a commercial perspective, the technology aims to develop batteries with improved energy density (targeting >400 Wh/kg), extended cycle life (>1000 cycles at 80% capacity retention), and enhanced safety profiles compared to conventional lithium-ion technologies. These improvements would directly address current market demands for electric vehicles and grid storage applications.
The evaluation of lithium chloride in battery systems also encompasses environmental and economic considerations. The technology trend is moving toward more sustainable and cost-effective battery solutions, with lithium chloride potentially offering advantages in terms of resource availability and manufacturing processes.
Recent technological developments suggest a convergence of lithium chloride applications in both solid-state and advanced liquid electrolyte systems, indicating a versatile future for this material in next-generation energy storage. The ultimate technological objective is to establish standardized evaluation protocols that can accurately predict real-world battery performance with lithium chloride across diverse operating conditions and applications.
The historical trajectory of lithium chloride in battery applications can be traced back to fundamental research in the 1970s on ionic conductivity in solid materials. However, it wasn't until recent advances in materials science and electrochemistry that its potential has been more fully recognized. The current technological push toward safer, higher-capacity energy storage solutions has positioned lithium chloride as a material of considerable interest.
The primary objective of evaluating battery performance with lithium chloride is to determine its efficacy in enhancing critical battery parameters including ionic conductivity, interfacial stability, and electrochemical performance across varying temperature ranges. Specifically, researchers aim to understand how lithium chloride influences the formation and stability of the solid electrolyte interphase (SEI) layer, which is crucial for battery longevity and safety.
Another key goal is to assess lithium chloride's role in mitigating dendrite formation—a common failure mechanism in lithium-based batteries that can lead to short circuits and safety hazards. The evaluation seeks to quantify how different concentrations and formulations of lithium chloride affect this phenomenon.
From a commercial perspective, the technology aims to develop batteries with improved energy density (targeting >400 Wh/kg), extended cycle life (>1000 cycles at 80% capacity retention), and enhanced safety profiles compared to conventional lithium-ion technologies. These improvements would directly address current market demands for electric vehicles and grid storage applications.
The evaluation of lithium chloride in battery systems also encompasses environmental and economic considerations. The technology trend is moving toward more sustainable and cost-effective battery solutions, with lithium chloride potentially offering advantages in terms of resource availability and manufacturing processes.
Recent technological developments suggest a convergence of lithium chloride applications in both solid-state and advanced liquid electrolyte systems, indicating a versatile future for this material in next-generation energy storage. The ultimate technological objective is to establish standardized evaluation protocols that can accurately predict real-world battery performance with lithium chloride across diverse operating conditions and applications.
Market Analysis for LiCl-based Battery Applications
The global market for lithium-ion batteries has experienced exponential growth, reaching approximately $46.2 billion in 2022 with projections to exceed $182.5 billion by 2030, representing a CAGR of 18.7%. Within this expanding landscape, LiCl-based battery applications are emerging as a significant segment due to their potential performance advantages and cost efficiencies.
The automotive sector represents the largest market opportunity for LiCl-based battery technologies, driven by the rapid adoption of electric vehicles worldwide. EV sales surpassed 10 million units globally in 2022, with major markets including China, Europe, and North America showing continued strong growth trajectories. LiCl-modified electrolytes and electrode materials offer potential solutions to range anxiety and charging speed limitations that currently constrain broader EV adoption.
Consumer electronics constitutes the second most promising application area, with demand for longer-lasting, faster-charging, and safer battery solutions continuing to rise. The global smartphone market alone, with annual shipments exceeding 1.2 billion units, represents a massive potential market for improved battery technologies. LiCl additives in battery electrolytes have demonstrated capacity retention improvements of up to 25% in laboratory testing, which could translate to significant competitive advantages in commercial applications.
Grid-scale energy storage represents a rapidly expanding market segment, projected to grow at 32.8% CAGR through 2030. The integration of renewable energy sources into power grids worldwide is creating unprecedented demand for efficient, long-duration energy storage solutions. LiCl-based technologies show particular promise in this sector due to their potential for improved cycle life and thermal stability characteristics.
Regional market analysis indicates that Asia-Pacific currently dominates the battery manufacturing landscape, accounting for over 70% of global production capacity. However, significant investments in North America and Europe aim to reduce dependency on Asian supply chains, creating new market opportunities for innovative battery technologies including those utilizing LiCl.
Customer demand patterns increasingly prioritize three key performance metrics: energy density, charging speed, and cycle life. LiCl-based solutions have demonstrated improvements in all three areas under laboratory conditions, with particularly notable enhancements in cycle life (up to 40% improvement in some studies) and fast-charging capabilities.
Market barriers to adoption include supply chain constraints for high-purity LiCl, integration challenges with existing manufacturing processes, and regulatory uncertainties regarding new battery chemistries. However, the potential performance benefits and relatively modest implementation costs compared to entirely new battery chemistries suggest a favorable cost-benefit ratio for commercial development.
The automotive sector represents the largest market opportunity for LiCl-based battery technologies, driven by the rapid adoption of electric vehicles worldwide. EV sales surpassed 10 million units globally in 2022, with major markets including China, Europe, and North America showing continued strong growth trajectories. LiCl-modified electrolytes and electrode materials offer potential solutions to range anxiety and charging speed limitations that currently constrain broader EV adoption.
Consumer electronics constitutes the second most promising application area, with demand for longer-lasting, faster-charging, and safer battery solutions continuing to rise. The global smartphone market alone, with annual shipments exceeding 1.2 billion units, represents a massive potential market for improved battery technologies. LiCl additives in battery electrolytes have demonstrated capacity retention improvements of up to 25% in laboratory testing, which could translate to significant competitive advantages in commercial applications.
Grid-scale energy storage represents a rapidly expanding market segment, projected to grow at 32.8% CAGR through 2030. The integration of renewable energy sources into power grids worldwide is creating unprecedented demand for efficient, long-duration energy storage solutions. LiCl-based technologies show particular promise in this sector due to their potential for improved cycle life and thermal stability characteristics.
Regional market analysis indicates that Asia-Pacific currently dominates the battery manufacturing landscape, accounting for over 70% of global production capacity. However, significant investments in North America and Europe aim to reduce dependency on Asian supply chains, creating new market opportunities for innovative battery technologies including those utilizing LiCl.
Customer demand patterns increasingly prioritize three key performance metrics: energy density, charging speed, and cycle life. LiCl-based solutions have demonstrated improvements in all three areas under laboratory conditions, with particularly notable enhancements in cycle life (up to 40% improvement in some studies) and fast-charging capabilities.
Market barriers to adoption include supply chain constraints for high-purity LiCl, integration challenges with existing manufacturing processes, and regulatory uncertainties regarding new battery chemistries. However, the potential performance benefits and relatively modest implementation costs compared to entirely new battery chemistries suggest a favorable cost-benefit ratio for commercial development.
Current Challenges in LiCl Battery Performance Evaluation
Despite significant advancements in lithium-ion battery technology, the evaluation of battery performance using lithium chloride (LiCl) as an electrolyte component faces numerous challenges that impede both research progress and commercial application. The hygroscopic nature of LiCl presents a fundamental obstacle, as it readily absorbs moisture from the environment, compromising the integrity of performance measurements and accelerating battery degradation. This necessitates stringent environmental controls during testing, significantly increasing evaluation complexity and cost.
Temperature sensitivity represents another critical challenge, as LiCl-based electrolytes exhibit substantial performance variations across different temperature ranges. This variability complicates the standardization of testing protocols and makes comparative analysis between different battery systems problematic. Researchers must conduct evaluations across wide temperature spectrums to generate comprehensive performance profiles, extending development timelines.
The interface dynamics between LiCl-containing electrolytes and electrode materials introduce additional evaluation complexities. The formation and evolution of the solid-electrolyte interphase (SEI) layer with LiCl present differs markedly from conventional electrolyte systems, requiring specialized analytical techniques and modified testing methodologies. Current evaluation frameworks often fail to adequately characterize these unique interfacial phenomena.
Long-term stability assessment presents perhaps the most significant challenge. Accelerated aging tests frequently fail to accurately predict real-world performance of LiCl-containing batteries, as degradation mechanisms specific to these systems may not manifest under standard testing conditions. This disconnect between laboratory evaluation and practical application creates uncertainty regarding battery lifespan and reliability projections.
Standardization issues further complicate the landscape, as the battery industry lacks universally accepted protocols for evaluating LiCl-based systems. This absence of standardized methodologies makes cross-comparison between research findings difficult and hinders knowledge transfer between academic and industrial sectors. The resulting fragmentation of evaluation approaches impedes collective progress in the field.
Analytical instrumentation limitations also constrain effective performance evaluation. Many conventional battery testing instruments are not optimized for the unique characteristics of LiCl-containing systems, particularly regarding corrosion resistance and sensitivity to trace contaminants. This technical gap necessitates either significant modification of existing equipment or development of specialized testing apparatus, both representing substantial resource investments.
The multifaceted nature of these challenges underscores the need for innovative approaches to battery performance evaluation that specifically address the unique properties of LiCl-based systems. Overcoming these obstacles requires interdisciplinary collaboration between electrochemists, materials scientists, and instrumentation specialists to develop robust, standardized evaluation methodologies.
Temperature sensitivity represents another critical challenge, as LiCl-based electrolytes exhibit substantial performance variations across different temperature ranges. This variability complicates the standardization of testing protocols and makes comparative analysis between different battery systems problematic. Researchers must conduct evaluations across wide temperature spectrums to generate comprehensive performance profiles, extending development timelines.
The interface dynamics between LiCl-containing electrolytes and electrode materials introduce additional evaluation complexities. The formation and evolution of the solid-electrolyte interphase (SEI) layer with LiCl present differs markedly from conventional electrolyte systems, requiring specialized analytical techniques and modified testing methodologies. Current evaluation frameworks often fail to adequately characterize these unique interfacial phenomena.
Long-term stability assessment presents perhaps the most significant challenge. Accelerated aging tests frequently fail to accurately predict real-world performance of LiCl-containing batteries, as degradation mechanisms specific to these systems may not manifest under standard testing conditions. This disconnect between laboratory evaluation and practical application creates uncertainty regarding battery lifespan and reliability projections.
Standardization issues further complicate the landscape, as the battery industry lacks universally accepted protocols for evaluating LiCl-based systems. This absence of standardized methodologies makes cross-comparison between research findings difficult and hinders knowledge transfer between academic and industrial sectors. The resulting fragmentation of evaluation approaches impedes collective progress in the field.
Analytical instrumentation limitations also constrain effective performance evaluation. Many conventional battery testing instruments are not optimized for the unique characteristics of LiCl-containing systems, particularly regarding corrosion resistance and sensitivity to trace contaminants. This technical gap necessitates either significant modification of existing equipment or development of specialized testing apparatus, both representing substantial resource investments.
The multifaceted nature of these challenges underscores the need for innovative approaches to battery performance evaluation that specifically address the unique properties of LiCl-based systems. Overcoming these obstacles requires interdisciplinary collaboration between electrochemists, materials scientists, and instrumentation specialists to develop robust, standardized evaluation methodologies.
Established Protocols for LiCl Battery Performance Assessment
01 Lithium chloride as electrolyte additive
Lithium chloride can be used as an electrolyte additive in lithium-ion batteries to improve battery performance. The addition of lithium chloride to the electrolyte can enhance ionic conductivity, improve the stability of the solid electrolyte interphase (SEI) layer, and reduce unwanted side reactions. This results in improved cycling stability, enhanced capacity retention, and extended battery life.- Lithium chloride as electrolyte additive: Lithium chloride can be used as an electrolyte additive in lithium batteries to enhance performance. When added to the electrolyte solution, it can improve ionic conductivity and stabilize the solid electrolyte interphase (SEI) layer. This results in better cycling stability, reduced impedance, and improved overall battery performance. The chloride ions can also help suppress unwanted side reactions at the electrode-electrolyte interface.
- Lithium chloride in solid-state electrolytes: Incorporating lithium chloride into solid-state electrolytes can significantly enhance battery performance. The addition of lithium chloride helps improve ionic conductivity in solid electrolytes, addressing one of the major limitations of solid-state batteries. These electrolytes demonstrate better thermal stability and safety characteristics compared to liquid electrolytes, while the presence of lithium chloride enhances lithium ion transport through the solid matrix, resulting in improved power density and cycling performance.
- Lithium chloride for electrode modification: Lithium chloride can be used to modify electrode surfaces in lithium batteries, enhancing their performance and stability. When applied to cathode or anode materials, lithium chloride creates a protective layer that prevents unwanted side reactions and reduces electrode degradation. This modification helps improve capacity retention, cycling stability, and rate capability of the battery. The chloride ions can also facilitate lithium ion diffusion at the electrode interface, resulting in better high-rate performance.
- Lithium chloride in composite electrode materials: Incorporating lithium chloride into composite electrode materials can enhance battery performance through improved structural stability and ionic conductivity. When lithium chloride is integrated into the electrode material composition, it can help maintain structural integrity during charge-discharge cycles, reducing volume changes and mechanical stress. These composite materials show enhanced electrochemical properties, including higher capacity, better rate capability, and improved cycling stability compared to conventional electrode materials.
- Lithium chloride for pre-lithiation processes: Lithium chloride can be utilized in pre-lithiation processes to improve initial capacity and coulombic efficiency of lithium batteries. By using lithium chloride as a lithium source for pre-lithiation of anode materials, the initial irreversible capacity loss can be significantly reduced. This process compensates for lithium ions consumed during SEI formation in the first cycle, resulting in higher initial capacity and better overall energy density. Pre-lithiation with lithium chloride also helps stabilize the electrode-electrolyte interface, leading to improved cycling performance.
02 Lithium chloride in solid-state electrolytes
Lithium chloride is utilized in the development of solid-state electrolytes for next-generation batteries. When incorporated into solid polymer or ceramic electrolytes, lithium chloride can significantly enhance ionic conductivity and mechanical properties. These solid-state electrolytes offer advantages such as improved safety, higher energy density, and better thermal stability compared to conventional liquid electrolytes, leading to overall enhanced battery performance.Expand Specific Solutions03 Lithium chloride for electrode modification
Lithium chloride can be used to modify electrode materials in lithium-ion batteries. When applied as a coating or dopant for cathode or anode materials, lithium chloride can improve the structural stability, enhance the electronic/ionic conductivity, and mitigate volume changes during charge-discharge cycles. These modifications lead to improved rate capability, higher capacity, and better cycling performance of the battery.Expand Specific Solutions04 Lithium chloride in pre-lithiation processes
Lithium chloride serves as a precursor in pre-lithiation processes for battery electrodes. Pre-lithiation helps compensate for the irreversible capacity loss during the first charge-discharge cycle by providing additional lithium ions. Using lithium chloride in these processes can lead to higher initial coulombic efficiency, improved energy density, and better overall battery performance, particularly for silicon and graphite-based anodes.Expand Specific Solutions05 Lithium chloride for interface engineering
Lithium chloride is employed in interface engineering between electrodes and electrolytes in batteries. By modifying the interface with lithium chloride, the formation of a stable and ion-conductive interphase layer is promoted. This engineered interface reduces interfacial resistance, suppresses dendrite formation, and enhances ion transport kinetics, resulting in improved battery safety, longer cycle life, and better rate performance.Expand Specific Solutions
Key Industry Players in LiCl Battery Development
The lithium chloride battery performance evaluation landscape is evolving rapidly, with the market currently in a growth phase characterized by increasing demand for advanced energy storage solutions. The global market size is expanding significantly, driven by electric vehicle adoption and renewable energy integration. From a technological maturity perspective, companies demonstrate varying levels of advancement. Leading players like LG Energy Solution, Toyota Motor Corp., and Hefei Guoxuan High-Tech are making substantial investments in lithium chloride battery research, while research institutions such as CNRS and Caltech are contributing fundamental breakthroughs. Emerging competitors like Corvus Energy and Xilectric are developing specialized applications. The competitive landscape features both established automotive manufacturers (Hyundai Mobis, Ford) and dedicated battery manufacturers (Tianjin Lishen, VITZROCELL) working to optimize lithium chloride's potential for improved energy density, cycle life, and safety performance.
Centre National de la Recherche Scientifique
Technical Solution: The Centre National de la Recherche Scientifique (CNRS) has developed a comprehensive methodology for evaluating battery performance with lithium chloride, particularly focused on fundamental electrochemical mechanisms. Their approach combines advanced spectroscopic techniques with computational modeling to understand the molecular-level interactions between lithium chloride and various battery components. CNRS researchers employ operando neutron diffraction to track lithium ion movement in real-time during battery cycling, providing unique insights into transport phenomena affected by lithium chloride. Their evaluation protocol includes sophisticated nuclear magnetic resonance (NMR) spectroscopy to characterize the local chemical environment around lithium ions in the presence of chloride ions. The CNRS methodology also incorporates density functional theory (DFT) calculations to predict and interpret experimental observations, creating a powerful feedback loop between theoretical and practical aspects of battery research. Their studies have demonstrated that lithium chloride can significantly modify the solvation structure of lithium ions in electrolytes, potentially reducing desolvation energy barriers at electrode interfaces by up to 25%, which translates to improved rate capability in practical cells.
Strengths: Unparalleled depth of fundamental scientific understanding; access to world-class characterization facilities enabling atomic-level insights; strong integration of experimental and computational approaches. Weaknesses: Their evaluation methods often prioritize scientific understanding over practical application metrics; the sophisticated techniques employed may not be readily transferable to industrial quality control settings.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has developed advanced lithium chloride-based battery evaluation protocols that focus on enhancing the stability of solid-state electrolytes. Their approach involves incorporating lithium chloride as a critical component in the interface between cathode materials and solid electrolytes to mitigate interfacial resistance issues. The company employs specialized electrochemical impedance spectroscopy (EIS) techniques to quantify the impact of lithium chloride on ion transport across interfaces. Their evaluation methodology includes accelerated aging tests under various temperature conditions (from -20°C to 60°C) to assess the long-term stability of batteries containing lithium chloride additives. LG Energy Solution has also pioneered a multi-parameter assessment framework that simultaneously monitors capacity retention, internal resistance changes, and self-discharge rates to provide a comprehensive performance profile of lithium chloride-modified battery systems.
Strengths: Superior interface engineering expertise allowing precise quantification of lithium chloride effects on battery performance; comprehensive testing infrastructure capable of simulating diverse operating conditions. Weaknesses: Their evaluation methods require sophisticated equipment not readily available in standard laboratories, and the testing protocols are time-intensive, potentially delaying product development cycles.
Critical Patents and Research on LiCl Battery Evaluation
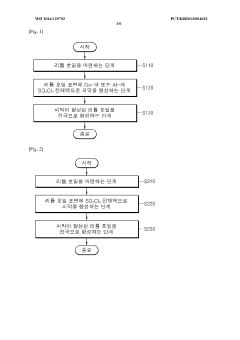
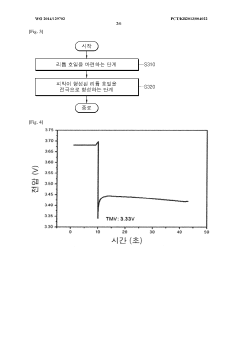
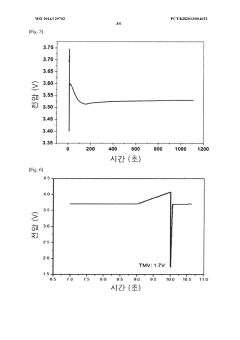
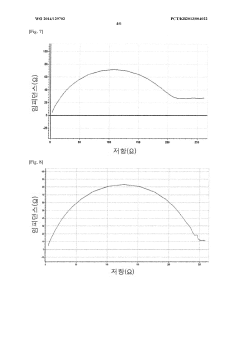
Method for manufacturing lithium-thionyl chloride battery with excellent voltage performance and method for evaluating same
PatentWO2014129702A1
Innovation
- A method involving the reaction of lithium metal with SO2Cl2 electrolyte to form a film on the lithium electrode, which improves the minimum drop voltage (TMV) and impedance characteristics by using aluminum-based or gallium-based compounds in the electrolyte solution and specific coating methods like roll coating or dip coating.
Method of evaluating performance of a flexible battery
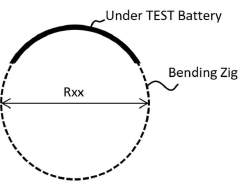
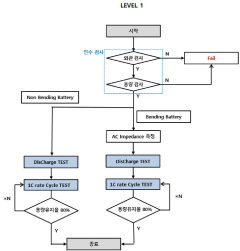
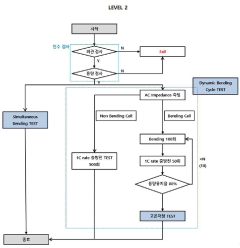
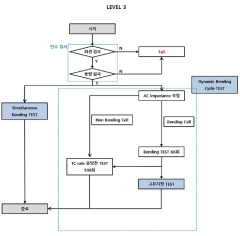
PatentInactiveKR1020200045686A
Innovation
- A method involving acceptance tests followed by static and dynamic bending tests, including charge/discharge performance evaluations under controlled bending conditions, to simulate real-world usage scenarios and detect failure causes.
Environmental Impact of LiCl Battery Technologies
The environmental implications of lithium chloride (LiCl) battery technologies extend across their entire lifecycle, from raw material extraction to disposal. Mining operations for lithium resources significantly impact local ecosystems, particularly in the lithium-rich regions of South America's "Lithium Triangle," where extensive water consumption—approximately 500,000 gallons per ton of lithium—threatens water tables and biodiversity. These operations also generate substantial carbon emissions, contributing to the overall environmental footprint of battery production.
Manufacturing processes for LiCl-based batteries involve energy-intensive procedures and potentially hazardous chemicals. The production phase accounts for approximately 30-40% of the total lifecycle emissions of these batteries, with energy consumption during electrolyte preparation and cell assembly being particularly significant contributors. Chemical handling during manufacturing presents additional environmental risks through potential leakage or improper disposal of processing agents.
During the operational phase, LiCl battery technologies demonstrate certain environmental advantages. Their enhanced energy density and cycle life compared to conventional lithium-ion batteries can reduce the frequency of replacement, thereby decreasing waste generation. Studies indicate that LiCl-modified batteries can achieve up to 20% longer lifespans under optimal conditions, translating to proportional reductions in manufacturing-related environmental impacts over time.
End-of-life management presents both challenges and opportunities. Currently, less than 5% of lithium batteries globally undergo proper recycling processes. LiCl batteries contain valuable materials that could be recovered, but existing recycling infrastructure is inadequately equipped to handle these specific chemistries. Improper disposal can lead to soil and water contamination, as lithium compounds may leach into groundwater systems.
Recent life cycle assessments (LCAs) comparing LiCl battery technologies with conventional alternatives show a complex environmental trade-off. While LiCl batteries may offer reduced impacts during use phases due to improved performance characteristics, their production and end-of-life stages currently present higher environmental burdens unless recycling rates improve significantly.
Emerging technologies for more environmentally friendly LiCl battery production include water-based processing methods that reduce solvent usage by up to 80%, and closed-loop manufacturing systems that recapture and reuse processing chemicals. These innovations, coupled with renewable energy integration in manufacturing facilities, could substantially reduce the environmental footprint of LiCl battery technologies in the coming decade.
Manufacturing processes for LiCl-based batteries involve energy-intensive procedures and potentially hazardous chemicals. The production phase accounts for approximately 30-40% of the total lifecycle emissions of these batteries, with energy consumption during electrolyte preparation and cell assembly being particularly significant contributors. Chemical handling during manufacturing presents additional environmental risks through potential leakage or improper disposal of processing agents.
During the operational phase, LiCl battery technologies demonstrate certain environmental advantages. Their enhanced energy density and cycle life compared to conventional lithium-ion batteries can reduce the frequency of replacement, thereby decreasing waste generation. Studies indicate that LiCl-modified batteries can achieve up to 20% longer lifespans under optimal conditions, translating to proportional reductions in manufacturing-related environmental impacts over time.
End-of-life management presents both challenges and opportunities. Currently, less than 5% of lithium batteries globally undergo proper recycling processes. LiCl batteries contain valuable materials that could be recovered, but existing recycling infrastructure is inadequately equipped to handle these specific chemistries. Improper disposal can lead to soil and water contamination, as lithium compounds may leach into groundwater systems.
Recent life cycle assessments (LCAs) comparing LiCl battery technologies with conventional alternatives show a complex environmental trade-off. While LiCl batteries may offer reduced impacts during use phases due to improved performance characteristics, their production and end-of-life stages currently present higher environmental burdens unless recycling rates improve significantly.
Emerging technologies for more environmentally friendly LiCl battery production include water-based processing methods that reduce solvent usage by up to 80%, and closed-loop manufacturing systems that recapture and reuse processing chemicals. These innovations, coupled with renewable energy integration in manufacturing facilities, could substantially reduce the environmental footprint of LiCl battery technologies in the coming decade.
Safety Standards and Regulatory Compliance for LiCl Batteries
The regulatory landscape for lithium chloride (LiCl) batteries encompasses multiple layers of standards designed to ensure safety, performance, and environmental compliance. At the international level, the IEC 62133 standard provides comprehensive guidelines for safety requirements of portable sealed secondary cells and batteries containing alkaline or non-acid electrolytes. For LiCl batteries specifically, this standard addresses critical safety concerns including thermal stability, electrical safety parameters, and mechanical integrity under various operating conditions.
In the United States, UL 1642 serves as the primary safety standard for lithium batteries, with the Department of Transportation (DOT) imposing strict regulations on the transportation of LiCl batteries through 49 CFR 173.185. These regulations mandate specific packaging requirements, state-of-charge limitations during transport, and comprehensive documentation of battery specifications and safety testing results.
The European Union enforces compliance through the Battery Directive (2006/66/EC) and subsequent amendments, which establish requirements for battery labeling, collection, recycling, and restrictions on hazardous substances. Additionally, LiCl batteries must conform to the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) regulation and the Restriction of Hazardous Substances (RoHS) Directive, limiting the use of certain hazardous materials.
Performance evaluation protocols for LiCl batteries must adhere to UN 38.3 testing requirements, which include altitude simulation, thermal testing, vibration, shock, external short circuit, impact, overcharge, and forced discharge tests. These tests are mandatory for batteries intended for commercial transport and serve as a baseline for safety certification.
Emerging regulatory trends indicate a shift toward more stringent requirements for battery management systems (BMS) in LiCl applications, with increased focus on thermal runaway prevention and early detection systems. The International Air Transport Association (IATA) Dangerous Goods Regulations are continuously updated to address evolving safety concerns related to lithium-based batteries, imposing additional restrictions on air transport.
Manufacturers must implement robust quality management systems compliant with ISO 9001 standards, with specialized battery producers often pursuing ISO/TS 16949 certification for automotive applications. Environmental considerations are addressed through ISO 14001 compliance, which governs waste management practices and environmental impact throughout the battery lifecycle.
Regulatory compliance testing for LiCl batteries typically requires third-party certification from accredited laboratories, with documentation maintained throughout the product lifecycle to demonstrate ongoing conformity with applicable standards and regulations.
In the United States, UL 1642 serves as the primary safety standard for lithium batteries, with the Department of Transportation (DOT) imposing strict regulations on the transportation of LiCl batteries through 49 CFR 173.185. These regulations mandate specific packaging requirements, state-of-charge limitations during transport, and comprehensive documentation of battery specifications and safety testing results.
The European Union enforces compliance through the Battery Directive (2006/66/EC) and subsequent amendments, which establish requirements for battery labeling, collection, recycling, and restrictions on hazardous substances. Additionally, LiCl batteries must conform to the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) regulation and the Restriction of Hazardous Substances (RoHS) Directive, limiting the use of certain hazardous materials.
Performance evaluation protocols for LiCl batteries must adhere to UN 38.3 testing requirements, which include altitude simulation, thermal testing, vibration, shock, external short circuit, impact, overcharge, and forced discharge tests. These tests are mandatory for batteries intended for commercial transport and serve as a baseline for safety certification.
Emerging regulatory trends indicate a shift toward more stringent requirements for battery management systems (BMS) in LiCl applications, with increased focus on thermal runaway prevention and early detection systems. The International Air Transport Association (IATA) Dangerous Goods Regulations are continuously updated to address evolving safety concerns related to lithium-based batteries, imposing additional restrictions on air transport.
Manufacturers must implement robust quality management systems compliant with ISO 9001 standards, with specialized battery producers often pursuing ISO/TS 16949 certification for automotive applications. Environmental considerations are addressed through ISO 14001 compliance, which governs waste management practices and environmental impact throughout the battery lifecycle.
Regulatory compliance testing for LiCl batteries typically requires third-party certification from accredited laboratories, with documentation maintained throughout the product lifecycle to demonstrate ongoing conformity with applicable standards and regulations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!