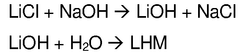How to Synthesize Lithium Chloride with High Purity
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Chloride Synthesis Background and Objectives
Lithium chloride (LiCl) has emerged as a critical compound in various industrial applications, particularly in the rapidly expanding lithium-ion battery sector. The historical development of lithium chloride synthesis can be traced back to the early 20th century, with significant advancements occurring during the post-World War II industrial expansion. Initially utilized primarily in metallurgical processes and as a desiccant, lithium chloride has evolved to become a fundamental component in modern energy storage solutions, pharmaceutical manufacturing, and advanced materials production.
The technological evolution in lithium chloride synthesis has progressed from basic mineral extraction methods to sophisticated chemical processes designed to achieve increasingly higher purity levels. This progression has been driven by the growing demand for ultra-pure lithium compounds, particularly in electronic applications where impurities at even parts-per-million levels can significantly impact performance characteristics.
Current global trends indicate an accelerating demand trajectory for high-purity lithium chloride, with annual growth rates exceeding 8% in specialized application segments. This growth is predominantly fueled by the expansion of electric vehicle production, renewable energy storage systems, and advanced electronic devices requiring high-performance power sources.
The primary technical objective in modern lithium chloride synthesis focuses on achieving purity levels exceeding 99.9% while simultaneously developing economically viable and environmentally sustainable production methodologies. This represents a significant challenge given the reactive nature of lithium and the difficulty in separating it from chemically similar elements, particularly sodium, which frequently occurs as a contaminant in lithium sources.
Secondary objectives include reducing energy consumption in the purification process, minimizing waste generation, and developing closed-loop systems that allow for the recovery and reuse of process chemicals. These objectives align with broader industry sustainability goals and increasingly stringent environmental regulations governing chemical manufacturing processes.
The technological roadmap for lithium chloride synthesis advancement anticipates several breakthrough opportunities, including the development of novel selective extraction techniques, advanced membrane separation technologies, and process intensification methodologies that could substantially reduce production costs while improving product quality. Recent research has demonstrated promising results with electrochemical purification techniques that may offer significant advantages over traditional thermal crystallization methods.
As global lithium demand continues to rise, driven primarily by energy transition initiatives, the importance of efficient high-purity lithium chloride synthesis will become increasingly critical to supply chain security and technological advancement across multiple industries.
The technological evolution in lithium chloride synthesis has progressed from basic mineral extraction methods to sophisticated chemical processes designed to achieve increasingly higher purity levels. This progression has been driven by the growing demand for ultra-pure lithium compounds, particularly in electronic applications where impurities at even parts-per-million levels can significantly impact performance characteristics.
Current global trends indicate an accelerating demand trajectory for high-purity lithium chloride, with annual growth rates exceeding 8% in specialized application segments. This growth is predominantly fueled by the expansion of electric vehicle production, renewable energy storage systems, and advanced electronic devices requiring high-performance power sources.
The primary technical objective in modern lithium chloride synthesis focuses on achieving purity levels exceeding 99.9% while simultaneously developing economically viable and environmentally sustainable production methodologies. This represents a significant challenge given the reactive nature of lithium and the difficulty in separating it from chemically similar elements, particularly sodium, which frequently occurs as a contaminant in lithium sources.
Secondary objectives include reducing energy consumption in the purification process, minimizing waste generation, and developing closed-loop systems that allow for the recovery and reuse of process chemicals. These objectives align with broader industry sustainability goals and increasingly stringent environmental regulations governing chemical manufacturing processes.
The technological roadmap for lithium chloride synthesis advancement anticipates several breakthrough opportunities, including the development of novel selective extraction techniques, advanced membrane separation technologies, and process intensification methodologies that could substantially reduce production costs while improving product quality. Recent research has demonstrated promising results with electrochemical purification techniques that may offer significant advantages over traditional thermal crystallization methods.
As global lithium demand continues to rise, driven primarily by energy transition initiatives, the importance of efficient high-purity lithium chloride synthesis will become increasingly critical to supply chain security and technological advancement across multiple industries.
Market Demand Analysis for High-Purity Lithium Chloride
The global market for high-purity lithium chloride has experienced significant growth in recent years, driven primarily by the expanding lithium-ion battery industry. As electric vehicles (EVs) continue to gain market share, the demand for high-purity lithium compounds has surged, with lithium chloride serving as a crucial intermediate in lithium metal and lithium compound production processes.
Market research indicates that the high-purity lithium chloride market reached approximately 12,000 metric tons in 2022, with projections suggesting a compound annual growth rate of 8-10% through 2030. This growth trajectory is substantially higher than traditional chemical markets, reflecting the strategic importance of lithium in the global energy transition.
The battery sector represents the largest demand driver, accounting for roughly 65% of high-purity lithium chloride consumption. Within this segment, manufacturers require lithium chloride with purity levels exceeding 99.5%, with premium applications demanding 99.9% purity or higher. Even minor impurities can significantly impact battery performance, safety, and longevity, making purification technology a critical competitive advantage.
Beyond batteries, high-purity lithium chloride finds applications in pharmaceuticals, specialty glass manufacturing, air conditioning systems, and metallurgical processes. The pharmaceutical industry, in particular, demands ultra-high purity grades (99.99%) for specific applications in psychiatric medications and enzyme preparations, representing a smaller but higher-margin market segment.
Regional analysis reveals Asia-Pacific as the dominant market, accounting for approximately 70% of global demand, with China leading consumption. North America and Europe follow with growing demand driven by domestic battery production initiatives and pharmaceutical applications. Recent government policies supporting domestic supply chains have accelerated investment in lithium processing capabilities in these regions.
Price trends show significant volatility, with high-purity lithium chloride commanding premium pricing compared to technical grades. Between 2020 and 2023, prices have fluctuated substantially, reflecting supply constraints and rapidly expanding demand. Current market prices for 99.9% purity lithium chloride range between $15-25 per kilogram, depending on volume and specific impurity profiles.
Industry forecasts suggest that demand will continue to outpace supply in the near term, creating opportunities for producers who can develop efficient, scalable purification technologies. The market increasingly values consistent quality, traceability, and sustainability credentials, with customers willing to pay premiums for products with lower environmental footprints and consistent specifications.
Market research indicates that the high-purity lithium chloride market reached approximately 12,000 metric tons in 2022, with projections suggesting a compound annual growth rate of 8-10% through 2030. This growth trajectory is substantially higher than traditional chemical markets, reflecting the strategic importance of lithium in the global energy transition.
The battery sector represents the largest demand driver, accounting for roughly 65% of high-purity lithium chloride consumption. Within this segment, manufacturers require lithium chloride with purity levels exceeding 99.5%, with premium applications demanding 99.9% purity or higher. Even minor impurities can significantly impact battery performance, safety, and longevity, making purification technology a critical competitive advantage.
Beyond batteries, high-purity lithium chloride finds applications in pharmaceuticals, specialty glass manufacturing, air conditioning systems, and metallurgical processes. The pharmaceutical industry, in particular, demands ultra-high purity grades (99.99%) for specific applications in psychiatric medications and enzyme preparations, representing a smaller but higher-margin market segment.
Regional analysis reveals Asia-Pacific as the dominant market, accounting for approximately 70% of global demand, with China leading consumption. North America and Europe follow with growing demand driven by domestic battery production initiatives and pharmaceutical applications. Recent government policies supporting domestic supply chains have accelerated investment in lithium processing capabilities in these regions.
Price trends show significant volatility, with high-purity lithium chloride commanding premium pricing compared to technical grades. Between 2020 and 2023, prices have fluctuated substantially, reflecting supply constraints and rapidly expanding demand. Current market prices for 99.9% purity lithium chloride range between $15-25 per kilogram, depending on volume and specific impurity profiles.
Industry forecasts suggest that demand will continue to outpace supply in the near term, creating opportunities for producers who can develop efficient, scalable purification technologies. The market increasingly values consistent quality, traceability, and sustainability credentials, with customers willing to pay premiums for products with lower environmental footprints and consistent specifications.
Current Synthesis Methods and Technical Challenges
The synthesis of high-purity lithium chloride currently employs several established methodologies, each with distinct advantages and limitations. The most common approach involves the reaction of lithium carbonate with hydrochloric acid, followed by crystallization processes to remove impurities. This method achieves purities of approximately 99.0-99.5%, but struggles to eliminate trace metal contaminants, particularly sodium and calcium ions that share similar chemical properties with lithium.
Alternative synthesis routes include direct reaction of lithium metal or lithium hydroxide with hydrochloric acid. The lithium metal route offers higher theoretical purity but presents significant safety challenges due to the highly reactive nature of lithium metal in air and moisture. The lithium hydroxide pathway provides better handling characteristics but introduces additional purification steps to remove hydroxide-related impurities.
Electrolytic processes represent another approach, where lithium-containing brines undergo selective electrolysis to produce lithium chloride. While this method can be scaled for industrial production, it faces challenges in energy efficiency and electrode degradation during continuous operation. The electrolytic approach typically achieves purities of 99.0-99.8%, depending on the sophistication of the cell design and operational parameters.
The primary technical challenges in high-purity lithium chloride synthesis center around three critical areas. First, the removal of isomorphic impurities that co-crystallize with lithium chloride presents significant separation difficulties. Elements like sodium, potassium, and magnesium form chloride salts with similar crystallization behaviors, making conventional purification methods insufficient for ultra-high purity requirements.
Second, the hygroscopic nature of lithium chloride complicates handling and storage, as moisture absorption introduces contamination and degrades product quality. Current dehydration techniques often involve high-temperature treatments that can lead to partial decomposition or introduction of container-derived impurities.
Third, analytical limitations pose challenges in accurately quantifying impurities at the parts-per-million or parts-per-billion levels required for advanced applications. This creates difficulties in process validation and quality control, particularly for emerging applications in battery technology and pharmaceuticals that demand 99.99% purity or higher.
Recent innovations have explored solvent extraction techniques and ion-exchange methodologies to overcome these challenges, but these approaches often introduce organic contaminants or require extensive additional purification steps. The development of membrane-based separation technologies shows promise but remains limited by membrane stability in concentrated chloride environments.
Alternative synthesis routes include direct reaction of lithium metal or lithium hydroxide with hydrochloric acid. The lithium metal route offers higher theoretical purity but presents significant safety challenges due to the highly reactive nature of lithium metal in air and moisture. The lithium hydroxide pathway provides better handling characteristics but introduces additional purification steps to remove hydroxide-related impurities.
Electrolytic processes represent another approach, where lithium-containing brines undergo selective electrolysis to produce lithium chloride. While this method can be scaled for industrial production, it faces challenges in energy efficiency and electrode degradation during continuous operation. The electrolytic approach typically achieves purities of 99.0-99.8%, depending on the sophistication of the cell design and operational parameters.
The primary technical challenges in high-purity lithium chloride synthesis center around three critical areas. First, the removal of isomorphic impurities that co-crystallize with lithium chloride presents significant separation difficulties. Elements like sodium, potassium, and magnesium form chloride salts with similar crystallization behaviors, making conventional purification methods insufficient for ultra-high purity requirements.
Second, the hygroscopic nature of lithium chloride complicates handling and storage, as moisture absorption introduces contamination and degrades product quality. Current dehydration techniques often involve high-temperature treatments that can lead to partial decomposition or introduction of container-derived impurities.
Third, analytical limitations pose challenges in accurately quantifying impurities at the parts-per-million or parts-per-billion levels required for advanced applications. This creates difficulties in process validation and quality control, particularly for emerging applications in battery technology and pharmaceuticals that demand 99.99% purity or higher.
Recent innovations have explored solvent extraction techniques and ion-exchange methodologies to overcome these challenges, but these approaches often introduce organic contaminants or require extensive additional purification steps. The development of membrane-based separation technologies shows promise but remains limited by membrane stability in concentrated chloride environments.
Current High-Purity Synthesis Solutions
01 Purification methods for lithium chloride
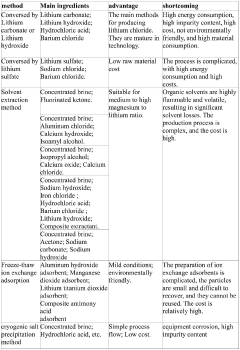
Various methods are employed to purify lithium chloride to achieve high purity levels. These include crystallization, ion exchange, solvent extraction, and membrane separation techniques. The purification processes aim to remove impurities such as sodium, calcium, magnesium, and other metal ions that can affect the quality and performance of lithium chloride in various applications.- Purification methods for lithium chloride: Various methods are employed to purify lithium chloride to achieve high purity levels. These include crystallization, precipitation, filtration, and ion exchange techniques. The purification processes often involve multiple steps to remove impurities such as sodium, calcium, magnesium, and other metal ions. Advanced separation technologies can achieve ultra-high purity lithium chloride suitable for specialized applications in pharmaceuticals and electronics.
- Analytical techniques for determining lithium chloride purity: Various analytical methods are used to determine the purity of lithium chloride, including atomic absorption spectroscopy, inductively coupled plasma mass spectrometry (ICP-MS), ion chromatography, and titration methods. These techniques allow for precise measurement of impurity levels and verification of lithium chloride purity. Quality control protocols often specify detection limits and acceptable impurity thresholds for different grades of lithium chloride.
- High-purity lithium chloride for battery applications: High-purity lithium chloride is essential for lithium-ion battery production. The purity requirements typically exceed 99.5%, with strict limits on metallic impurities that could affect battery performance and safety. Purification processes specifically designed for battery-grade lithium chloride focus on removing transition metals and other elements that might interfere with electrochemical processes. Advanced manufacturing techniques ensure consistent quality suitable for next-generation energy storage applications.
- Industrial production of high-purity lithium chloride: Industrial-scale production of high-purity lithium chloride involves specialized equipment and processes designed to maintain purity throughout manufacturing. This includes controlled reaction environments, specialized reactors, and dedicated purification systems. Continuous monitoring and in-process testing ensure consistent quality. Modern production facilities implement automated systems to minimize contamination risks and optimize yield while maintaining the required purity specifications.
- Applications requiring specific lithium chloride purity grades: Different applications require specific purity grades of lithium chloride. Pharmaceutical applications typically demand USP or pharmaceutical-grade purity with strict limits on heavy metals and other contaminants. Electronic applications require ultra-high purity with minimal metallic impurities. Air conditioning and dehumidification applications can use technical-grade lithium chloride. The specific purity requirements are determined by the intended use, with corresponding testing and certification protocols to verify compliance.
02 High-purity lithium chloride for battery applications
High-purity lithium chloride is essential for lithium-ion battery production. The battery-grade lithium chloride typically requires purity levels exceeding 99.5%, with strict limits on metallic impurities. Purification processes focus on removing elements that can negatively impact battery performance, such as sodium, calcium, potassium, and transition metals. Advanced purification techniques are developed specifically to meet the stringent requirements of the battery industry.Expand Specific Solutions03 Analytical methods for determining lithium chloride purity
Various analytical techniques are used to determine the purity of lithium chloride, including atomic absorption spectroscopy, inductively coupled plasma mass spectrometry (ICP-MS), ion chromatography, and titration methods. These techniques allow for precise quantification of lithium content and detection of trace impurities at parts per million or even parts per billion levels, ensuring quality control in production processes.Expand Specific Solutions04 Industrial production of high-purity lithium chloride
Industrial-scale production of high-purity lithium chloride involves multiple stages including extraction from raw materials (such as brines, ores, or recycled sources), initial concentration, and multi-step purification processes. Advanced equipment and process control systems are employed to maintain consistent quality. Continuous flow processes and automated monitoring systems help achieve high throughput while maintaining the required purity specifications.Expand Specific Solutions05 Applications requiring specific lithium chloride purity grades
Different applications require specific purity grades of lithium chloride. Pharmaceutical applications demand ultra-high purity with strict limits on heavy metals and other contaminants. Electronic applications require low levels of specific impurities that could affect performance. Air conditioning and dehumidification applications may tolerate lower purity grades. The specifications for each grade are tailored to the requirements of the end-use, with corresponding purification processes designed to meet these specifications cost-effectively.Expand Specific Solutions
Key Industry Players and Competitive Landscape
The high-purity lithium chloride synthesis market is currently in a growth phase, with increasing demand driven by battery technologies and electronics applications. The global market size is expanding rapidly, projected to reach significant volumes as lithium-based technologies proliferate. Technologically, the field shows varying maturity levels across different synthesis methods. Leading players include Ganfeng Lithium and Tianqi Lithium, who have established robust production capabilities, while companies like Sumitomo Metal Mining and BASF bring advanced chemical processing expertise. Specialized firms such as Terralithium and SinoLithium Materials are developing innovative purification techniques. Research institutions including Central South University and KU Leuven are advancing fundamental synthesis methodologies, creating a competitive landscape balanced between established industrial giants and emerging technology-focused enterprises.
Ganfeng Lithium Group Co., Ltd.
Technical Solution: Ganfeng Lithium has developed a proprietary high-purity lithium chloride synthesis process that combines direct lithium extraction (DLE) technology with advanced purification techniques. Their method starts with extracting lithium from various sources including brine, clay, and hard rock using selective adsorption materials. The extracted lithium solution undergoes multi-stage purification including ion exchange, chemical precipitation, and membrane filtration to remove impurities such as sodium, calcium, magnesium, and boron. The final crystallization process is conducted under precisely controlled temperature and pH conditions, resulting in lithium chloride with purity exceeding 99.9%. Their process incorporates real-time monitoring systems that adjust parameters based on feed composition variations, ensuring consistent product quality. Ganfeng has also implemented a closed-loop water recycling system that reduces water consumption by approximately 70% compared to traditional evaporation methods.
Strengths: Versatile feedstock capability allowing processing of multiple lithium sources; advanced impurity removal achieving pharmaceutical-grade purity; significantly reduced water consumption and processing time compared to solar evaporation. Weaknesses: Higher energy requirements than conventional methods; requires specialized equipment and technical expertise; more complex process control systems needed to maintain quality consistency.
Rockwood Lithium
Technical Solution: Rockwood Lithium (now part of Albemarle Corporation) has developed a sophisticated lithium chloride synthesis process optimized for brine resources. Their technology employs a selective extraction process using proprietary ion exchange materials that preferentially adsorb lithium ions while rejecting competing ions like sodium and magnesium. After extraction, the lithium-rich solution undergoes a multi-stage purification process including chemical precipitation to remove divalent impurities, followed by specialized membrane filtration techniques. A key innovation in their approach is the use of countercurrent ion exchange columns that achieve higher lithium recovery rates while minimizing reagent consumption. The purified lithium solution is then concentrated through low-temperature evaporation under vacuum conditions to preserve energy efficiency. Final crystallization is performed using controlled cooling crystallization techniques that produce uniform, high-purity lithium chloride crystals. Their process achieves consistent purity levels exceeding 99.95%, with particularly low levels of sodium, calcium, and magnesium impurities that are critical for battery applications.
Strengths: Highly efficient lithium recovery from brines with minimal loss; exceptional sodium and magnesium rejection capabilities; energy-efficient concentration and crystallization processes. Weaknesses: Process is optimized primarily for brine resources rather than hard rock sources; requires specialized ion exchange materials that need periodic replacement; higher initial capital investment compared to traditional evaporation methods.
Critical Patents and Technical Literature Review
Process for producing lithium hydroxide monohydrate
PatentWO2025036769A1
Innovation
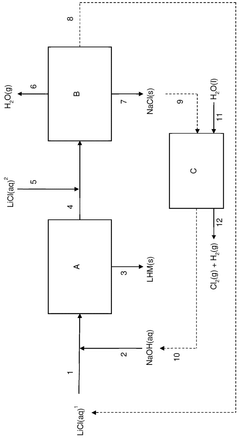
- A process involving the addition of an aqueous sodium hydroxide solution to a lithium chloride solution, followed by cooling to precipitate lithium hydroxide monohydrate, and subsequent separation and purification steps to achieve high purity and efficiency.
A method and device for preparing high-purity lithium chloride based on lithium-ion solid electrolyte
PatentWO2024061312A1
Innovation
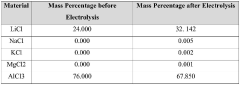
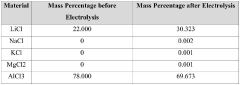
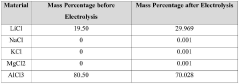
- Using lithium-ion solid electrolyte for direct electrolytic reaction to produce high-purity lithium chloride from low-purity lithium chloride salt.
- Utilizing various raw material sources including salt lake brine, seawater, or solid minerals to produce dry low-purity lithium chloride salt as starting material.
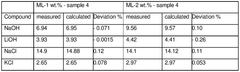
- Providing a cost-effective method to meet the high demand for high-purity lithium chloride (≥99.3 wt%) required for metallic lithium production and other applications.
Raw Material Supply Chain Analysis
The global lithium supply chain represents a critical component in the production of high-purity lithium chloride, with significant implications for both cost and quality of the final product. Primary lithium sources include brine operations concentrated in South America's "Lithium Triangle" (Chile, Argentina, and Bolivia), hard rock mining operations predominantly in Australia, and emerging extraction from clay deposits in the United States. Each source presents distinct advantages and challenges in terms of purity profiles and processing requirements.
Brine-based lithium extraction typically yields lithium carbonate as an intermediate product, which requires additional processing steps to convert to lithium chloride. This pathway generally offers cost advantages but may introduce specific impurity profiles, particularly magnesium and boron compounds that can be challenging to remove in subsequent purification stages.
Hard rock mining, primarily of spodumene, provides an alternative supply chain with different impurity characteristics. Australian operations dominate this segment, with significant processing capacity developed in China. The conversion of spodumene concentrate to lithium compounds involves energy-intensive thermal and hydrometallurgical processes, potentially affecting both cost structures and environmental footprints.
Supply chain resilience represents a growing concern for high-purity lithium chloride production. Current market dynamics reveal significant geographic concentration, with approximately 75% of lithium processing capacity located in China. This concentration creates potential vulnerabilities in supply security, particularly for applications requiring consistent high-purity materials.
Quality consistency across the supply chain presents another critical challenge. Variations in raw material composition necessitate adaptive processing techniques to achieve high-purity specifications. Establishing robust supplier qualification protocols and implementing comprehensive material characterization methodologies are essential practices for maintaining quality standards.
Emerging trends in the lithium supply chain include the development of direct lithium extraction (DLE) technologies, which promise reduced environmental impact and potentially higher recovery rates from lower-grade resources. These technologies may significantly alter the economics and geographical distribution of lithium production in the coming decade.
Price volatility in lithium markets introduces additional complexity to supply chain management for high-purity lithium chloride production. Strategic approaches, including long-term supply agreements and vertical integration strategies, are increasingly being adopted by manufacturers to mitigate these risks and ensure consistent access to suitable raw materials.
Brine-based lithium extraction typically yields lithium carbonate as an intermediate product, which requires additional processing steps to convert to lithium chloride. This pathway generally offers cost advantages but may introduce specific impurity profiles, particularly magnesium and boron compounds that can be challenging to remove in subsequent purification stages.
Hard rock mining, primarily of spodumene, provides an alternative supply chain with different impurity characteristics. Australian operations dominate this segment, with significant processing capacity developed in China. The conversion of spodumene concentrate to lithium compounds involves energy-intensive thermal and hydrometallurgical processes, potentially affecting both cost structures and environmental footprints.
Supply chain resilience represents a growing concern for high-purity lithium chloride production. Current market dynamics reveal significant geographic concentration, with approximately 75% of lithium processing capacity located in China. This concentration creates potential vulnerabilities in supply security, particularly for applications requiring consistent high-purity materials.
Quality consistency across the supply chain presents another critical challenge. Variations in raw material composition necessitate adaptive processing techniques to achieve high-purity specifications. Establishing robust supplier qualification protocols and implementing comprehensive material characterization methodologies are essential practices for maintaining quality standards.
Emerging trends in the lithium supply chain include the development of direct lithium extraction (DLE) technologies, which promise reduced environmental impact and potentially higher recovery rates from lower-grade resources. These technologies may significantly alter the economics and geographical distribution of lithium production in the coming decade.
Price volatility in lithium markets introduces additional complexity to supply chain management for high-purity lithium chloride production. Strategic approaches, including long-term supply agreements and vertical integration strategies, are increasingly being adopted by manufacturers to mitigate these risks and ensure consistent access to suitable raw materials.
Environmental Impact and Sustainability Considerations
The synthesis of high-purity lithium chloride presents significant environmental challenges that must be addressed through sustainable practices. Traditional extraction methods often involve extensive mining operations that disrupt ecosystems, deplete water resources, and generate substantial waste. The brine extraction process, commonly used in salt flats, can alter hydrological systems and impact biodiversity in sensitive areas. Chemical processing routes typically require large volumes of water and energy, contributing to resource depletion and carbon emissions.
Water consumption represents a critical environmental concern, particularly in arid regions where lithium resources are abundant. Advanced synthesis methods are now incorporating closed-loop water systems that recycle up to 90% of process water, significantly reducing the environmental footprint. Additionally, emerging technologies utilize renewable energy sources for evaporation and crystallization processes, decreasing carbon emissions associated with high-purity lithium chloride production.
Waste management constitutes another substantial challenge. The synthesis process generates various byproducts, including magnesium and calcium compounds, which require proper disposal or valorization. Leading manufacturers have developed innovative approaches to transform these byproducts into marketable materials for construction and agricultural applications, moving toward zero-waste production models.
Life cycle assessment (LCA) studies indicate that the environmental impact of lithium chloride synthesis varies significantly depending on the production method. Direct extraction technologies demonstrate 30-50% lower carbon footprints compared to conventional evaporation techniques. These newer methods also reduce land disturbance by up to 70%, preserving natural habitats and minimizing ecological disruption.
Regulatory frameworks worldwide are increasingly mandating stricter environmental standards for lithium compound production. Companies pursuing high-purity lithium chloride synthesis must now implement comprehensive environmental management systems, conduct regular impact assessments, and engage with local communities to ensure responsible production practices.
The industry is witnessing a paradigm shift toward circular economy principles, with manufacturers developing processes to recover lithium from spent batteries and industrial waste streams. This approach not only reduces the need for primary resource extraction but also addresses end-of-life product management. Research indicates that recycled lithium can achieve comparable purity levels to virgin material while consuming 50-70% less energy and water.
Water consumption represents a critical environmental concern, particularly in arid regions where lithium resources are abundant. Advanced synthesis methods are now incorporating closed-loop water systems that recycle up to 90% of process water, significantly reducing the environmental footprint. Additionally, emerging technologies utilize renewable energy sources for evaporation and crystallization processes, decreasing carbon emissions associated with high-purity lithium chloride production.
Waste management constitutes another substantial challenge. The synthesis process generates various byproducts, including magnesium and calcium compounds, which require proper disposal or valorization. Leading manufacturers have developed innovative approaches to transform these byproducts into marketable materials for construction and agricultural applications, moving toward zero-waste production models.
Life cycle assessment (LCA) studies indicate that the environmental impact of lithium chloride synthesis varies significantly depending on the production method. Direct extraction technologies demonstrate 30-50% lower carbon footprints compared to conventional evaporation techniques. These newer methods also reduce land disturbance by up to 70%, preserving natural habitats and minimizing ecological disruption.
Regulatory frameworks worldwide are increasingly mandating stricter environmental standards for lithium compound production. Companies pursuing high-purity lithium chloride synthesis must now implement comprehensive environmental management systems, conduct regular impact assessments, and engage with local communities to ensure responsible production practices.
The industry is witnessing a paradigm shift toward circular economy principles, with manufacturers developing processes to recover lithium from spent batteries and industrial waste streams. This approach not only reduces the need for primary resource extraction but also addresses end-of-life product management. Research indicates that recycled lithium can achieve comparable purity levels to virgin material while consuming 50-70% less energy and water.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!