Quantifying Lithium Chloride Crystal Habit Modification
AUG 28, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
LiCl Crystal Habit Modification Background & Objectives
Lithium chloride (LiCl) crystal habit modification represents a critical area of research in materials science and chemical engineering, with applications spanning pharmaceutical manufacturing, battery technology, and industrial chemical processing. The evolution of crystal habit modification techniques for LiCl has progressed significantly over the past several decades, driven by the increasing demand for precise control over crystal morphology to enhance performance characteristics in various applications.
Historically, LiCl crystallization studies began in the mid-20th century with rudimentary approaches focused primarily on temperature and concentration control. By the 1970s, researchers had begun exploring the use of additives to influence crystal growth patterns, though quantification methods remained largely qualitative and observational. The 1990s marked a significant turning point with the introduction of computational modeling techniques that enabled more systematic approaches to crystal habit prediction and modification.
Recent technological advancements have shifted focus toward nanoscale control of LiCl crystallization, particularly important for lithium battery applications where crystal morphology directly impacts electrochemical performance. The integration of in-situ monitoring technologies has further revolutionized the field, allowing real-time observation and quantification of crystal habit changes during formation processes.
The primary technical objective of current research in LiCl crystal habit modification is to develop robust, reproducible methodologies for quantifying the effects of various parameters on crystal morphology. This includes establishing standardized metrics for crystal habit characterization, developing predictive models that accurately correlate process conditions with resulting crystal properties, and creating automated systems for real-time crystal habit analysis and control.
Additionally, researchers aim to elucidate the fundamental molecular mechanisms governing LiCl crystal growth and modification, particularly the interaction between crystal surfaces and habit-modifying additives. Understanding these mechanisms at the molecular level would enable more precise engineering of crystal properties for specific applications.
From an industrial perspective, key objectives include scaling laboratory findings to commercial production environments while maintaining precise control over crystal habits, reducing energy consumption in crystallization processes through optimized habit modification, and developing environmentally sustainable approaches to LiCl processing that minimize waste and resource utilization.
The convergence of advanced imaging technologies, computational modeling, and machine learning approaches presents unprecedented opportunities for quantifying and controlling LiCl crystal habits with nanometer-scale precision. This technological evolution is expected to continue accelerating, driven by the growing importance of lithium compounds in energy storage, pharmaceutical, and advanced materials applications.
Historically, LiCl crystallization studies began in the mid-20th century with rudimentary approaches focused primarily on temperature and concentration control. By the 1970s, researchers had begun exploring the use of additives to influence crystal growth patterns, though quantification methods remained largely qualitative and observational. The 1990s marked a significant turning point with the introduction of computational modeling techniques that enabled more systematic approaches to crystal habit prediction and modification.
Recent technological advancements have shifted focus toward nanoscale control of LiCl crystallization, particularly important for lithium battery applications where crystal morphology directly impacts electrochemical performance. The integration of in-situ monitoring technologies has further revolutionized the field, allowing real-time observation and quantification of crystal habit changes during formation processes.
The primary technical objective of current research in LiCl crystal habit modification is to develop robust, reproducible methodologies for quantifying the effects of various parameters on crystal morphology. This includes establishing standardized metrics for crystal habit characterization, developing predictive models that accurately correlate process conditions with resulting crystal properties, and creating automated systems for real-time crystal habit analysis and control.
Additionally, researchers aim to elucidate the fundamental molecular mechanisms governing LiCl crystal growth and modification, particularly the interaction between crystal surfaces and habit-modifying additives. Understanding these mechanisms at the molecular level would enable more precise engineering of crystal properties for specific applications.
From an industrial perspective, key objectives include scaling laboratory findings to commercial production environments while maintaining precise control over crystal habits, reducing energy consumption in crystallization processes through optimized habit modification, and developing environmentally sustainable approaches to LiCl processing that minimize waste and resource utilization.
The convergence of advanced imaging technologies, computational modeling, and machine learning approaches presents unprecedented opportunities for quantifying and controlling LiCl crystal habits with nanometer-scale precision. This technological evolution is expected to continue accelerating, driven by the growing importance of lithium compounds in energy storage, pharmaceutical, and advanced materials applications.
Market Applications & Demand Analysis
The market for lithium chloride crystal habit modification technologies is experiencing significant growth, driven primarily by the expanding lithium-ion battery sector. As electric vehicles and renewable energy storage solutions gain mainstream adoption, the demand for high-quality lithium compounds with specific crystalline properties has intensified. The global lithium compounds market, valued at approximately 43 billion USD in 2022, is projected to grow at a compound annual growth rate of 12.3% through 2030, with crystal habit modification technologies representing an increasingly important segment.
Battery manufacturers require precise control over lithium chloride crystal morphology to optimize electrode performance, enhance energy density, and improve charging efficiency. Quantitative methods for crystal habit modification enable manufacturers to achieve consistent product quality while reducing production costs through more efficient material utilization. This has created a specialized market segment focused on advanced crystallization control technologies, estimated to be worth 3.2 billion USD globally.
Beyond energy storage applications, pharmaceutical companies are increasingly adopting lithium chloride crystal habit modification techniques for drug formulation. The controlled crystallization of active pharmaceutical ingredients containing lithium compounds can significantly impact bioavailability, stability, and manufacturing efficiency. This pharmaceutical application segment is growing at approximately 9.7% annually, driven by the need for more precise drug delivery systems and improved therapeutic outcomes.
The water treatment industry represents another significant market for lithium chloride crystal modification technologies. Specialized crystalline forms of lithium chloride demonstrate enhanced performance in desalination processes and water purification systems. As water scarcity becomes a global concern, the demand for advanced materials with optimized crystal habits for efficient water treatment is projected to grow substantially, with market analysts forecasting a 14.5% annual growth rate in this segment.
Regional analysis indicates that Asia-Pacific dominates the market demand, accounting for over 45% of global consumption, primarily due to the concentration of battery manufacturing facilities in China, South Korea, and Japan. North America and Europe follow with growing demand driven by pharmaceutical applications and renewable energy initiatives. Emerging economies in Latin America, particularly those with lithium mining operations, are developing downstream processing capabilities that incorporate advanced crystal habit modification technologies to capture greater value from their natural resources.
Battery manufacturers require precise control over lithium chloride crystal morphology to optimize electrode performance, enhance energy density, and improve charging efficiency. Quantitative methods for crystal habit modification enable manufacturers to achieve consistent product quality while reducing production costs through more efficient material utilization. This has created a specialized market segment focused on advanced crystallization control technologies, estimated to be worth 3.2 billion USD globally.
Beyond energy storage applications, pharmaceutical companies are increasingly adopting lithium chloride crystal habit modification techniques for drug formulation. The controlled crystallization of active pharmaceutical ingredients containing lithium compounds can significantly impact bioavailability, stability, and manufacturing efficiency. This pharmaceutical application segment is growing at approximately 9.7% annually, driven by the need for more precise drug delivery systems and improved therapeutic outcomes.
The water treatment industry represents another significant market for lithium chloride crystal modification technologies. Specialized crystalline forms of lithium chloride demonstrate enhanced performance in desalination processes and water purification systems. As water scarcity becomes a global concern, the demand for advanced materials with optimized crystal habits for efficient water treatment is projected to grow substantially, with market analysts forecasting a 14.5% annual growth rate in this segment.
Regional analysis indicates that Asia-Pacific dominates the market demand, accounting for over 45% of global consumption, primarily due to the concentration of battery manufacturing facilities in China, South Korea, and Japan. North America and Europe follow with growing demand driven by pharmaceutical applications and renewable energy initiatives. Emerging economies in Latin America, particularly those with lithium mining operations, are developing downstream processing capabilities that incorporate advanced crystal habit modification technologies to capture greater value from their natural resources.
Current Quantification Methods & Technical Challenges
The quantification of lithium chloride crystal habit modification currently employs several methodologies, each with distinct advantages and limitations. Optical microscopy remains the most traditional approach, allowing researchers to visually observe crystal morphology changes and measure basic dimensional parameters. However, this method suffers from subjectivity in interpretation and limited precision when analyzing complex three-dimensional structures.
X-ray diffraction (XRD) techniques have emerged as more sophisticated quantification tools, providing detailed information about crystal lattice parameters and orientation. The Face Index Analysis derived from XRD data enables precise determination of which crystal faces are preferentially developed or inhibited during habit modification. Nevertheless, XRD requires specialized equipment and expertise for proper data interpretation.
Scanning Electron Microscopy (SEM) offers high-resolution imaging of crystal surfaces and morphologies, revealing nanoscale features that optical microscopy cannot detect. When coupled with Energy Dispersive X-ray Spectroscopy (EDS), it provides elemental composition data at specific crystal locations, helping correlate morphological changes with chemical modifications. The main drawback is the sample preparation process, which may introduce artifacts.
Particle size analyzers using laser diffraction provide statistical distribution data on crystal dimensions but often fail to capture the nuanced aspects of habit modification beyond size parameters. This limitation becomes particularly problematic when studying lithium chloride crystals, whose functionality depends on specific facet development rather than mere size alterations.
Image analysis software has recently gained prominence, offering automated quantification of crystal parameters from microscopy images. These systems can process large datasets and extract metrics such as aspect ratio, circularity, and surface area. However, algorithm limitations often struggle with crystal aggregates and overlapping particles, leading to data misinterpretation.
The technical challenges in quantifying lithium chloride crystal habit modification are multifaceted. Sample preparation inconsistencies introduce variability in measurements, while the dynamic nature of crystallization processes makes real-time quantification exceptionally difficult. Environmental factors such as humidity and temperature fluctuations can significantly impact crystal habits, necessitating strictly controlled experimental conditions.
Standardization represents another major challenge, as different research groups employ varied methodologies and metrics, complicating cross-study comparisons. The lack of universally accepted parameters for quantifying habit modification has led to fragmented research approaches and inconsistent reporting standards in the literature.
Advanced techniques like Atomic Force Microscopy (AFM) and Raman spectroscopy show promise for more comprehensive quantification but remain underutilized due to accessibility limitations and complex data interpretation requirements. Integrating multiple complementary techniques appears necessary to overcome the limitations of individual methods and achieve holistic quantification of lithium chloride crystal habit modification.
X-ray diffraction (XRD) techniques have emerged as more sophisticated quantification tools, providing detailed information about crystal lattice parameters and orientation. The Face Index Analysis derived from XRD data enables precise determination of which crystal faces are preferentially developed or inhibited during habit modification. Nevertheless, XRD requires specialized equipment and expertise for proper data interpretation.
Scanning Electron Microscopy (SEM) offers high-resolution imaging of crystal surfaces and morphologies, revealing nanoscale features that optical microscopy cannot detect. When coupled with Energy Dispersive X-ray Spectroscopy (EDS), it provides elemental composition data at specific crystal locations, helping correlate morphological changes with chemical modifications. The main drawback is the sample preparation process, which may introduce artifacts.
Particle size analyzers using laser diffraction provide statistical distribution data on crystal dimensions but often fail to capture the nuanced aspects of habit modification beyond size parameters. This limitation becomes particularly problematic when studying lithium chloride crystals, whose functionality depends on specific facet development rather than mere size alterations.
Image analysis software has recently gained prominence, offering automated quantification of crystal parameters from microscopy images. These systems can process large datasets and extract metrics such as aspect ratio, circularity, and surface area. However, algorithm limitations often struggle with crystal aggregates and overlapping particles, leading to data misinterpretation.
The technical challenges in quantifying lithium chloride crystal habit modification are multifaceted. Sample preparation inconsistencies introduce variability in measurements, while the dynamic nature of crystallization processes makes real-time quantification exceptionally difficult. Environmental factors such as humidity and temperature fluctuations can significantly impact crystal habits, necessitating strictly controlled experimental conditions.
Standardization represents another major challenge, as different research groups employ varied methodologies and metrics, complicating cross-study comparisons. The lack of universally accepted parameters for quantifying habit modification has led to fragmented research approaches and inconsistent reporting standards in the literature.
Advanced techniques like Atomic Force Microscopy (AFM) and Raman spectroscopy show promise for more comprehensive quantification but remain underutilized due to accessibility limitations and complex data interpretation requirements. Integrating multiple complementary techniques appears necessary to overcome the limitations of individual methods and achieve holistic quantification of lithium chloride crystal habit modification.
Established Quantification Methodologies
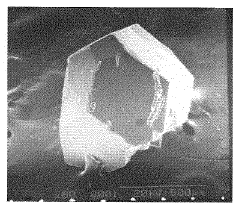
01 Cubic crystal habit of lithium chloride
Lithium chloride typically crystallizes in a cubic crystal habit under standard conditions. The cubic structure is characterized by its regular, six-faced geometric form with equal dimensions along all axes. This crystal habit affects various properties including solubility, stability, and processing characteristics. The cubic crystal structure of lithium chloride makes it useful in various applications including moisture absorption, heat storage, and as a precursor in lithium compound synthesis.- Cubic crystal habit of lithium chloride: Lithium chloride typically crystallizes in a cubic crystal habit under standard conditions. This cubic structure is characterized by its regular, symmetrical arrangement of atoms in a face-centered cubic lattice. The formation of these cubic crystals can be controlled through specific crystallization parameters such as temperature, concentration, and cooling rate. The cubic habit provides certain physical properties that make lithium chloride useful in various applications.
- Modification of lithium chloride crystal habit: The crystal habit of lithium chloride can be modified through various techniques including the addition of specific additives, controlling crystallization conditions, or applying mechanical treatments. These modifications can alter the crystal morphology from cubic to other forms such as needle-like, dendritic, or plate-like structures. By controlling the crystal habit, properties such as flowability, dissolution rate, and stability can be enhanced for specific applications in pharmaceuticals, battery technology, and other industrial processes.
- Lithium chloride crystal growth in composite materials: Lithium chloride crystals can be grown within composite materials to create functional structures with unique properties. The crystal habit within these composites can be controlled to achieve specific orientations and distributions. This approach is particularly useful in developing materials for optical applications, energy storage systems, and specialized coatings. The interaction between the host material and the growing lithium chloride crystals influences the final crystal habit and the overall performance of the composite.
- Influence of impurities on lithium chloride crystal habit: Impurities present during the crystallization process can significantly affect the crystal habit of lithium chloride. Certain impurities can selectively adsorb onto specific crystal faces, inhibiting growth in those directions and promoting growth in others, resulting in modified crystal morphologies. Understanding and controlling these impurity effects is crucial for producing lithium chloride crystals with desired habits for applications in pharmaceuticals, catalysis, and electronic materials. The type, concentration, and introduction timing of impurities can be manipulated to achieve specific crystal habits.
- Applications of controlled lithium chloride crystal habit: The controlled crystal habit of lithium chloride has important applications across various industries. In pharmaceutical formulations, specific crystal habits can improve drug delivery and stability. In battery technology, optimized crystal morphologies enhance ion transport and electrode performance. For humidity control applications, certain crystal habits provide better moisture absorption properties. Additionally, specialized crystal habits are utilized in optical materials, catalysts, and chemical synthesis processes where surface area and crystal orientation significantly impact performance.
02 Modification of lithium chloride crystal habit
The crystal habit of lithium chloride can be modified through various processing techniques and additives. Factors such as crystallization temperature, solvent composition, pH, and the presence of specific additives can influence the resulting crystal morphology. Modified crystal habits may exhibit different shapes such as needle-like, dendritic, or plate-like structures. These modifications can be employed to enhance specific properties for targeted applications, including improved flow characteristics, controlled dissolution rates, or better incorporation into composite materials.Expand Specific Solutions03 Crystal growth control for lithium chloride production
Controlling the growth of lithium chloride crystals is essential for producing materials with specific characteristics. Various methods are employed to regulate crystal growth, including seeding techniques, temperature gradient control, and agitation parameters. These approaches allow for the development of crystals with uniform size distribution, reduced agglomeration, and consistent morphology. Advanced crystal growth control techniques enable the production of high-purity lithium chloride with tailored physical properties suitable for specialized applications in batteries, pharmaceuticals, and other industries.Expand Specific Solutions04 Lithium chloride crystal habit in composite materials
The incorporation of lithium chloride crystals into composite materials is influenced by the crystal habit. Different crystal habits affect how lithium chloride integrates with matrix materials, impacting the overall performance of the composite. The interface between lithium chloride crystals and host materials can be engineered by selecting appropriate crystal habits to enhance properties such as ionic conductivity, mechanical strength, or thermal stability. This approach is particularly relevant in the development of advanced materials for energy storage, catalysis, and specialized industrial applications.Expand Specific Solutions05 Characterization techniques for lithium chloride crystal habit
Various analytical methods are employed to characterize the crystal habit of lithium chloride, including X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), and optical microscopy. These techniques provide detailed information about crystal morphology, size distribution, surface features, and internal structure. Advanced characterization approaches may also include thermal analysis, spectroscopic methods, and computational modeling to understand the relationship between processing conditions and resulting crystal habits. Comprehensive characterization is essential for quality control and the development of lithium chloride materials with specific performance attributes.Expand Specific Solutions
Key Industry Players & Research Institutions
The lithium chloride crystal habit modification market is in a growth phase, characterized by increasing research and development activities across pharmaceutical, semiconductor, and energy sectors. The market is expanding due to applications in battery technology, pharmaceuticals, and electronic displays, with an estimated global value exceeding $500 million. Technologically, the field shows moderate maturity with established players like Novartis AG and Semiconductor Energy Laboratory leading pharmaceutical applications, while companies such as LG Display, Samsung Display, and FUJIFILM focus on display technology implementations. Energy sector innovation is driven by Hunan Shanshan Energy Technology and Beijing Easpring Material Technology, particularly in lithium-ion battery development. Academic institutions including Shandong University and University of Florida contribute significant research advancements, creating a competitive ecosystem balancing commercial applications with fundamental research.
Novartis AG
Technical Solution: Novartis AG has developed advanced crystallization techniques for lithium chloride crystal habit modification specifically for pharmaceutical applications. Their approach combines controlled nucleation environments with precise temperature gradient manipulation to achieve desired crystal morphologies. The company employs a proprietary solvent system that incorporates specific additives functioning as crystal habit modifiers, allowing for tailored crystal growth along preferred crystallographic planes. Quantification methods include real-time monitoring using in-situ Raman spectroscopy coupled with image analysis algorithms that track crystal habit evolution throughout the crystallization process. This enables precise measurement of aspect ratios, crystal size distributions, and surface area characteristics. Novartis has also implemented machine learning models that correlate processing parameters with resulting crystal habits, creating predictive tools for pharmaceutical formulation development that optimize bioavailability and stability profiles of active pharmaceutical ingredients containing lithium compounds.
Strengths: Highly precise control over crystal morphology with pharmaceutical-grade quality standards; integrated analytical quantification methods provide comprehensive data for regulatory documentation. Weaknesses: System optimized primarily for pharmaceutical applications may have limited transferability to other industrial sectors; requires sophisticated equipment and expertise for implementation.
Shandong University
Technical Solution: Shandong University has developed a systematic approach to quantifying lithium chloride crystal habit modification through a combination of experimental and computational methods. Their technique employs advanced microscopy coupled with image analysis algorithms to precisely characterize crystal morphology changes. The university's research team has created a standardized protocol that utilizes Fourier Transform analysis to quantify symmetry changes in crystal habits, allowing for numerical comparison between different modification conditions. Their methodology incorporates in-situ monitoring of crystallization processes using specialized flow cells with optical access, enabling real-time quantification of habit evolution. The university has also pioneered the use of molecular dynamics simulations calibrated with experimental data to predict crystal habit modifications under various conditions, creating a powerful predictive tool. Additionally, they've developed a comprehensive database correlating specific additives and process parameters with resulting crystal habits, establishing quantitative structure-property relationships that guide rational design of crystal modification strategies for lithium chloride and related compounds.
Strengths: Strong integration of computational and experimental approaches provides both fundamental understanding and practical applications; systematic database development enables knowledge transfer across applications. Weaknesses: Academic focus may limit industrial implementation readiness; requires specialized expertise in both crystallography and computational modeling.
Critical Patents & Research in LiCl Crystal Modification
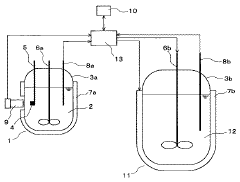
Crystallization method and crystallization apparatus
PatentInactiveJP2007044639A
Innovation
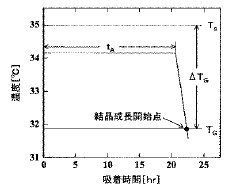
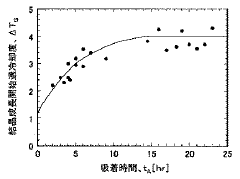
- A crystallization method involving the adsorption of a crystal habit modifier to a seed crystal, with pre-determined adsorption equilibrium time and crystal growth initiation temperature, followed by controlled cooling to achieve desired crystal shapes.
Process for production of glycine enriched sodium chloride crystals with improved flow
PatentWO2005066075A1
Innovation
- A cyclic process involving the addition of glycine to saturated brine, evaporation, washing with saturated brine to remove excess glycine, and solar evaporation to produce rhombic dodecahedron shaped NaCl crystals with 0.5-1.0% glycine content, maintaining the crystal morphology and improving flow characteristics.
Regulatory Framework for Lithium Compounds
The regulatory landscape governing lithium compounds is complex and multifaceted, reflecting the strategic importance of lithium in various industries, particularly in battery technology and pharmaceuticals. For lithium chloride crystal habit modification processes, compliance with international and national regulatory frameworks is essential for commercial viability and market access.
At the international level, organizations such as the International Conference on Harmonisation (ICH) provide guidelines on pharmaceutical ingredient quality, which directly impact lithium chloride crystal production when intended for medicinal applications. The Q3D Elemental Impurities guideline is particularly relevant, establishing permissible limits for metal impurities in pharmaceutical substances.
National regulatory bodies impose varying requirements for lithium compounds. The U.S. Food and Drug Administration (FDA) classifies lithium chloride under different regulatory categories depending on its intended use, with pharmaceutical applications facing stringent Good Manufacturing Practice (GMP) requirements. Similarly, the European Medicines Agency (EMA) enforces the European Pharmacopoeia standards, which include specific monographs for lithium salts.
Environmental regulations significantly impact lithium chloride production processes. The U.S. Environmental Protection Agency (EPA) regulates waste discharge through the Clean Water Act, while the European Union's REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation requires comprehensive safety data for lithium compounds produced or imported in quantities exceeding one ton annually.
Occupational safety frameworks also apply to lithium chloride handling. The Occupational Safety and Health Administration (OSHA) in the United States sets exposure limits for lithium compounds, requiring appropriate engineering controls and personal protective equipment. Comparable standards exist internationally through organizations like the International Labour Organization (ILO).
For crystal habit modification techniques specifically, regulatory considerations extend to the additives and processing aids employed. These substances must meet food-grade or pharmaceutical-grade standards when the resulting lithium chloride is intended for human consumption or medical applications. Novel modification agents may require separate regulatory approval before implementation in commercial processes.
Transportation and storage of lithium compounds are subject to hazardous materials regulations, including the International Maritime Dangerous Goods (IMDG) Code and regional equivalents. These regulations mandate specific packaging, labeling, and handling procedures to ensure safety throughout the supply chain.
Emerging regulatory trends indicate increasing scrutiny of the environmental footprint of lithium production, with several jurisdictions developing sustainability criteria that may impact crystal habit modification processes. Companies engaged in lithium chloride production must maintain regulatory intelligence capabilities to navigate this evolving landscape effectively.
At the international level, organizations such as the International Conference on Harmonisation (ICH) provide guidelines on pharmaceutical ingredient quality, which directly impact lithium chloride crystal production when intended for medicinal applications. The Q3D Elemental Impurities guideline is particularly relevant, establishing permissible limits for metal impurities in pharmaceutical substances.
National regulatory bodies impose varying requirements for lithium compounds. The U.S. Food and Drug Administration (FDA) classifies lithium chloride under different regulatory categories depending on its intended use, with pharmaceutical applications facing stringent Good Manufacturing Practice (GMP) requirements. Similarly, the European Medicines Agency (EMA) enforces the European Pharmacopoeia standards, which include specific monographs for lithium salts.
Environmental regulations significantly impact lithium chloride production processes. The U.S. Environmental Protection Agency (EPA) regulates waste discharge through the Clean Water Act, while the European Union's REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation requires comprehensive safety data for lithium compounds produced or imported in quantities exceeding one ton annually.
Occupational safety frameworks also apply to lithium chloride handling. The Occupational Safety and Health Administration (OSHA) in the United States sets exposure limits for lithium compounds, requiring appropriate engineering controls and personal protective equipment. Comparable standards exist internationally through organizations like the International Labour Organization (ILO).
For crystal habit modification techniques specifically, regulatory considerations extend to the additives and processing aids employed. These substances must meet food-grade or pharmaceutical-grade standards when the resulting lithium chloride is intended for human consumption or medical applications. Novel modification agents may require separate regulatory approval before implementation in commercial processes.
Transportation and storage of lithium compounds are subject to hazardous materials regulations, including the International Maritime Dangerous Goods (IMDG) Code and regional equivalents. These regulations mandate specific packaging, labeling, and handling procedures to ensure safety throughout the supply chain.
Emerging regulatory trends indicate increasing scrutiny of the environmental footprint of lithium production, with several jurisdictions developing sustainability criteria that may impact crystal habit modification processes. Companies engaged in lithium chloride production must maintain regulatory intelligence capabilities to navigate this evolving landscape effectively.
Environmental Impact Assessment
The environmental implications of lithium chloride crystal habit modification processes are significant and multifaceted, requiring thorough assessment to ensure sustainable implementation. The modification of crystal habits involves various chemical processes that can generate waste streams containing lithium compounds, solvents, and other additives used during crystallization procedures.
Primary environmental concerns include water consumption and contamination. Lithium chloride crystallization processes typically require substantial water resources, both as a solvent and for cooling purposes. Wastewater from these operations may contain elevated concentrations of lithium, chloride ions, and process additives that could potentially affect aquatic ecosystems if released without adequate treatment. Studies indicate that lithium concentrations exceeding 0.3 mg/L can adversely impact aquatic organisms, highlighting the importance of effective wastewater management systems.
Air quality considerations must also be addressed, particularly when processes involve volatile organic compounds (VOCs) as additives or solvents in crystal habit modification. Emissions from these operations may contribute to local air pollution and potentially to greenhouse gas emissions, depending on the energy sources used for heating, cooling, and other process requirements.
Energy consumption represents another significant environmental factor. Crystal habit modification often requires precise temperature control and extended processing times, resulting in substantial energy demands. The carbon footprint of these operations varies considerably based on regional energy sources, with renewable energy integration offering potential for significant environmental impact reduction.
Solid waste generation from failed batches, filter materials, and packaging also contributes to the environmental footprint. While lithium compounds themselves are valuable resources that can be recovered and recycled, the economic feasibility of recovery processes depends on concentration levels and available technologies.
Life cycle assessment (LCA) studies comparing different crystal habit modification techniques reveal varying environmental profiles. Techniques utilizing organic solvents typically show higher environmental impacts than aqueous-based approaches, though the latter may require more energy for subsequent drying operations. Recent innovations in solvent-free or green solvent approaches demonstrate promising reductions in environmental impact while maintaining effective crystal habit control.
Regulatory frameworks governing these processes continue to evolve, with increasing emphasis on circular economy principles and resource efficiency. Companies implementing lithium chloride crystal habit modification technologies must navigate complex compliance requirements spanning water discharge permits, air emission standards, and waste management regulations across different jurisdictions.
Primary environmental concerns include water consumption and contamination. Lithium chloride crystallization processes typically require substantial water resources, both as a solvent and for cooling purposes. Wastewater from these operations may contain elevated concentrations of lithium, chloride ions, and process additives that could potentially affect aquatic ecosystems if released without adequate treatment. Studies indicate that lithium concentrations exceeding 0.3 mg/L can adversely impact aquatic organisms, highlighting the importance of effective wastewater management systems.
Air quality considerations must also be addressed, particularly when processes involve volatile organic compounds (VOCs) as additives or solvents in crystal habit modification. Emissions from these operations may contribute to local air pollution and potentially to greenhouse gas emissions, depending on the energy sources used for heating, cooling, and other process requirements.
Energy consumption represents another significant environmental factor. Crystal habit modification often requires precise temperature control and extended processing times, resulting in substantial energy demands. The carbon footprint of these operations varies considerably based on regional energy sources, with renewable energy integration offering potential for significant environmental impact reduction.
Solid waste generation from failed batches, filter materials, and packaging also contributes to the environmental footprint. While lithium compounds themselves are valuable resources that can be recovered and recycled, the economic feasibility of recovery processes depends on concentration levels and available technologies.
Life cycle assessment (LCA) studies comparing different crystal habit modification techniques reveal varying environmental profiles. Techniques utilizing organic solvents typically show higher environmental impacts than aqueous-based approaches, though the latter may require more energy for subsequent drying operations. Recent innovations in solvent-free or green solvent approaches demonstrate promising reductions in environmental impact while maintaining effective crystal habit control.
Regulatory frameworks governing these processes continue to evolve, with increasing emphasis on circular economy principles and resource efficiency. Companies implementing lithium chloride crystal habit modification technologies must navigate complex compliance requirements spanning water discharge permits, air emission standards, and waste management regulations across different jurisdictions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!