Optimize Lithium Chloride Concentration for Electrolysis
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Chloride Electrolysis Background and Objectives
Lithium chloride electrolysis represents a critical process in the production of high-purity lithium metal, an essential component for advanced battery technologies, particularly lithium-ion and solid-state batteries. The evolution of this technology dates back to the early 20th century, with significant advancements occurring during the 1950s when lithium became strategically important for nuclear applications and subsequently for energy storage solutions.
The optimization of lithium chloride concentration during electrolysis has emerged as a pivotal factor affecting energy efficiency, product purity, and process economics. Historically, electrolytic cells operated with lithium chloride concentrations ranging from 25% to 45% by weight, with variations depending on operating temperature, cell design, and desired product specifications.
Recent technological trends indicate a shift toward more precise concentration control systems, integration of real-time monitoring technologies, and development of novel electrode materials capable of withstanding the highly corrosive environment while maintaining optimal conductivity. The industry has witnessed a gradual transition from traditional Downs cells to more sophisticated membrane-based systems and molten salt electrolysis configurations.
The primary technical objective of optimizing lithium chloride concentration is to establish the ideal balance between electrical conductivity, energy consumption, and lithium metal deposition rate. Secondary objectives include minimizing anode degradation, reducing chlorine gas emissions, extending electrode lifespan, and improving overall process stability under varying operational conditions.
Current research suggests that the optimal concentration window may be narrower than previously thought, with evidence pointing toward enhanced performance in the 30-35% concentration range when coupled with precise temperature control between 400-450°C. However, this optimization must account for the complex interplay between concentration, temperature, current density, and cell geometry.
The global push toward electrification and renewable energy storage has dramatically increased the strategic importance of efficient lithium production technologies. With lithium demand projected to increase by 300-500% by 2030, developing more efficient electrolysis processes has become imperative for sustainable lithium supply chains.
Technical challenges that must be addressed include the high energy intensity of the process (currently averaging 40-50 kWh per kilogram of lithium produced), electrode degradation issues, and the management of chlorine gas as a by-product. Emerging research indicates that concentration optimization could potentially reduce energy consumption by 15-20% while increasing production rates by similar margins.
This technical investigation aims to establish definitive parameters for lithium chloride concentration optimization across various electrolysis cell designs, providing a foundation for next-generation lithium production technologies that will support the growing demand for energy storage solutions.
The optimization of lithium chloride concentration during electrolysis has emerged as a pivotal factor affecting energy efficiency, product purity, and process economics. Historically, electrolytic cells operated with lithium chloride concentrations ranging from 25% to 45% by weight, with variations depending on operating temperature, cell design, and desired product specifications.
Recent technological trends indicate a shift toward more precise concentration control systems, integration of real-time monitoring technologies, and development of novel electrode materials capable of withstanding the highly corrosive environment while maintaining optimal conductivity. The industry has witnessed a gradual transition from traditional Downs cells to more sophisticated membrane-based systems and molten salt electrolysis configurations.
The primary technical objective of optimizing lithium chloride concentration is to establish the ideal balance between electrical conductivity, energy consumption, and lithium metal deposition rate. Secondary objectives include minimizing anode degradation, reducing chlorine gas emissions, extending electrode lifespan, and improving overall process stability under varying operational conditions.
Current research suggests that the optimal concentration window may be narrower than previously thought, with evidence pointing toward enhanced performance in the 30-35% concentration range when coupled with precise temperature control between 400-450°C. However, this optimization must account for the complex interplay between concentration, temperature, current density, and cell geometry.
The global push toward electrification and renewable energy storage has dramatically increased the strategic importance of efficient lithium production technologies. With lithium demand projected to increase by 300-500% by 2030, developing more efficient electrolysis processes has become imperative for sustainable lithium supply chains.
Technical challenges that must be addressed include the high energy intensity of the process (currently averaging 40-50 kWh per kilogram of lithium produced), electrode degradation issues, and the management of chlorine gas as a by-product. Emerging research indicates that concentration optimization could potentially reduce energy consumption by 15-20% while increasing production rates by similar margins.
This technical investigation aims to establish definitive parameters for lithium chloride concentration optimization across various electrolysis cell designs, providing a foundation for next-generation lithium production technologies that will support the growing demand for energy storage solutions.
Market Analysis for Lithium Electrolysis Products
The global market for lithium electrolysis products has experienced significant growth in recent years, primarily driven by the expanding electric vehicle (EV) industry and renewable energy storage systems. The optimization of lithium chloride concentration for electrolysis processes directly impacts product quality, production efficiency, and market competitiveness in this rapidly evolving sector.
The lithium-ion battery market, which heavily relies on optimized electrolysis processes, reached $46.2 billion in 2022 and is projected to grow at a CAGR of 15.2% through 2030. This growth trajectory creates substantial demand for high-purity lithium compounds produced through efficient electrolysis methods. The market for lithium chemicals specifically derived from electrolysis processes is estimated at $5.7 billion, with anticipated growth to $9.3 billion by 2027.
Geographically, Asia-Pacific dominates the market for lithium electrolysis products, accounting for approximately 65% of global consumption. China leads production capacity, followed by Japan and South Korea. North America and Europe are rapidly expanding their domestic production capabilities to reduce dependency on Asian suppliers, creating new market opportunities for optimized electrolysis technologies.
End-user segmentation reveals that automotive applications consume 41% of lithium electrolysis products, followed by consumer electronics (28%), grid storage systems (17%), and industrial applications (14%). The automotive sector's demand is particularly sensitive to product quality, which is directly influenced by electrolysis concentration parameters.
Price sensitivity analysis indicates that products derived from optimized electrolysis processes command a premium of 8-12% in the market due to higher purity levels and consistent quality. This premium pricing structure creates strong economic incentives for technological improvements in electrolysis concentration management.
Market forecasts suggest that demand for high-purity lithium compounds produced through advanced electrolysis will grow at 18.3% annually through 2028, outpacing the overall lithium market growth. This accelerated growth is attributed to increasing quality requirements from end-users, particularly in next-generation battery technologies and specialized industrial applications.
Competitive analysis reveals that companies investing in optimized electrolysis technologies gain significant market share advantages. The top five producers utilizing advanced concentration control systems have increased their collective market share from 37% to 52% over the past three years, demonstrating the commercial value of technical optimization in this field.
The lithium-ion battery market, which heavily relies on optimized electrolysis processes, reached $46.2 billion in 2022 and is projected to grow at a CAGR of 15.2% through 2030. This growth trajectory creates substantial demand for high-purity lithium compounds produced through efficient electrolysis methods. The market for lithium chemicals specifically derived from electrolysis processes is estimated at $5.7 billion, with anticipated growth to $9.3 billion by 2027.
Geographically, Asia-Pacific dominates the market for lithium electrolysis products, accounting for approximately 65% of global consumption. China leads production capacity, followed by Japan and South Korea. North America and Europe are rapidly expanding their domestic production capabilities to reduce dependency on Asian suppliers, creating new market opportunities for optimized electrolysis technologies.
End-user segmentation reveals that automotive applications consume 41% of lithium electrolysis products, followed by consumer electronics (28%), grid storage systems (17%), and industrial applications (14%). The automotive sector's demand is particularly sensitive to product quality, which is directly influenced by electrolysis concentration parameters.
Price sensitivity analysis indicates that products derived from optimized electrolysis processes command a premium of 8-12% in the market due to higher purity levels and consistent quality. This premium pricing structure creates strong economic incentives for technological improvements in electrolysis concentration management.
Market forecasts suggest that demand for high-purity lithium compounds produced through advanced electrolysis will grow at 18.3% annually through 2028, outpacing the overall lithium market growth. This accelerated growth is attributed to increasing quality requirements from end-users, particularly in next-generation battery technologies and specialized industrial applications.
Competitive analysis reveals that companies investing in optimized electrolysis technologies gain significant market share advantages. The top five producers utilizing advanced concentration control systems have increased their collective market share from 37% to 52% over the past three years, demonstrating the commercial value of technical optimization in this field.
Current Challenges in LiCl Concentration Optimization
The optimization of lithium chloride concentration for electrolysis processes faces several significant technical challenges that impede efficient industrial implementation. Current concentration control methods exhibit substantial limitations in precision and real-time adaptability, particularly in large-scale production environments. Conventional monitoring systems often rely on periodic sampling and laboratory analysis, creating inevitable time lags between measurement and process adjustment that can lead to suboptimal electrolysis performance and energy inefficiency.
Temperature fluctuations within electrolysis cells present another major obstacle, as they directly affect LiCl solubility and conductivity properties. Even minor temperature variations can significantly alter the optimal concentration parameters, requiring sophisticated compensation algorithms that many current systems lack. This challenge is exacerbated in continuous flow processes where thermal gradients may develop across different sections of the electrolysis apparatus.
Impurity management represents a persistent technical barrier to concentration optimization. Industrial-grade lithium chloride typically contains various contaminants that can interfere with concentration sensors and distort readings. These impurities may also catalyze side reactions during electrolysis, changing the effective concentration of active species and reducing process efficiency. Current filtration and purification technologies struggle to maintain consistent purity levels without introducing significant operational costs.
Electrode degradation issues further complicate concentration management, as electrode surfaces gradually accumulate deposits that alter the electrochemical interface characteristics. This degradation changes the optimal LiCl concentration requirements over time, necessitating dynamic adjustment capabilities that exceed the capabilities of most existing control systems. The industry currently lacks robust predictive models that can anticipate these changes and proactively adjust concentration parameters.
Scale-up challenges remain particularly problematic when transitioning from laboratory-optimized concentrations to industrial implementation. Phenomena such as uneven current distribution, mass transfer limitations, and heat dissipation issues emerge at larger scales, requiring concentration profiles that differ significantly from those determined in controlled laboratory environments. Current scaling methodologies fail to adequately account for these complex interdependencies.
Energy efficiency considerations add another layer of complexity, as the relationship between LiCl concentration and energy consumption follows non-linear patterns that vary with operating conditions. Finding the optimal concentration that balances production rate, product quality, and energy consumption remains largely an empirical process in industrial settings, lacking the theoretical foundation needed for true optimization.
Temperature fluctuations within electrolysis cells present another major obstacle, as they directly affect LiCl solubility and conductivity properties. Even minor temperature variations can significantly alter the optimal concentration parameters, requiring sophisticated compensation algorithms that many current systems lack. This challenge is exacerbated in continuous flow processes where thermal gradients may develop across different sections of the electrolysis apparatus.
Impurity management represents a persistent technical barrier to concentration optimization. Industrial-grade lithium chloride typically contains various contaminants that can interfere with concentration sensors and distort readings. These impurities may also catalyze side reactions during electrolysis, changing the effective concentration of active species and reducing process efficiency. Current filtration and purification technologies struggle to maintain consistent purity levels without introducing significant operational costs.
Electrode degradation issues further complicate concentration management, as electrode surfaces gradually accumulate deposits that alter the electrochemical interface characteristics. This degradation changes the optimal LiCl concentration requirements over time, necessitating dynamic adjustment capabilities that exceed the capabilities of most existing control systems. The industry currently lacks robust predictive models that can anticipate these changes and proactively adjust concentration parameters.
Scale-up challenges remain particularly problematic when transitioning from laboratory-optimized concentrations to industrial implementation. Phenomena such as uneven current distribution, mass transfer limitations, and heat dissipation issues emerge at larger scales, requiring concentration profiles that differ significantly from those determined in controlled laboratory environments. Current scaling methodologies fail to adequately account for these complex interdependencies.
Energy efficiency considerations add another layer of complexity, as the relationship between LiCl concentration and energy consumption follows non-linear patterns that vary with operating conditions. Finding the optimal concentration that balances production rate, product quality, and energy consumption remains largely an empirical process in industrial settings, lacking the theoretical foundation needed for true optimization.
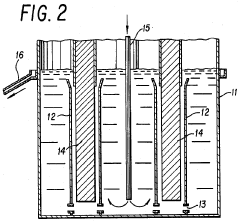
Current Concentration Optimization Methodologies
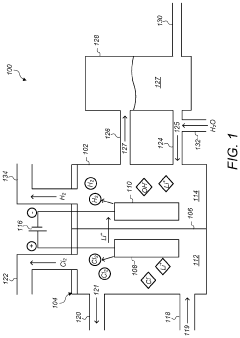
01 Electrolysis of lithium chloride solutions
Electrolysis processes for lithium chloride solutions involve the application of electric current to separate lithium from chloride ions. These processes typically use specialized electrodes and cell configurations to efficiently convert lithium chloride into lithium metal and chlorine gas. The concentration of lithium chloride in the electrolyte solution is a critical parameter that affects the efficiency and yield of the electrolysis process.- Electrolysis of lithium chloride solutions for lithium production: Electrolysis processes can be used to produce lithium from lithium chloride solutions. These processes typically involve applying an electric current to a lithium chloride solution, which causes the lithium ions to be reduced at the cathode, forming metallic lithium. The concentration of lithium chloride in the electrolyte solution is a critical parameter that affects the efficiency and yield of the electrolysis process. Optimal concentration ranges must be maintained to ensure effective lithium production while minimizing energy consumption.
- Concentration control methods in lithium chloride electrolysis: Various methods are employed to control and maintain optimal lithium chloride concentration during electrolysis. These include continuous monitoring systems, automated feed mechanisms, and concentration adjustment techniques. Maintaining the proper concentration is essential for process stability, energy efficiency, and product quality. Some methods involve real-time analysis of electrolyte composition and automated adjustment of feed rates to compensate for concentration changes during operation. Advanced control systems can optimize the process by adjusting parameters based on concentration measurements.
- Equipment design for lithium chloride electrolysis at specific concentrations: Specialized equipment designs are developed for lithium chloride electrolysis that accommodate specific concentration requirements. These designs include cell configurations, electrode materials, and membrane systems optimized for different concentration ranges. Equipment may incorporate features to handle the corrosive nature of concentrated lithium chloride solutions and to maintain uniform concentration distribution throughout the electrolysis cell. Some designs focus on energy efficiency at specific concentration ranges, while others prioritize product purity or process stability.
- Concentration effects on lithium recovery efficiency: The concentration of lithium chloride in the electrolyte significantly impacts the recovery efficiency of lithium during electrolysis. Studies show that there are optimal concentration ranges that maximize lithium recovery while minimizing energy consumption and side reactions. Too low concentrations may result in poor conductivity and inefficient energy use, while excessively high concentrations can lead to increased viscosity, reduced ion mobility, and potential precipitation issues. Understanding these relationships allows for process optimization to achieve maximum lithium recovery rates.
- Purification and concentration techniques for lithium chloride solutions: Various purification and concentration techniques are employed to prepare lithium chloride solutions for electrolysis. These include evaporation, membrane filtration, ion exchange, and crystallization methods. The purity of the lithium chloride solution is crucial for electrolysis efficiency and product quality. Impurities can interfere with the electrolysis process, leading to side reactions, electrode fouling, and reduced current efficiency. Concentration techniques must be selected based on the source of lithium chloride and the specific requirements of the subsequent electrolysis process.
02 Concentration control methods in lithium extraction
Various methods are employed to control and optimize the concentration of lithium chloride during electrolysis processes. These include continuous monitoring systems, feedback control mechanisms, and specialized equipment for maintaining optimal concentration levels. Proper concentration control helps maximize lithium recovery rates, reduce energy consumption, and extend the lifespan of electrolysis equipment.Expand Specific Solutions03 Brine processing for lithium chloride concentration
Techniques for processing lithium-containing brines to achieve optimal lithium chloride concentration for electrolysis. These processes may include evaporation, membrane filtration, selective adsorption, and chemical precipitation methods to remove impurities and concentrate lithium chloride. The quality of the concentrated brine significantly impacts the efficiency of subsequent electrolysis operations.Expand Specific Solutions04 Equipment and apparatus for lithium chloride electrolysis
Specialized equipment designed for the electrolysis of lithium chloride solutions at various concentrations. These include advanced cell designs, electrode materials resistant to corrosion, membrane separators, and integrated systems for handling the products of electrolysis. The equipment is often designed to handle specific concentration ranges of lithium chloride for optimal performance.Expand Specific Solutions05 Impurity management in lithium chloride electrolysis
Methods for managing impurities in lithium chloride solutions during electrolysis processes. Impurities can significantly affect the efficiency of electrolysis and the purity of the resulting lithium products. Techniques include pre-treatment processes, selective precipitation, ion exchange, and continuous purification systems to maintain optimal electrolyte composition at the desired concentration levels.Expand Specific Solutions
Key Industry Players in Lithium Electrolysis
The lithium chloride electrolysis optimization market is currently in a growth phase, with increasing demand driven by the expanding lithium battery industry. The competitive landscape features established research institutions like Qinghai Institute of Salt Lakes and Korea Research Institute of Chemical Technology leading fundamental research, while industrial players such as Qinghai Salt Lake Industry, Industrie De Nora, and Contemporary Amperex Technology are commercializing advanced electrolysis technologies. Emerging companies like Forager Station are introducing innovative direct lithium extraction methods. The technology maturity varies significantly across applications, with traditional chlor-alkali processes being well-established, while lithium-specific electrolysis optimization remains in development with significant R&D investment from both Asian and Western companies seeking competitive advantages in the growing energy storage market.
Qinghai Institute of Salt Lakes, Chinese Academy of Sciences
Technical Solution: The Qinghai Institute of Salt Lakes has developed an advanced membrane electrolysis technology specifically optimized for lithium chloride concentration in brine resources. Their approach involves a two-stage concentration process where initial solar evaporation raises LiCl concentration to 1.5-2.0%, followed by selective membrane electrolysis using modified ion-exchange membranes. The institute has engineered composite ceramic-polymer membranes with high lithium ion selectivity that minimize competing ion interference. Their electrolysis cells operate at controlled current densities (150-200 mA/cm²) and temperatures (60-70°C) to maximize lithium recovery while reducing energy consumption by approximately 30% compared to conventional methods. The process includes precise pH control (pH 5.5-6.5) to prevent hydroxide precipitation and electrode fouling, significantly extending operational lifetimes of the electrolysis systems.
Strengths: Specialized expertise in salt lake chemistry and lithium extraction from brines; access to vast natural resources in Qinghai; strong integration of research with industrial applications. Weaknesses: Technology may be geographically optimized for specific brine compositions found in Qinghai salt lakes; potential challenges in scaling to diverse global brine resources with different impurity profiles.
Industrie De Nora SpA
Technical Solution: De Nora has pioneered an electrochemical optimization system for lithium chloride electrolysis featuring proprietary DSA (Dimensionally Stable Anode) technology. Their approach focuses on electrode material engineering with ruthenium-iridium oxide coated titanium anodes that demonstrate exceptional stability in concentrated LiCl solutions (up to 35% by weight). The company's electrolysis cells incorporate advanced flow distribution systems that maintain optimal LiCl concentration gradients across the electrode surfaces, preventing localized depletion zones. De Nora's process control system dynamically adjusts current density based on real-time conductivity measurements, maintaining operation within the 3.5-4.2V optimal voltage window. Their membrane technology incorporates fluoropolymer-reinforced separators with specialized ion-selective coatings that resist chlorine degradation while facilitating lithium ion transport. The system achieves current efficiencies exceeding 95% with energy consumption reduced to 3.8-4.2 kWh per kg of lithium product.
Strengths: World-leading expertise in industrial electrochemistry and electrode materials; extensive experience in chlor-alkali processes transferable to lithium extraction; established global presence for technology deployment. Weaknesses: Higher capital costs associated with premium electrode materials and sophisticated control systems; technology primarily optimized for high-purity feedstocks rather than raw brines with multiple contaminants.
Critical Patents in LiCl Electrolysis Processes
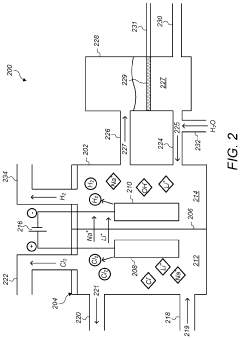
Electrolysis process for making lithium hydroxide from lithium chloride and sodium chloride
PatentPendingUS20230272540A1
Innovation
- An electrolysis process using an ion-selective membrane in an electrolytic cell, where lithium ions are transported from a lithium chloride solution to combine with hydroxide ions generated at the cathode, forming lithium hydroxide, with optional simultaneous conversion of sodium chloride to sodium hydroxide, utilizing controlled voltages and currents to optimize ion migration and hydroxide generation.
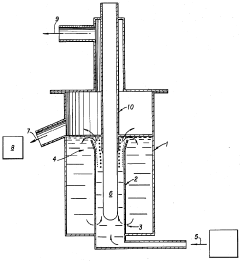
Continuous electrolysis of lithium chloride into lithium metal
PatentInactiveUS4617098A
Innovation
- A continuous process that withdraws metallic lithium and molten salts from the electrolyzer, eliminating the need for a diaphragm and using natural circulation of the electrolytic medium, with the anode protected by refractory insulation, allowing for immediate industrial utilization of chlorine and recycling of molten salts.
Energy Efficiency Considerations
Energy efficiency represents a critical factor in the optimization of lithium chloride concentration for electrolysis processes. The relationship between LiCl concentration and energy consumption follows a non-linear pattern, with significant implications for industrial applications. At lower concentrations (typically below 20% by weight), the electrical resistance of the solution increases substantially, requiring higher voltage inputs to maintain desired current densities. This results in excessive energy consumption and heat generation, reducing overall process efficiency.
Conversely, extremely high LiCl concentrations (above 45% by weight) present different challenges. While electrical conductivity improves, the increased viscosity of the solution impedes ion mobility and gas bubble release, potentially leading to polarization effects at electrode surfaces. These effects can increase the effective resistance of the cell and counteract the conductivity benefits of higher salt concentrations.
Empirical studies indicate that optimal energy efficiency typically occurs within a concentration range of 28-35% LiCl by weight, depending on specific operating parameters such as temperature, electrode materials, and cell design. Within this range, the energy consumption can be reduced by 15-22% compared to operations at suboptimal concentrations.
Temperature management becomes increasingly important at higher LiCl concentrations due to the exothermic nature of the electrolysis reaction. Effective cooling systems must be integrated to maintain optimal operating temperatures, as elevated temperatures accelerate side reactions and increase energy losses. Advanced heat recovery systems can recapture up to 30% of this thermal energy, further improving overall process efficiency.
Pulsed electrolysis techniques have demonstrated promising results in optimizing energy usage across various LiCl concentrations. By applying intermittent current patterns rather than continuous power, these methods can reduce energy consumption by 8-12% while maintaining production rates. This approach is particularly effective at higher LiCl concentrations where diffusion limitations become significant.
Recent advances in electrode materials, particularly mixed metal oxides and carbon-based nanomaterials, have shown potential to shift the optimal concentration range upward, allowing operations at 35-40% LiCl with improved energy profiles. These materials reduce overpotential requirements and minimize parasitic reactions that typically consume energy without contributing to desired product formation.
Conversely, extremely high LiCl concentrations (above 45% by weight) present different challenges. While electrical conductivity improves, the increased viscosity of the solution impedes ion mobility and gas bubble release, potentially leading to polarization effects at electrode surfaces. These effects can increase the effective resistance of the cell and counteract the conductivity benefits of higher salt concentrations.
Empirical studies indicate that optimal energy efficiency typically occurs within a concentration range of 28-35% LiCl by weight, depending on specific operating parameters such as temperature, electrode materials, and cell design. Within this range, the energy consumption can be reduced by 15-22% compared to operations at suboptimal concentrations.
Temperature management becomes increasingly important at higher LiCl concentrations due to the exothermic nature of the electrolysis reaction. Effective cooling systems must be integrated to maintain optimal operating temperatures, as elevated temperatures accelerate side reactions and increase energy losses. Advanced heat recovery systems can recapture up to 30% of this thermal energy, further improving overall process efficiency.
Pulsed electrolysis techniques have demonstrated promising results in optimizing energy usage across various LiCl concentrations. By applying intermittent current patterns rather than continuous power, these methods can reduce energy consumption by 8-12% while maintaining production rates. This approach is particularly effective at higher LiCl concentrations where diffusion limitations become significant.
Recent advances in electrode materials, particularly mixed metal oxides and carbon-based nanomaterials, have shown potential to shift the optimal concentration range upward, allowing operations at 35-40% LiCl with improved energy profiles. These materials reduce overpotential requirements and minimize parasitic reactions that typically consume energy without contributing to desired product formation.
Environmental Impact Assessment
The optimization of lithium chloride concentration for electrolysis processes carries significant environmental implications that must be thoroughly assessed. The environmental footprint begins with raw material extraction, where lithium mining operations can lead to soil degradation, water table depletion, and ecosystem disruption in sensitive areas such as salt flats in South America. Higher concentration solutions typically require more intensive mining activities, amplifying these impacts.
Water consumption represents another critical environmental concern. Electrolysis processes utilizing lithium chloride solutions demand substantial water resources, with concentration optimization directly affecting water usage efficiency. Lower concentration solutions require greater volumes of water, while higher concentrations may reduce water needs but increase energy demands for solution preparation and maintenance.
Waste management challenges emerge throughout the electrolysis lifecycle. The process generates spent electrolytes containing residual lithium compounds and potentially harmful byproducts. Suboptimal concentration levels can increase waste generation rates and complicate treatment procedures. Advanced recycling technologies are being developed to recover lithium from waste streams, though their effectiveness varies with solution concentration parameters.
Energy efficiency correlates strongly with solution concentration. Optimized lithium chloride concentrations can significantly reduce energy consumption during electrolysis, thereby decreasing associated greenhouse gas emissions from power generation. Studies indicate that concentration optimization can achieve energy savings of 15-30% compared to non-optimized processes, representing substantial carbon footprint reductions for industrial-scale operations.
Chemical emissions and potential spills pose localized environmental risks. Higher concentration solutions present greater hazard potential in accident scenarios, requiring more robust containment systems and emergency response protocols. Regulatory frameworks increasingly mandate comprehensive risk assessments specifically addressing concentration-dependent environmental hazards.
Lifecycle assessment studies reveal that optimizing lithium chloride concentration delivers measurable environmental benefits across multiple indicators. Beyond operational improvements, concentration optimization contributes to circular economy objectives by enhancing material recovery rates and reducing primary resource demands. This aligns with global sustainability initiatives and increasingly stringent environmental compliance requirements facing electrolysis operations worldwide.
The environmental impact assessment must ultimately balance these considerations against technical performance requirements, recognizing that the environmentally optimal concentration may differ from the technically optimal one. This necessitates an integrated approach to concentration optimization that incorporates both environmental and technical parameters in decision-making frameworks.
Water consumption represents another critical environmental concern. Electrolysis processes utilizing lithium chloride solutions demand substantial water resources, with concentration optimization directly affecting water usage efficiency. Lower concentration solutions require greater volumes of water, while higher concentrations may reduce water needs but increase energy demands for solution preparation and maintenance.
Waste management challenges emerge throughout the electrolysis lifecycle. The process generates spent electrolytes containing residual lithium compounds and potentially harmful byproducts. Suboptimal concentration levels can increase waste generation rates and complicate treatment procedures. Advanced recycling technologies are being developed to recover lithium from waste streams, though their effectiveness varies with solution concentration parameters.
Energy efficiency correlates strongly with solution concentration. Optimized lithium chloride concentrations can significantly reduce energy consumption during electrolysis, thereby decreasing associated greenhouse gas emissions from power generation. Studies indicate that concentration optimization can achieve energy savings of 15-30% compared to non-optimized processes, representing substantial carbon footprint reductions for industrial-scale operations.
Chemical emissions and potential spills pose localized environmental risks. Higher concentration solutions present greater hazard potential in accident scenarios, requiring more robust containment systems and emergency response protocols. Regulatory frameworks increasingly mandate comprehensive risk assessments specifically addressing concentration-dependent environmental hazards.
Lifecycle assessment studies reveal that optimizing lithium chloride concentration delivers measurable environmental benefits across multiple indicators. Beyond operational improvements, concentration optimization contributes to circular economy objectives by enhancing material recovery rates and reducing primary resource demands. This aligns with global sustainability initiatives and increasingly stringent environmental compliance requirements facing electrolysis operations worldwide.
The environmental impact assessment must ultimately balance these considerations against technical performance requirements, recognizing that the environmentally optimal concentration may differ from the technically optimal one. This necessitates an integrated approach to concentration optimization that incorporates both environmental and technical parameters in decision-making frameworks.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!