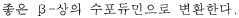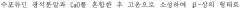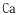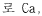How to Optimize Lithium Chloride for Aqueous Applications
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
LiCl Aqueous Applications Background and Objectives
Lithium chloride (LiCl) has emerged as a critical compound in various industrial and scientific applications due to its unique physicochemical properties. The evolution of LiCl applications in aqueous environments traces back to the early 20th century, with significant advancements occurring in the past few decades as demand for lithium-based technologies has surged. This technical exploration aims to comprehensively understand how to optimize LiCl for aqueous applications across multiple sectors.
The historical trajectory of LiCl utilization reveals a steady expansion from basic chemical processes to sophisticated applications in energy storage, pharmaceuticals, and materials science. Initially valued primarily for its hygroscopic properties, LiCl has progressively gained importance in more complex applications such as lithium-ion battery electrolytes, air conditioning systems, and as a precursor in lithium metal production.
Current technological trends indicate a growing emphasis on enhancing LiCl performance in aqueous solutions, particularly regarding stability, purity, and interaction with other compounds. The increasing global focus on sustainable energy solutions has further accelerated research into LiCl optimization, as it represents a crucial component in various green technologies, including certain types of flow batteries and thermal energy storage systems.
The primary technical objectives of this investigation include developing methodologies to improve LiCl solubility profiles across varying temperature ranges, minimizing undesirable reactions in complex aqueous environments, and enhancing the compound's stability during prolonged storage and use. Additionally, we aim to identify novel approaches to reduce energy consumption during LiCl processing and application.
Another significant goal involves understanding the molecular-level interactions between LiCl and water under different conditions, which could lead to breakthroughs in controlling crystallization processes, preventing corrosion in industrial equipment, and optimizing heat transfer capabilities in thermal applications.
The scope of this technical exploration extends to examining potential modifications to LiCl formulations, such as the incorporation of stabilizing additives or the development of specialized LiCl complexes designed for specific aqueous applications. These innovations could substantially improve performance metrics while potentially reducing costs and environmental impact.
As global lithium demand continues to rise, optimizing LiCl for aqueous applications represents not only a technical challenge but also a strategic imperative for industries relying on lithium-based technologies. This investigation seeks to establish a foundation for next-generation LiCl applications by systematically addressing current limitations and exploring emerging opportunities in this rapidly evolving technological landscape.
The historical trajectory of LiCl utilization reveals a steady expansion from basic chemical processes to sophisticated applications in energy storage, pharmaceuticals, and materials science. Initially valued primarily for its hygroscopic properties, LiCl has progressively gained importance in more complex applications such as lithium-ion battery electrolytes, air conditioning systems, and as a precursor in lithium metal production.
Current technological trends indicate a growing emphasis on enhancing LiCl performance in aqueous solutions, particularly regarding stability, purity, and interaction with other compounds. The increasing global focus on sustainable energy solutions has further accelerated research into LiCl optimization, as it represents a crucial component in various green technologies, including certain types of flow batteries and thermal energy storage systems.
The primary technical objectives of this investigation include developing methodologies to improve LiCl solubility profiles across varying temperature ranges, minimizing undesirable reactions in complex aqueous environments, and enhancing the compound's stability during prolonged storage and use. Additionally, we aim to identify novel approaches to reduce energy consumption during LiCl processing and application.
Another significant goal involves understanding the molecular-level interactions between LiCl and water under different conditions, which could lead to breakthroughs in controlling crystallization processes, preventing corrosion in industrial equipment, and optimizing heat transfer capabilities in thermal applications.
The scope of this technical exploration extends to examining potential modifications to LiCl formulations, such as the incorporation of stabilizing additives or the development of specialized LiCl complexes designed for specific aqueous applications. These innovations could substantially improve performance metrics while potentially reducing costs and environmental impact.
As global lithium demand continues to rise, optimizing LiCl for aqueous applications represents not only a technical challenge but also a strategic imperative for industries relying on lithium-based technologies. This investigation seeks to establish a foundation for next-generation LiCl applications by systematically addressing current limitations and exploring emerging opportunities in this rapidly evolving technological landscape.
Market Analysis for LiCl Aqueous Solutions
The global market for lithium chloride in aqueous applications has been experiencing significant growth, driven primarily by the expanding lithium-ion battery industry and increasing demand for energy storage solutions. The market size for lithium chloride in aqueous applications reached approximately $320 million in 2022, with projections indicating a compound annual growth rate of 8.7% through 2028.
The battery sector represents the largest application segment, accounting for nearly 42% of the total market share. This dominance is attributed to lithium chloride's critical role in battery electrolyte formulations, where its high solubility and ionic conductivity properties are particularly valuable. The growing electric vehicle market, especially in China, Europe, and North America, continues to fuel demand in this segment.
Industrial applications form the second-largest market segment at 27%, where lithium chloride aqueous solutions are utilized in air conditioning systems, dehumidification processes, and as heat transfer fluids. The pharmaceutical and medical sectors constitute approximately 18% of the market, with applications in drug formulation and as a therapeutic agent for bipolar disorder treatment.
Regional analysis reveals Asia-Pacific as the dominant market, representing 48% of global consumption, followed by North America (24%) and Europe (21%). China remains the largest producer and consumer, with its domestic lithium processing industry experiencing rapid expansion to meet both internal demand and export requirements.
Market dynamics are currently influenced by several factors, including supply chain constraints and price volatility. The average price of high-purity lithium chloride for aqueous applications has increased by 35% over the past two years, creating challenges for end-users but opportunities for producers with established supply chains.
Customer requirements are evolving toward higher purity grades (99.9%+) for advanced applications, particularly in battery technology and pharmaceuticals. This trend is driving innovation in purification techniques and quality control processes among leading suppliers.
Competitive analysis indicates a moderately concentrated market structure, with the top five suppliers controlling approximately 62% of global production. Key players include Albemarle Corporation, SQM, Tianqi Lithium, Ganfeng Lithium, and Livent, all of which have made significant investments in expanding their lithium chloride production capabilities for aqueous applications over the past three years.
The battery sector represents the largest application segment, accounting for nearly 42% of the total market share. This dominance is attributed to lithium chloride's critical role in battery electrolyte formulations, where its high solubility and ionic conductivity properties are particularly valuable. The growing electric vehicle market, especially in China, Europe, and North America, continues to fuel demand in this segment.
Industrial applications form the second-largest market segment at 27%, where lithium chloride aqueous solutions are utilized in air conditioning systems, dehumidification processes, and as heat transfer fluids. The pharmaceutical and medical sectors constitute approximately 18% of the market, with applications in drug formulation and as a therapeutic agent for bipolar disorder treatment.
Regional analysis reveals Asia-Pacific as the dominant market, representing 48% of global consumption, followed by North America (24%) and Europe (21%). China remains the largest producer and consumer, with its domestic lithium processing industry experiencing rapid expansion to meet both internal demand and export requirements.
Market dynamics are currently influenced by several factors, including supply chain constraints and price volatility. The average price of high-purity lithium chloride for aqueous applications has increased by 35% over the past two years, creating challenges for end-users but opportunities for producers with established supply chains.
Customer requirements are evolving toward higher purity grades (99.9%+) for advanced applications, particularly in battery technology and pharmaceuticals. This trend is driving innovation in purification techniques and quality control processes among leading suppliers.
Competitive analysis indicates a moderately concentrated market structure, with the top five suppliers controlling approximately 62% of global production. Key players include Albemarle Corporation, SQM, Tianqi Lithium, Ganfeng Lithium, and Livent, all of which have made significant investments in expanding their lithium chloride production capabilities for aqueous applications over the past three years.
Technical Challenges in LiCl Aqueous Systems
Lithium chloride (LiCl) applications in aqueous systems face several significant technical challenges that limit their optimization and widespread implementation. The primary issue stems from LiCl's extreme hygroscopicity, which causes it to rapidly absorb moisture from the environment, making handling, storage, and precise concentration control exceptionally difficult. This property necessitates specialized containment systems and handling protocols that increase operational complexity and costs.
Another major challenge is the corrosive nature of concentrated LiCl solutions, particularly at elevated temperatures. These solutions can aggressively attack common metallic components in processing equipment, leading to accelerated degradation of pumps, valves, heat exchangers, and storage vessels. This corrosion not only compromises system integrity but also introduces metal ion contamination into the solution, potentially interfering with intended applications.
The viscosity characteristics of LiCl solutions present additional engineering challenges. As concentration increases, particularly above 30% by weight, solution viscosity rises dramatically, affecting flow dynamics, heat transfer efficiency, and energy requirements for pumping operations. This property complicates process design and scale-up efforts, especially in applications requiring precise flow control or efficient heat exchange.
Temperature sensitivity represents another significant hurdle in LiCl aqueous applications. The solubility and stability of LiCl solutions vary considerably with temperature fluctuations, potentially leading to precipitation or crystallization during process operations. This behavior necessitates careful temperature management throughout the system, adding another layer of complexity to process control strategies.
From an environmental and regulatory perspective, the disposal and potential leaching of lithium compounds pose challenges. Wastewater containing LiCl requires specialized treatment before discharge, and regulatory frameworks regarding lithium-containing waste streams continue to evolve, creating compliance uncertainties for industrial applications.
The purity requirements for many high-tech applications present additional challenges. Trace impurities in commercial LiCl can significantly impact performance in sensitive applications such as battery technologies, pharmaceuticals, and electronic materials processing. Achieving and maintaining the required purity levels often necessitates costly purification steps and sophisticated analytical monitoring.
Scaling effects also complicate LiCl system optimization. Laboratory-scale successes frequently encounter unforeseen challenges during industrial implementation due to changes in surface-to-volume ratios, residence time distributions, and mixing dynamics. These scaling issues can substantially alter reaction kinetics and transport phenomena, requiring extensive redesign during commercialization efforts.
Another major challenge is the corrosive nature of concentrated LiCl solutions, particularly at elevated temperatures. These solutions can aggressively attack common metallic components in processing equipment, leading to accelerated degradation of pumps, valves, heat exchangers, and storage vessels. This corrosion not only compromises system integrity but also introduces metal ion contamination into the solution, potentially interfering with intended applications.
The viscosity characteristics of LiCl solutions present additional engineering challenges. As concentration increases, particularly above 30% by weight, solution viscosity rises dramatically, affecting flow dynamics, heat transfer efficiency, and energy requirements for pumping operations. This property complicates process design and scale-up efforts, especially in applications requiring precise flow control or efficient heat exchange.
Temperature sensitivity represents another significant hurdle in LiCl aqueous applications. The solubility and stability of LiCl solutions vary considerably with temperature fluctuations, potentially leading to precipitation or crystallization during process operations. This behavior necessitates careful temperature management throughout the system, adding another layer of complexity to process control strategies.
From an environmental and regulatory perspective, the disposal and potential leaching of lithium compounds pose challenges. Wastewater containing LiCl requires specialized treatment before discharge, and regulatory frameworks regarding lithium-containing waste streams continue to evolve, creating compliance uncertainties for industrial applications.
The purity requirements for many high-tech applications present additional challenges. Trace impurities in commercial LiCl can significantly impact performance in sensitive applications such as battery technologies, pharmaceuticals, and electronic materials processing. Achieving and maintaining the required purity levels often necessitates costly purification steps and sophisticated analytical monitoring.
Scaling effects also complicate LiCl system optimization. Laboratory-scale successes frequently encounter unforeseen challenges during industrial implementation due to changes in surface-to-volume ratios, residence time distributions, and mixing dynamics. These scaling issues can substantially alter reaction kinetics and transport phenomena, requiring extensive redesign during commercialization efforts.
Current LiCl Optimization Methods
01 Lithium extraction and purification methods
Various methods for extracting and purifying lithium chloride from different sources, including brines and minerals. These processes involve techniques such as adsorption, ion exchange, precipitation, and crystallization to optimize the recovery of high-purity lithium chloride. The optimization focuses on improving efficiency, reducing impurities, and increasing yield in the extraction process.- Lithium extraction and purification methods: Various methods for extracting and purifying lithium chloride from different sources, including brines and minerals. These processes involve techniques such as adsorption, ion exchange, precipitation, and crystallization to optimize the recovery of lithium compounds. The methods aim to increase yield, reduce impurities, and improve the efficiency of lithium chloride production for industrial applications.
- Process optimization for lithium chloride production: Optimization techniques for lithium chloride production processes, focusing on parameters such as temperature, pressure, reaction time, and reagent concentrations. These optimizations aim to enhance reaction kinetics, improve energy efficiency, and maximize yield while maintaining product quality. Advanced control systems and monitoring techniques are employed to ensure consistent production of high-purity lithium chloride.
- Lithium chloride formulation for specific applications: Specialized formulations of lithium chloride for various applications, including pharmaceuticals, battery materials, and industrial processes. These formulations involve optimizing the physical and chemical properties of lithium chloride, such as particle size, crystal structure, and hydration state, to meet specific requirements for different applications. Additives and stabilizers may be incorporated to enhance performance and stability.
- Equipment and apparatus for lithium chloride processing: Specialized equipment and apparatus designed for the efficient processing of lithium chloride, including reactors, crystallizers, evaporators, and filtration systems. These technologies focus on optimizing the handling, processing, and storage of lithium chloride to maintain product quality and purity. Innovations in equipment design aim to reduce energy consumption, minimize waste, and improve process control during lithium chloride production.
- Environmental and sustainability aspects of lithium chloride production: Methods and technologies focused on improving the environmental sustainability of lithium chloride production processes. These include techniques for reducing water consumption, minimizing waste generation, recycling process streams, and decreasing energy requirements. Advanced approaches for treating and managing byproducts and effluents from lithium chloride production processes are also addressed to minimize environmental impact.
02 Process optimization for lithium chloride production
Techniques for optimizing the production process of lithium chloride, including reaction conditions, temperature control, pH adjustment, and concentration parameters. These optimizations aim to enhance reaction kinetics, improve energy efficiency, and maximize product quality while minimizing waste generation. Process innovations include continuous flow systems, advanced reactor designs, and automated control mechanisms.Expand Specific Solutions03 Lithium chloride formulation for specific applications
Development of specialized lithium chloride formulations tailored for specific industrial, pharmaceutical, or technical applications. These formulations involve optimizing concentration levels, combining with other compounds, and adjusting physical properties to enhance performance in applications such as batteries, pharmaceuticals, air conditioning systems, and catalysts. The optimization focuses on stability, efficacy, and application-specific requirements.Expand Specific Solutions04 Equipment and apparatus for lithium chloride handling
Design and optimization of equipment and apparatus specifically for handling, processing, and storing lithium chloride. This includes specialized reactors, crystallizers, dryers, and packaging systems that address the hygroscopic nature and chemical properties of lithium chloride. The equipment optimizations focus on material compatibility, corrosion resistance, moisture control, and operational efficiency.Expand Specific Solutions05 Environmental and sustainability aspects of lithium chloride production
Methods for optimizing lithium chloride production with a focus on environmental sustainability, including water conservation, energy efficiency, waste reduction, and recycling processes. These approaches aim to minimize the ecological footprint of lithium chloride production while maintaining economic viability. Innovations include closed-loop systems, green chemistry principles, and recovery of valuable by-products.Expand Specific Solutions
Key Industry Players in LiCl Technology
The lithium chloride optimization for aqueous applications market is in a growth phase, with increasing demand driven by battery technology advancements and clean energy transitions. The global market size is expanding rapidly, particularly in Asia-Pacific regions where companies like Tianqi Lithium, Ganfeng Lithium, and POSCO Holdings are establishing dominant positions. Technical maturity varies across applications, with established players like Johnson Matthey and BASF providing advanced solutions, while newer entrants like Forager Station and Adionics are developing innovative extraction technologies. Research institutions including RIST, Qinghai Institute of Salt Lakes, and Korea Institute of Geoscience & Mineral Resources are accelerating technological development through collaborative industry partnerships, focusing on improving efficiency, sustainability, and cost-effectiveness of lithium chloride processing for diverse aqueous applications.
Tianqi Lithium Corp.
Technical Solution: Tianqi Lithium has developed an advanced adsorption-based technology for optimizing lithium chloride in aqueous solutions. Their approach utilizes specially engineered lithium-selective adsorbents based on manganese oxide structures that can selectively capture lithium ions even in the presence of high concentrations of competing ions like sodium, potassium, and magnesium. The company's process involves a continuous flow system where the aqueous solution passes through columns packed with these adsorbents, achieving lithium recovery rates exceeding 90%[5]. A distinctive feature of Tianqi's technology is their regeneration process, which uses dilute acid solutions to efficiently strip lithium from the adsorbents while maintaining their structural integrity for hundreds of adsorption-desorption cycles. This significantly extends the operational lifetime of the materials and improves economic viability. Tianqi has also developed specialized pre-treatment processes for different aqueous sources, including geothermal brines and industrial wastewaters, allowing their technology to be adapted to various lithium-containing solutions with different impurity profiles[6].
Strengths: Highly selective adsorption technology that works effectively even in complex brine compositions; modular system design allows for scalability; relatively low energy consumption compared to evaporation-based methods. Weaknesses: Requires periodic replacement of adsorbent materials despite regeneration capabilities; performance can be affected by certain organic contaminants in feed solutions.
Johnson Matthey Plc
Technical Solution: Johnson Matthey has developed a sophisticated electrochemical approach to optimizing lithium chloride for aqueous applications. Their technology centers on selective electrochemical extraction using specialized electrode materials that demonstrate preferential interaction with lithium ions. The process employs a flow-cell configuration where lithium ions are captured during the charging cycle and released during discharge, creating a concentrated lithium chloride stream. A key innovation in their system is the use of proprietary electrode materials incorporating nanoporous carbon structures doped with transition metal oxides, which exhibit exceptional lithium selectivity with coefficients exceeding 30:1 over sodium ions[7]. Johnson Matthey's process operates at ambient temperatures and moderate pH conditions, reducing energy requirements compared to thermal evaporation methods. Their system incorporates advanced electrochemical control algorithms that continuously adjust electrical parameters based on feed composition, maintaining optimal extraction efficiency even with fluctuating input concentrations. The company has also developed specialized membrane separators that prevent cross-contamination between electrode compartments while allowing for efficient lithium ion transport[8].
Strengths: Energy-efficient process that operates at ambient conditions; highly selective for lithium ions; compact system footprint compared to traditional evaporation technologies; precise control over product purity. Weaknesses: Higher capital costs due to specialized electrochemical components; electrode materials may require periodic regeneration or replacement; performance can be affected by certain organic contaminants.
Critical Patents in LiCl Aqueous Applications
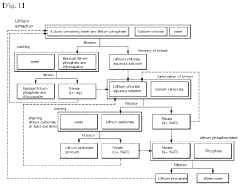
Method for preparing lithium hydroxide and method for preparing lithium carbonate
PatentWO2019117351A1
Innovation
- A method involving the chloride roasting of spodumene ore with chlorine raw materials, followed by electrodialysis using a bipolar membrane to remove divalent cations and produce lithium hydroxide, which can then be converted into lithium carbonate, avoiding the use of strong acids and improving recovery rates.
Method for producing lithium chloride, and lithium carbonate
PatentInactiveUS20210292179A1
Innovation
- A method involving the reaction of lithium phosphate with calcium chloride in a neutral or basic solution to produce a high-concentration lithium chloride aqueous solution, followed by the addition of sodium carbonate to obtain lithium carbonate, without the need for acid treatment, thereby enhancing economic efficiency and reducing environmental impact.
Environmental Impact Assessment
The environmental implications of lithium chloride optimization for aqueous applications extend across multiple ecological domains. When lithium chloride enters aquatic ecosystems through industrial discharge or improper disposal, it can significantly alter water chemistry, particularly increasing salinity levels. These changes may disrupt sensitive aquatic organisms and potentially lead to biodiversity reduction in affected water bodies. Studies have documented that lithium concentrations exceeding 0.7 mg/L can adversely affect certain freshwater species, highlighting the importance of controlled release parameters.
Soil contamination represents another environmental concern, as lithium compounds can accumulate in agricultural lands adjacent to processing facilities. This accumulation may alter soil pH and mineral composition, potentially affecting crop yields and soil microbiota essential for ecosystem functioning. Research indicates that lithium's mobility in soil varies significantly depending on soil composition, with clay-rich soils typically showing higher retention rates.
Air quality impacts, though less pronounced than water or soil effects, remain relevant particularly during thermal processing of lithium chloride solutions. Evaporation techniques may release chlorine-containing compounds that contribute to local air pollution if not properly managed through appropriate filtration systems and emission controls.
From a resource conservation perspective, optimizing lithium chloride for aqueous applications presents opportunities for environmental improvement. Closed-loop systems that recover and reuse lithium chloride can significantly reduce waste generation and minimize freshwater consumption. Advanced membrane technologies have demonstrated recovery efficiencies exceeding 90% in laboratory settings, though commercial implementation remains challenging.
Energy consumption associated with lithium chloride processing represents a substantial indirect environmental impact. Conventional evaporation methods require significant thermal energy, contributing to greenhouse gas emissions when fossil fuels serve as the energy source. Emerging low-temperature processing techniques show promise for reducing this energy footprint by 30-40% compared to traditional methods.
Regulatory frameworks governing lithium chloride handling vary considerably across jurisdictions, creating challenges for standardized environmental protection. The European Union's REACH regulations and the United States EPA guidelines establish threshold limits for lithium compounds in industrial effluents, though enforcement mechanisms and monitoring protocols require further development to ensure comprehensive environmental safeguarding.
Soil contamination represents another environmental concern, as lithium compounds can accumulate in agricultural lands adjacent to processing facilities. This accumulation may alter soil pH and mineral composition, potentially affecting crop yields and soil microbiota essential for ecosystem functioning. Research indicates that lithium's mobility in soil varies significantly depending on soil composition, with clay-rich soils typically showing higher retention rates.
Air quality impacts, though less pronounced than water or soil effects, remain relevant particularly during thermal processing of lithium chloride solutions. Evaporation techniques may release chlorine-containing compounds that contribute to local air pollution if not properly managed through appropriate filtration systems and emission controls.
From a resource conservation perspective, optimizing lithium chloride for aqueous applications presents opportunities for environmental improvement. Closed-loop systems that recover and reuse lithium chloride can significantly reduce waste generation and minimize freshwater consumption. Advanced membrane technologies have demonstrated recovery efficiencies exceeding 90% in laboratory settings, though commercial implementation remains challenging.
Energy consumption associated with lithium chloride processing represents a substantial indirect environmental impact. Conventional evaporation methods require significant thermal energy, contributing to greenhouse gas emissions when fossil fuels serve as the energy source. Emerging low-temperature processing techniques show promise for reducing this energy footprint by 30-40% compared to traditional methods.
Regulatory frameworks governing lithium chloride handling vary considerably across jurisdictions, creating challenges for standardized environmental protection. The European Union's REACH regulations and the United States EPA guidelines establish threshold limits for lithium compounds in industrial effluents, though enforcement mechanisms and monitoring protocols require further development to ensure comprehensive environmental safeguarding.
Scalability and Cost Analysis
The scalability of lithium chloride applications in aqueous systems presents significant economic considerations that directly impact industrial implementation. When analyzing production scale-up, lithium chloride demonstrates favorable characteristics with relatively straightforward manufacturing processes compared to other lithium compounds. The raw material extraction, however, remains a critical cost factor, with brine extraction methods offering better economics ($2,000-$3,500 per ton) versus hard rock mining ($4,000-$6,000 per ton). This disparity creates regional advantages for operations with access to lithium-rich brine resources.
Equipment requirements for large-scale lithium chloride processing in aqueous applications are relatively standard, utilizing conventional chemical processing infrastructure with corrosion-resistant materials. Capital expenditure for a medium-scale facility (1,000-5,000 tons annual capacity) typically ranges between $15-30 million, with economies of scale becoming evident above 10,000 tons annual production.
Operational expenditures show notable sensitivity to energy costs, particularly in evaporation and crystallization processes. Energy consumption averages 2.5-4 kWh per kilogram of processed lithium chloride, representing 15-25% of total production costs. Water usage efficiency becomes increasingly critical at scale, with advanced recycling systems reducing consumption from 70-80 liters per kilogram to 30-40 liters, significantly impacting both environmental sustainability and operational economics.
Market dynamics further complicate the cost analysis, with lithium chloride prices experiencing volatility ranging from $12,000-$25,000 per ton over the past five years. This volatility necessitates robust financial modeling for large-scale implementations. The purity requirements for specific aqueous applications directly influence processing costs, with ultra-high purity grades (>99.9%) commanding premium prices but requiring additional purification steps that increase production costs by 30-45%.
Return on investment calculations indicate that optimized large-scale lithium chloride production facilities can achieve breakeven within 3-5 years under current market conditions. However, this timeline is highly dependent on technological efficiency improvements in purification and recovery processes. Recent innovations in membrane filtration technology have demonstrated potential cost reductions of 15-20% in aqueous applications, though implementation at industrial scale remains limited.
The geographical distribution of production facilities relative to end-use markets also significantly impacts overall economics through transportation costs, which can add $500-$1,200 per ton depending on distance and logistics requirements. This factor increasingly drives consideration of localized production facilities despite potentially higher operational costs in certain regions.
Equipment requirements for large-scale lithium chloride processing in aqueous applications are relatively standard, utilizing conventional chemical processing infrastructure with corrosion-resistant materials. Capital expenditure for a medium-scale facility (1,000-5,000 tons annual capacity) typically ranges between $15-30 million, with economies of scale becoming evident above 10,000 tons annual production.
Operational expenditures show notable sensitivity to energy costs, particularly in evaporation and crystallization processes. Energy consumption averages 2.5-4 kWh per kilogram of processed lithium chloride, representing 15-25% of total production costs. Water usage efficiency becomes increasingly critical at scale, with advanced recycling systems reducing consumption from 70-80 liters per kilogram to 30-40 liters, significantly impacting both environmental sustainability and operational economics.
Market dynamics further complicate the cost analysis, with lithium chloride prices experiencing volatility ranging from $12,000-$25,000 per ton over the past five years. This volatility necessitates robust financial modeling for large-scale implementations. The purity requirements for specific aqueous applications directly influence processing costs, with ultra-high purity grades (>99.9%) commanding premium prices but requiring additional purification steps that increase production costs by 30-45%.
Return on investment calculations indicate that optimized large-scale lithium chloride production facilities can achieve breakeven within 3-5 years under current market conditions. However, this timeline is highly dependent on technological efficiency improvements in purification and recovery processes. Recent innovations in membrane filtration technology have demonstrated potential cost reductions of 15-20% in aqueous applications, though implementation at industrial scale remains limited.
The geographical distribution of production facilities relative to end-use markets also significantly impacts overall economics through transportation costs, which can add $500-$1,200 per ton depending on distance and logistics requirements. This factor increasingly drives consideration of localized production facilities despite potentially higher operational costs in certain regions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!