Evaluate Lithium Chloride Solubility in Non-Aqueous Media
AUG 28, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
LiCl Solubility Background and Research Objectives
Lithium chloride (LiCl) has emerged as a critical compound in various industrial applications, particularly in battery technologies, pharmaceuticals, and chemical synthesis. The solubility behavior of LiCl in non-aqueous media represents a fundamental area of research with significant implications for advancing these applications. Historically, research on lithium salt solubility has predominantly focused on aqueous systems, leaving considerable knowledge gaps regarding non-aqueous environments.
The evolution of lithium-based technologies has accelerated dramatically over the past two decades, driven primarily by the exponential growth in energy storage demands. From the early use of lithium salts in specialized applications, the technology has progressed to become integral to modern energy systems, particularly in lithium-ion batteries. This progression has heightened the importance of understanding LiCl solubility characteristics across diverse solvent systems.
Current technological trends indicate a shift toward more efficient and sustainable energy storage solutions, where lithium salts play a pivotal role. The manipulation of LiCl solubility in non-aqueous media offers promising avenues for enhancing battery performance, extending operational lifespans, and reducing environmental impacts. These trends underscore the strategic importance of comprehensive solubility studies.
The primary objective of this research is to systematically evaluate and quantify LiCl solubility across a spectrum of non-aqueous solvents under varying temperature and pressure conditions. This evaluation aims to establish predictive models that can accurately forecast solubility behavior in complex solvent systems, thereby facilitating more efficient formulation processes in industrial applications.
Secondary objectives include identifying optimal solvent compositions for specific applications, understanding the thermodynamic and kinetic factors governing LiCl dissolution in non-aqueous media, and developing novel methodologies for enhancing solubility where beneficial. These objectives align with broader industry goals of improving energy density in batteries and optimizing chemical processes.
The research also seeks to address existing technical limitations, particularly the challenges associated with LiCl's hygroscopic nature and its tendency to form complex coordination structures in non-aqueous environments. By systematically investigating these phenomena, the study aims to provide practical solutions to current technological bottlenecks.
Long-term goals include establishing a comprehensive database of LiCl solubility parameters across diverse non-aqueous systems, developing theoretical frameworks that explain observed solubility patterns, and translating these insights into practical applications that advance industrial capabilities. This knowledge foundation will support innovation in battery technologies, pharmaceutical formulations, and chemical synthesis processes.
The evolution of lithium-based technologies has accelerated dramatically over the past two decades, driven primarily by the exponential growth in energy storage demands. From the early use of lithium salts in specialized applications, the technology has progressed to become integral to modern energy systems, particularly in lithium-ion batteries. This progression has heightened the importance of understanding LiCl solubility characteristics across diverse solvent systems.
Current technological trends indicate a shift toward more efficient and sustainable energy storage solutions, where lithium salts play a pivotal role. The manipulation of LiCl solubility in non-aqueous media offers promising avenues for enhancing battery performance, extending operational lifespans, and reducing environmental impacts. These trends underscore the strategic importance of comprehensive solubility studies.
The primary objective of this research is to systematically evaluate and quantify LiCl solubility across a spectrum of non-aqueous solvents under varying temperature and pressure conditions. This evaluation aims to establish predictive models that can accurately forecast solubility behavior in complex solvent systems, thereby facilitating more efficient formulation processes in industrial applications.
Secondary objectives include identifying optimal solvent compositions for specific applications, understanding the thermodynamic and kinetic factors governing LiCl dissolution in non-aqueous media, and developing novel methodologies for enhancing solubility where beneficial. These objectives align with broader industry goals of improving energy density in batteries and optimizing chemical processes.
The research also seeks to address existing technical limitations, particularly the challenges associated with LiCl's hygroscopic nature and its tendency to form complex coordination structures in non-aqueous environments. By systematically investigating these phenomena, the study aims to provide practical solutions to current technological bottlenecks.
Long-term goals include establishing a comprehensive database of LiCl solubility parameters across diverse non-aqueous systems, developing theoretical frameworks that explain observed solubility patterns, and translating these insights into practical applications that advance industrial capabilities. This knowledge foundation will support innovation in battery technologies, pharmaceutical formulations, and chemical synthesis processes.
Market Applications and Demand Analysis for Non-Aqueous LiCl Solutions
The non-aqueous lithium chloride solutions market is experiencing significant growth driven by the expanding lithium-ion battery industry. With the global electric vehicle market projected to reach $823 billion by 2030, the demand for advanced battery technologies and materials continues to surge. Non-aqueous LiCl solutions play a crucial role in this ecosystem, particularly in battery electrolyte formulations, where they contribute to enhanced performance characteristics.
The pharmaceutical sector represents another substantial market for non-aqueous LiCl solutions, valued at approximately $1.4 trillion globally. These solutions are utilized in drug synthesis processes, particularly for compounds that are sensitive to water or require anhydrous conditions. The growing emphasis on novel drug development and green chemistry approaches has further accelerated demand in this segment.
Electronics manufacturing constitutes a third major application area, with the global electronics market exceeding $2.9 trillion. Non-aqueous LiCl solutions are employed in specialized coating processes, semiconductor manufacturing, and as components in certain types of sensors and electronic devices. The miniaturization trend in electronics has intensified the need for high-purity materials with precise solubility characteristics.
Chemical processing industries utilize non-aqueous LiCl solutions as catalysts, drying agents, and in separation processes. This sector's demand is growing at 4.7% annually, driven by innovations in sustainable chemical manufacturing and process intensification techniques. The ability to precisely control LiCl solubility in various non-aqueous media enables more efficient and selective chemical transformations.
Regional market analysis reveals that Asia-Pacific dominates demand, accounting for 58% of global consumption, followed by North America (22%) and Europe (17%). This distribution closely mirrors global battery production capacity and electronics manufacturing hubs. China, South Korea, and Japan lead in consumption volumes, reflecting their dominant positions in battery and electronics manufacturing.
Market forecasts indicate that demand for non-aqueous LiCl solutions will grow at a compound annual rate of 8.3% through 2028, outpacing many specialty chemicals. This growth is driven by technological advancements in battery technologies, increasing electronics production, and expanding pharmaceutical applications. The highest growth rates are anticipated in emerging markets, particularly India and Southeast Asian countries, where manufacturing capacity is rapidly expanding.
Customer requirements are increasingly focused on purity levels, consistent solubility profiles across different non-aqueous solvents, and sustainability of production processes. These evolving demands are reshaping product development priorities and creating new market opportunities for specialized formulations with optimized performance characteristics.
The pharmaceutical sector represents another substantial market for non-aqueous LiCl solutions, valued at approximately $1.4 trillion globally. These solutions are utilized in drug synthesis processes, particularly for compounds that are sensitive to water or require anhydrous conditions. The growing emphasis on novel drug development and green chemistry approaches has further accelerated demand in this segment.
Electronics manufacturing constitutes a third major application area, with the global electronics market exceeding $2.9 trillion. Non-aqueous LiCl solutions are employed in specialized coating processes, semiconductor manufacturing, and as components in certain types of sensors and electronic devices. The miniaturization trend in electronics has intensified the need for high-purity materials with precise solubility characteristics.
Chemical processing industries utilize non-aqueous LiCl solutions as catalysts, drying agents, and in separation processes. This sector's demand is growing at 4.7% annually, driven by innovations in sustainable chemical manufacturing and process intensification techniques. The ability to precisely control LiCl solubility in various non-aqueous media enables more efficient and selective chemical transformations.
Regional market analysis reveals that Asia-Pacific dominates demand, accounting for 58% of global consumption, followed by North America (22%) and Europe (17%). This distribution closely mirrors global battery production capacity and electronics manufacturing hubs. China, South Korea, and Japan lead in consumption volumes, reflecting their dominant positions in battery and electronics manufacturing.
Market forecasts indicate that demand for non-aqueous LiCl solutions will grow at a compound annual rate of 8.3% through 2028, outpacing many specialty chemicals. This growth is driven by technological advancements in battery technologies, increasing electronics production, and expanding pharmaceutical applications. The highest growth rates are anticipated in emerging markets, particularly India and Southeast Asian countries, where manufacturing capacity is rapidly expanding.
Customer requirements are increasingly focused on purity levels, consistent solubility profiles across different non-aqueous solvents, and sustainability of production processes. These evolving demands are reshaping product development priorities and creating new market opportunities for specialized formulations with optimized performance characteristics.
Current Challenges in Non-Aqueous Solvent Systems
Despite significant advancements in lithium-based technologies, the solubility of lithium chloride in non-aqueous media presents persistent challenges that impede further development in energy storage applications. Current non-aqueous solvent systems face several critical limitations that affect both research progress and industrial implementation. These challenges stem from fundamental physicochemical properties and practical application requirements.
The primary challenge lies in achieving sufficient solubility of lithium chloride in organic solvents. Unlike its behavior in aqueous systems, LiCl exhibits dramatically reduced solubility in most non-aqueous media due to the strong ionic character of the salt and the typically lower dielectric constants of organic solvents. This limited solubility restricts the concentration of lithium ions available for electrochemical processes, directly impacting the energy density of resulting battery systems.
Solvent stability represents another significant hurdle. Many organic solvents that provide acceptable LiCl solubility suffer from chemical or electrochemical instability under operating conditions. Decomposition reactions at electrode interfaces create unwanted byproducts that can passivate electrodes, increase internal resistance, and ultimately lead to capacity fade and shortened device lifetimes.
Temperature dependence of solubility introduces additional complications. The solubility behavior of LiCl in non-aqueous media often exhibits strong temperature sensitivity, creating challenges for applications requiring operation across wide temperature ranges. This variability necessitates complex engineering solutions to maintain consistent performance in real-world conditions.
Ion coordination and solvation mechanisms in non-aqueous systems remain incompletely understood. The specific interactions between lithium ions, chloride counterions, and solvent molecules create complex solvation structures that affect ion mobility, conductivity, and electrochemical behavior. This knowledge gap hampers rational solvent design and optimization efforts.
Competitive solvation presents further difficulties when multiple salt species are present. In practical applications, electrolyte systems often contain multiple ionic species, leading to competitive solvation effects that can reduce LiCl solubility below levels observed in single-salt systems. These interactions are difficult to predict and control.
Environmental and safety concerns also constrain solvent selection. Many solvents offering favorable LiCl solubility properties present toxicity, flammability, or environmental persistence issues. Regulatory pressures and sustainability considerations increasingly limit the practical use of certain solvent systems despite their technical advantages.
Cost factors and scalability challenges complete the picture of current limitations. High-performance specialty solvents that address some of the above challenges often come with prohibitive costs or manufacturing complexities that prevent widespread commercial adoption, particularly for large-scale energy storage applications.
The primary challenge lies in achieving sufficient solubility of lithium chloride in organic solvents. Unlike its behavior in aqueous systems, LiCl exhibits dramatically reduced solubility in most non-aqueous media due to the strong ionic character of the salt and the typically lower dielectric constants of organic solvents. This limited solubility restricts the concentration of lithium ions available for electrochemical processes, directly impacting the energy density of resulting battery systems.
Solvent stability represents another significant hurdle. Many organic solvents that provide acceptable LiCl solubility suffer from chemical or electrochemical instability under operating conditions. Decomposition reactions at electrode interfaces create unwanted byproducts that can passivate electrodes, increase internal resistance, and ultimately lead to capacity fade and shortened device lifetimes.
Temperature dependence of solubility introduces additional complications. The solubility behavior of LiCl in non-aqueous media often exhibits strong temperature sensitivity, creating challenges for applications requiring operation across wide temperature ranges. This variability necessitates complex engineering solutions to maintain consistent performance in real-world conditions.
Ion coordination and solvation mechanisms in non-aqueous systems remain incompletely understood. The specific interactions between lithium ions, chloride counterions, and solvent molecules create complex solvation structures that affect ion mobility, conductivity, and electrochemical behavior. This knowledge gap hampers rational solvent design and optimization efforts.
Competitive solvation presents further difficulties when multiple salt species are present. In practical applications, electrolyte systems often contain multiple ionic species, leading to competitive solvation effects that can reduce LiCl solubility below levels observed in single-salt systems. These interactions are difficult to predict and control.
Environmental and safety concerns also constrain solvent selection. Many solvents offering favorable LiCl solubility properties present toxicity, flammability, or environmental persistence issues. Regulatory pressures and sustainability considerations increasingly limit the practical use of certain solvent systems despite their technical advantages.
Cost factors and scalability challenges complete the picture of current limitations. High-performance specialty solvents that address some of the above challenges often come with prohibitive costs or manufacturing complexities that prevent widespread commercial adoption, particularly for large-scale energy storage applications.
Existing Methodologies for Evaluating LiCl Solubility
01 Solubility characteristics of lithium chloride in various solvents
Lithium chloride exhibits different solubility characteristics in various solvents. It is highly soluble in water, with solubility increasing significantly with temperature. It also shows solubility in certain organic solvents like alcohols and ketones, though generally to a lesser extent than in water. The solubility properties of lithium chloride make it useful in various applications including as a desiccant and in chemical synthesis processes.- Solubility characteristics of lithium chloride in various solvents: Lithium chloride exhibits varying solubility in different solvents, which is important for various industrial applications. It is highly soluble in water, with solubility increasing significantly with temperature. It also shows good solubility in certain organic solvents like alcohols and ketones, while being less soluble in non-polar organic solvents. Understanding these solubility characteristics is crucial for processes involving lithium extraction, purification, and formulation of lithium-based products.
- Methods to enhance lithium chloride solubility: Various techniques can be employed to enhance the solubility of lithium chloride in different media. These include temperature manipulation, pH adjustment, use of co-solvents, and addition of specific solubilizing agents. Some processes utilize ultrasonic treatment or mechanical agitation to improve dissolution rates. Enhanced solubility is particularly important in applications requiring concentrated lithium chloride solutions or when working with mixed solvent systems where standard solubility might be limited.
- Lithium chloride solubility in extraction and recovery processes: The solubility properties of lithium chloride play a critical role in extraction and recovery processes from various sources including brines, ores, and recycled materials. These processes often involve selective dissolution steps where the differential solubility of lithium chloride compared to other salts is exploited. Precipitation techniques based on solubility differences are commonly used for purification, while evaporation and crystallization methods leverage solubility changes with temperature to recover lithium chloride from solutions.
- Impact of impurities on lithium chloride solubility: The presence of impurities significantly affects the solubility behavior of lithium chloride in various systems. Common impurities include other metal ions, organic compounds, and competing salts. These impurities can either enhance or suppress lithium chloride solubility through mechanisms such as common ion effect, complex formation, or alteration of solution properties. Understanding and controlling these interactions is essential for developing efficient purification processes and maintaining consistent product quality in lithium chloride production.
- Applications utilizing controlled lithium chloride solubility: Controlled solubility of lithium chloride is utilized in numerous applications across different industries. In battery technology, precise control of lithium chloride solubility is crucial for electrolyte formulation. In pharmaceutical applications, its solubility characteristics are important for drug delivery systems. Other applications include humidity control systems, heat storage media, and as a flux in metallurgical processes. The ability to predict and manipulate lithium chloride solubility enables optimization of these applications for improved performance and efficiency.
02 Methods to enhance lithium chloride solubility
Various methods can be employed to enhance the solubility of lithium chloride. These include heating the solution, adjusting the pH, adding co-solvents, or incorporating specific additives that can form complexes with lithium ions. Enhanced solubility can be achieved through the use of mixed solvent systems or by introducing certain organic compounds that interact favorably with lithium chloride.Expand Specific Solutions03 Lithium chloride solubility in extraction and separation processes
The solubility properties of lithium chloride are utilized in various extraction and separation processes. These include liquid-liquid extraction, crystallization, and selective precipitation techniques. The differential solubility of lithium chloride compared to other salts allows for effective separation in brine processing and lithium recovery operations from various sources including geothermal brines and spent lithium batteries.Expand Specific Solutions04 Impact of impurities on lithium chloride solubility
The presence of impurities can significantly affect the solubility of lithium chloride. Common impurities in lithium chloride solutions include other metal ions, organic compounds, and various salts. These impurities can either increase or decrease solubility through mechanisms such as common ion effect, formation of complexes, or alteration of solution properties. Understanding these effects is crucial for purification processes and applications requiring high-purity lithium chloride.Expand Specific Solutions05 Temperature and pressure effects on lithium chloride solubility
Temperature and pressure have significant effects on the solubility of lithium chloride. Generally, the solubility of lithium chloride in water increases with temperature, following a positive solubility curve. Pressure effects are less pronounced but can become significant under extreme conditions. These relationships are important in processes involving lithium chloride solutions at varying temperatures, such as in heat storage systems, absorption refrigeration, and certain industrial separation processes.Expand Specific Solutions
Key Industry Players and Research Institutions
The lithium chloride solubility in non-aqueous media market is in a growth phase, driven by expanding applications in lithium-ion batteries and energy storage systems. The global market size is projected to increase significantly due to rising demand for electric vehicles and portable electronics. Technologically, the field is moderately mature but continues to evolve with innovations in electrolyte formulations. Leading players like LG Energy Solution, Tianqi Lithium, and Ganfeng Lithium are investing heavily in R&D to improve solubility characteristics and performance. Companies such as CATL (Ningde Amperex Technology) and Jiangsu HSC New Energy Materials are developing specialized electrolyte additives, while research institutions like Central South University contribute fundamental knowledge. The competitive landscape features both established chemical manufacturers and emerging specialized materials companies focusing on novel solvent systems and lithium salt interactions.
LG Chem Ltd.
Technical Solution: LG Chem has developed advanced electrolyte systems focusing on lithium chloride solubility in non-aqueous media, particularly for lithium-ion battery applications. Their approach involves using mixed organic solvent systems combining cyclic carbonates (ethylene carbonate, propylene carbonate) with linear carbonates (dimethyl carbonate, ethyl methyl carbonate) to enhance LiCl solubility. The company has pioneered the use of additives such as crown ethers and glymes that act as chelating agents to coordinate with lithium ions, effectively breaking the strong ion-pairing between Li+ and Cl- ions. This technology has enabled them to achieve LiCl solubility up to 0.5M in certain non-aqueous formulations, significantly higher than conventional systems. LG Chem has also developed temperature-dependent solubility control mechanisms that allow for dynamic adjustment of LiCl concentration in electrolytes during battery operation cycles.
Strengths: Superior lithium-ion coordination chemistry that enables higher LiCl solubility than competitors; extensive intellectual property portfolio in electrolyte formulations; integrated supply chain for raw materials. Weaknesses: Higher production costs compared to standard electrolytes; some formulations show stability issues at extreme temperatures; limited compatibility with certain cathode materials.
Central Glass Co., Ltd.
Technical Solution: Central Glass has pioneered the "Fluorinated Solvent Enhancement Technology" (FSET) for improving lithium chloride solubility in non-aqueous media. Their approach leverages the company's expertise in fluorochemistry to develop partially fluorinated ethers and carbonates that demonstrate superior LiCl solvation properties. These fluorinated solvents feature modified electron density distributions that interact favorably with both Li+ and Cl- ions, disrupting the strong ionic lattice. Central Glass's research has shown that incorporating specific fluorinated structural motifs can increase LiCl solubility by up to 250% compared to their non-fluorinated analogs. Their technology also employs macrocyclic polyether additives that selectively coordinate with lithium ions, further enhancing dissolution. The company has developed a proprietary solvent blending process that creates stable microenvironments within the solution, allowing for higher overall LiCl concentrations without precipitation. This technology has been successfully implemented in their high-performance battery electrolyte formulations, demonstrating excellent electrochemical stability windows (up to 4.5V vs. Li/Li+) while maintaining good LiCl solubility.
Strengths: Unique fluorochemistry expertise provides differentiated technology; excellent electrochemical stability of resulting solutions; good compatibility with modern lithium battery systems. Weaknesses: Higher production costs for fluorinated solvents; some fluorinated compounds have environmental persistence concerns; technology requires specialized handling due to the reactive nature of some components.
Critical Patents and Literature on Non-Aqueous Lithium Salt Solubility
Non-aqueous electrolytic solution and lithium secondary battery
PatentInactiveUS7968220B2
Innovation
- A non-aqueous electrolytic solution containing a biphenyl derivative in a specific concentration range (0.001 to 0.8 weight %) is used, which forms a thin film on the positive electrode, enhancing cycling performance by preventing excessive decomposition and maintaining high electric capacity, especially at high temperatures and voltages.
Cathode materials having high energy density and lithium secondary battery containing the same
PatentActiveUS20120225343A1
Innovation
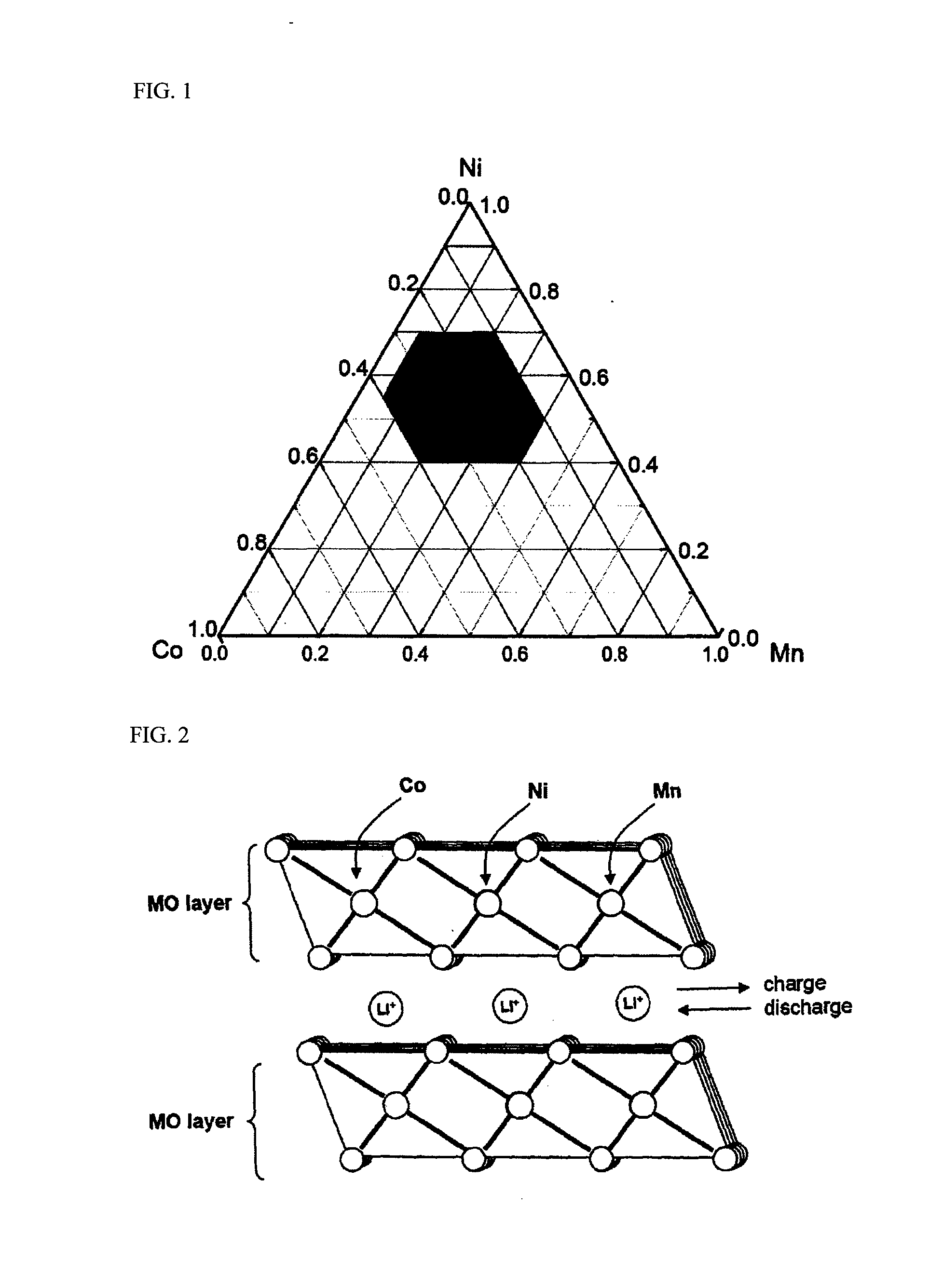
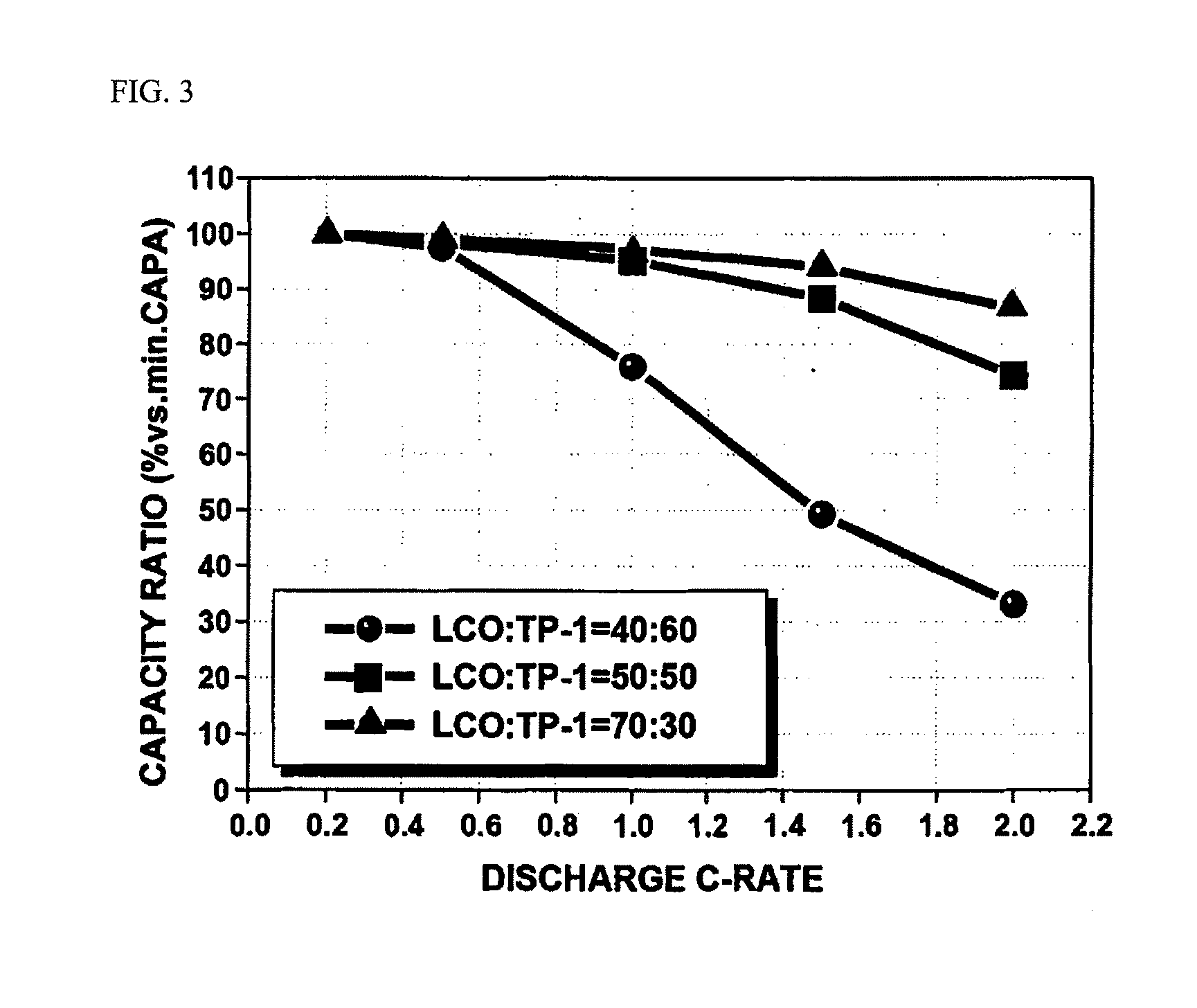
- A cathode material comprising a mixture of two lithium transition metal oxides with specific compositions, oxide powder (a) and oxide powder (b), in a 50:50 to 90:10 weight ratio, where oxide powder (b) has a transition metal mixture with an average oxidation number greater than +3, stabilizing crystal structures and enhancing energy density and capacity properties.
Thermodynamic Models for Predicting Salt Solubility
Thermodynamic models serve as essential tools for predicting lithium chloride solubility in non-aqueous media, offering theoretical frameworks that describe the complex interactions between solute and solvent molecules. These models provide systematic approaches to understanding solubility phenomena beyond empirical observations, enabling researchers to make predictions across various conditions without extensive experimental work.
The NRTL (Non-Random Two-Liquid) model has demonstrated particular efficacy for lithium salt systems in organic solvents. This model accounts for the non-ideality of liquid mixtures by considering local molecular compositions that differ from bulk composition due to molecular interactions. For lithium chloride in solvents like dimethyl sulfoxide or acetonitrile, NRTL parameters can be calibrated to predict solubility with error margins below 5% across wide temperature ranges.
UNIQUAC (UNIversal QUAsiChemical) represents another powerful approach, incorporating both combinatorial and residual contributions to activity coefficients. The combinatorial term accounts for molecular size and shape differences, while the residual term addresses energy interactions. This distinction makes UNIQUAC particularly valuable for lithium chloride systems where the ionic nature creates significant deviations from ideal behavior in non-aqueous media.
Modified Wilson equations have been adapted specifically for electrolyte systems like lithium chloride. These modifications incorporate terms for ion-solvent interactions that standard models often neglect. Research indicates that modified Wilson models can accurately predict lithium chloride solubility in mixed solvent systems, such as alcohol-ether combinations, which are relevant for battery electrolyte applications.
The Pitzer ion interaction model, though originally developed for aqueous systems, has been extended to non-aqueous media with promising results. By incorporating parameters that account for short-range and long-range interactions between ions and solvent molecules, the extended Pitzer model has demonstrated accuracy in predicting lithium chloride solubility in solvents like propylene carbonate and dimethoxyethane.
Molecular dynamics simulations complement these thermodynamic models by providing atomistic insights into solvation mechanisms. These simulations can generate parameters for continuum models, creating a multi-scale modeling approach that bridges microscopic interactions with macroscopic solubility predictions. Recent advances in computational efficiency have made these simulations increasingly practical for industrial applications.
Machine learning approaches are emerging as powerful tools for solubility prediction, particularly when trained on experimental data combined with descriptors derived from thermodynamic models. Neural networks and Gaussian process regression models have demonstrated superior predictive capability for lithium salt solubility compared to traditional correlation equations, especially when dealing with complex solvent mixtures relevant to advanced battery technologies.
The NRTL (Non-Random Two-Liquid) model has demonstrated particular efficacy for lithium salt systems in organic solvents. This model accounts for the non-ideality of liquid mixtures by considering local molecular compositions that differ from bulk composition due to molecular interactions. For lithium chloride in solvents like dimethyl sulfoxide or acetonitrile, NRTL parameters can be calibrated to predict solubility with error margins below 5% across wide temperature ranges.
UNIQUAC (UNIversal QUAsiChemical) represents another powerful approach, incorporating both combinatorial and residual contributions to activity coefficients. The combinatorial term accounts for molecular size and shape differences, while the residual term addresses energy interactions. This distinction makes UNIQUAC particularly valuable for lithium chloride systems where the ionic nature creates significant deviations from ideal behavior in non-aqueous media.
Modified Wilson equations have been adapted specifically for electrolyte systems like lithium chloride. These modifications incorporate terms for ion-solvent interactions that standard models often neglect. Research indicates that modified Wilson models can accurately predict lithium chloride solubility in mixed solvent systems, such as alcohol-ether combinations, which are relevant for battery electrolyte applications.
The Pitzer ion interaction model, though originally developed for aqueous systems, has been extended to non-aqueous media with promising results. By incorporating parameters that account for short-range and long-range interactions between ions and solvent molecules, the extended Pitzer model has demonstrated accuracy in predicting lithium chloride solubility in solvents like propylene carbonate and dimethoxyethane.
Molecular dynamics simulations complement these thermodynamic models by providing atomistic insights into solvation mechanisms. These simulations can generate parameters for continuum models, creating a multi-scale modeling approach that bridges microscopic interactions with macroscopic solubility predictions. Recent advances in computational efficiency have made these simulations increasingly practical for industrial applications.
Machine learning approaches are emerging as powerful tools for solubility prediction, particularly when trained on experimental data combined with descriptors derived from thermodynamic models. Neural networks and Gaussian process regression models have demonstrated superior predictive capability for lithium salt solubility compared to traditional correlation equations, especially when dealing with complex solvent mixtures relevant to advanced battery technologies.
Environmental Impact of Non-Aqueous Lithium Processing
The environmental implications of lithium processing in non-aqueous media represent a critical consideration in the sustainable development of lithium-based technologies. Traditional aqueous lithium extraction methods typically involve extensive water consumption, land disruption, and potential contamination of water resources with chemicals used in processing. Non-aqueous lithium processing offers potential alternatives that may mitigate some of these environmental concerns.
Non-aqueous solvents used for lithium chloride processing, such as organic solvents, ionic liquids, and deep eutectic solvents, generally require less water than conventional methods. This reduction in water usage is particularly significant in arid regions where lithium brine operations compete with agricultural and community water needs. However, these alternative solvents introduce their own environmental challenges, including potential toxicity, biodegradability issues, and atmospheric emissions.
The carbon footprint of non-aqueous processing must be carefully evaluated. While some non-aqueous methods may reduce direct water impacts, they often require more energy for solvent recovery and recycling. This energy demand can lead to increased greenhouse gas emissions unless powered by renewable energy sources. Life cycle assessments comparing aqueous and non-aqueous processing routes indicate varying environmental trade-offs depending on specific process configurations and regional energy mixes.
Waste management presents another significant environmental consideration. Non-aqueous processing generates different waste streams compared to traditional methods, potentially including spent organic solvents, degradation products, and contaminated filter materials. The environmental persistence of these materials varies widely, with some synthetic solvents having limited biodegradability and potential for bioaccumulation in ecosystems.
Regulatory frameworks for non-aqueous lithium processing are still evolving in many jurisdictions. Current environmental regulations may not adequately address the specific risks associated with novel solvents and processing techniques. This regulatory gap creates uncertainty for technology developers and potential environmental vulnerabilities if harmful practices are not properly controlled.
Emerging research in green chemistry offers promising directions for environmentally responsible non-aqueous lithium processing. Bio-derived solvents, closed-loop solvent recycling systems, and hybrid processing approaches that minimize environmental impacts while maintaining economic viability represent active areas of investigation. These innovations could potentially transform lithium processing into a more environmentally sustainable practice.
The geographical distribution of environmental impacts also shifts with non-aqueous processing. While traditional methods concentrate environmental burdens in lithium-rich regions, non-aqueous processing may distribute impacts differently across the supply chain, affecting areas where specialized chemicals are manufactured or where processing facilities are located.
Non-aqueous solvents used for lithium chloride processing, such as organic solvents, ionic liquids, and deep eutectic solvents, generally require less water than conventional methods. This reduction in water usage is particularly significant in arid regions where lithium brine operations compete with agricultural and community water needs. However, these alternative solvents introduce their own environmental challenges, including potential toxicity, biodegradability issues, and atmospheric emissions.
The carbon footprint of non-aqueous processing must be carefully evaluated. While some non-aqueous methods may reduce direct water impacts, they often require more energy for solvent recovery and recycling. This energy demand can lead to increased greenhouse gas emissions unless powered by renewable energy sources. Life cycle assessments comparing aqueous and non-aqueous processing routes indicate varying environmental trade-offs depending on specific process configurations and regional energy mixes.
Waste management presents another significant environmental consideration. Non-aqueous processing generates different waste streams compared to traditional methods, potentially including spent organic solvents, degradation products, and contaminated filter materials. The environmental persistence of these materials varies widely, with some synthetic solvents having limited biodegradability and potential for bioaccumulation in ecosystems.
Regulatory frameworks for non-aqueous lithium processing are still evolving in many jurisdictions. Current environmental regulations may not adequately address the specific risks associated with novel solvents and processing techniques. This regulatory gap creates uncertainty for technology developers and potential environmental vulnerabilities if harmful practices are not properly controlled.
Emerging research in green chemistry offers promising directions for environmentally responsible non-aqueous lithium processing. Bio-derived solvents, closed-loop solvent recycling systems, and hybrid processing approaches that minimize environmental impacts while maintaining economic viability represent active areas of investigation. These innovations could potentially transform lithium processing into a more environmentally sustainable practice.
The geographical distribution of environmental impacts also shifts with non-aqueous processing. While traditional methods concentrate environmental burdens in lithium-rich regions, non-aqueous processing may distribute impacts differently across the supply chain, affecting areas where specialized chemicals are manufactured or where processing facilities are located.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!