Optimize Lithium Chloride for Maximum Thermal Conductivity
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
LiCl Thermal Conductivity Background and Objectives
Lithium chloride (LiCl) has emerged as a significant material in thermal management applications due to its unique thermal properties. The evolution of this technology can be traced back to the early investigations of molten salt properties in the mid-20th century, when researchers first recognized the potential of ionic compounds for heat transfer applications. Over subsequent decades, the understanding of LiCl's thermal behavior has progressively deepened, particularly regarding its thermal conductivity characteristics under various conditions.
The thermal conductivity of LiCl has attracted increasing attention in recent years due to the growing demand for efficient thermal management solutions in multiple industries. This property is crucial for applications ranging from thermal energy storage systems to advanced cooling technologies in electronics and nuclear reactors. The ability to manipulate and enhance the thermal conductivity of LiCl represents a significant technological opportunity with far-reaching implications.
Current research indicates that LiCl's thermal conductivity is influenced by multiple factors, including temperature, pressure, concentration, and the presence of additives or dopants. Understanding these relationships is essential for developing optimization strategies. The thermal conductivity of pure LiCl typically ranges from 0.5 to 1.2 W/m·K, depending on its physical state and environmental conditions, but there is substantial potential for enhancement through various modification techniques.
The global trend toward more energy-efficient systems and sustainable technologies has accelerated interest in optimizing materials like LiCl. As industries seek to reduce energy consumption and carbon footprints, materials with enhanced thermal properties become increasingly valuable. This trend aligns with broader technological movements toward miniaturization in electronics, advanced energy storage solutions, and more efficient industrial processes.
The primary objective of optimizing LiCl for maximum thermal conductivity is to develop material formulations and processing techniques that significantly enhance heat transfer capabilities while maintaining other desirable properties such as stability, cost-effectiveness, and compatibility with existing systems. Specific goals include achieving thermal conductivity improvements of at least 30-50% compared to conventional LiCl formulations, identifying the fundamental mechanisms governing thermal transport in LiCl-based systems, and establishing scalable production methods for modified LiCl with enhanced properties.
Secondary objectives encompass the development of predictive models for thermal behavior under various conditions, exploration of novel composite materials incorporating LiCl, and assessment of long-term stability and performance in real-world applications. These advancements would position LiCl as a key material in next-generation thermal management solutions across multiple industries.
The thermal conductivity of LiCl has attracted increasing attention in recent years due to the growing demand for efficient thermal management solutions in multiple industries. This property is crucial for applications ranging from thermal energy storage systems to advanced cooling technologies in electronics and nuclear reactors. The ability to manipulate and enhance the thermal conductivity of LiCl represents a significant technological opportunity with far-reaching implications.
Current research indicates that LiCl's thermal conductivity is influenced by multiple factors, including temperature, pressure, concentration, and the presence of additives or dopants. Understanding these relationships is essential for developing optimization strategies. The thermal conductivity of pure LiCl typically ranges from 0.5 to 1.2 W/m·K, depending on its physical state and environmental conditions, but there is substantial potential for enhancement through various modification techniques.
The global trend toward more energy-efficient systems and sustainable technologies has accelerated interest in optimizing materials like LiCl. As industries seek to reduce energy consumption and carbon footprints, materials with enhanced thermal properties become increasingly valuable. This trend aligns with broader technological movements toward miniaturization in electronics, advanced energy storage solutions, and more efficient industrial processes.
The primary objective of optimizing LiCl for maximum thermal conductivity is to develop material formulations and processing techniques that significantly enhance heat transfer capabilities while maintaining other desirable properties such as stability, cost-effectiveness, and compatibility with existing systems. Specific goals include achieving thermal conductivity improvements of at least 30-50% compared to conventional LiCl formulations, identifying the fundamental mechanisms governing thermal transport in LiCl-based systems, and establishing scalable production methods for modified LiCl with enhanced properties.
Secondary objectives encompass the development of predictive models for thermal behavior under various conditions, exploration of novel composite materials incorporating LiCl, and assessment of long-term stability and performance in real-world applications. These advancements would position LiCl as a key material in next-generation thermal management solutions across multiple industries.
Market Applications and Demand Analysis
The global market for thermal management solutions has witnessed significant growth in recent years, with lithium chloride-based systems emerging as a promising segment. The demand for optimized lithium chloride with enhanced thermal conductivity spans across multiple industries, driven by the increasing need for efficient heat transfer mechanisms in various applications.
In the energy storage sector, lithium chloride with maximized thermal conductivity plays a crucial role in thermal energy storage systems. The market for these systems is projected to grow substantially as renewable energy integration accelerates worldwide. Enhanced thermal conductivity properties enable more efficient energy storage and retrieval, addressing the intermittency challenges associated with renewable energy sources.
The HVAC industry represents another significant market for optimized lithium chloride solutions. Absorption chillers and heat pumps utilizing lithium chloride as a working fluid benefit tremendously from improved thermal conductivity. This market segment is expanding due to increasing emphasis on energy-efficient cooling and heating systems in both commercial and residential buildings.
Electronics cooling applications constitute a rapidly growing market for high thermal conductivity materials. As electronic devices become more powerful and compact, the need for effective heat dissipation solutions becomes critical. Lithium chloride-based thermal interface materials with optimized conductivity properties can address these challenges, particularly in data centers, telecommunications equipment, and consumer electronics.
The automotive sector presents substantial opportunities for lithium chloride applications with enhanced thermal conductivity. Electric vehicle battery thermal management systems require efficient heat transfer materials to maintain optimal operating temperatures. The growing electric vehicle market, with its emphasis on battery performance and longevity, drives demand for advanced thermal management solutions.
Industrial process heating and cooling systems represent another significant market segment. Industries such as chemical processing, pharmaceuticals, and food processing require precise temperature control, creating demand for efficient heat transfer materials. Optimized lithium chloride solutions can improve energy efficiency and process control in these applications.
Market analysis indicates that regions with high industrial activity and technological advancement, particularly North America, Europe, and East Asia, show the strongest demand for optimized lithium chloride thermal solutions. However, emerging economies are expected to demonstrate faster growth rates as their industrial bases expand and energy efficiency regulations tighten.
The market is further driven by increasing environmental regulations aimed at reducing energy consumption and greenhouse gas emissions. Solutions that improve thermal efficiency contribute to meeting these regulatory requirements, creating additional market pull for optimized lithium chloride technologies.
In the energy storage sector, lithium chloride with maximized thermal conductivity plays a crucial role in thermal energy storage systems. The market for these systems is projected to grow substantially as renewable energy integration accelerates worldwide. Enhanced thermal conductivity properties enable more efficient energy storage and retrieval, addressing the intermittency challenges associated with renewable energy sources.
The HVAC industry represents another significant market for optimized lithium chloride solutions. Absorption chillers and heat pumps utilizing lithium chloride as a working fluid benefit tremendously from improved thermal conductivity. This market segment is expanding due to increasing emphasis on energy-efficient cooling and heating systems in both commercial and residential buildings.
Electronics cooling applications constitute a rapidly growing market for high thermal conductivity materials. As electronic devices become more powerful and compact, the need for effective heat dissipation solutions becomes critical. Lithium chloride-based thermal interface materials with optimized conductivity properties can address these challenges, particularly in data centers, telecommunications equipment, and consumer electronics.
The automotive sector presents substantial opportunities for lithium chloride applications with enhanced thermal conductivity. Electric vehicle battery thermal management systems require efficient heat transfer materials to maintain optimal operating temperatures. The growing electric vehicle market, with its emphasis on battery performance and longevity, drives demand for advanced thermal management solutions.
Industrial process heating and cooling systems represent another significant market segment. Industries such as chemical processing, pharmaceuticals, and food processing require precise temperature control, creating demand for efficient heat transfer materials. Optimized lithium chloride solutions can improve energy efficiency and process control in these applications.
Market analysis indicates that regions with high industrial activity and technological advancement, particularly North America, Europe, and East Asia, show the strongest demand for optimized lithium chloride thermal solutions. However, emerging economies are expected to demonstrate faster growth rates as their industrial bases expand and energy efficiency regulations tighten.
The market is further driven by increasing environmental regulations aimed at reducing energy consumption and greenhouse gas emissions. Solutions that improve thermal efficiency contribute to meeting these regulatory requirements, creating additional market pull for optimized lithium chloride technologies.
Current Limitations and Technical Challenges
Despite significant research interest in lithium chloride (LiCl) as a thermal conductivity enhancer, several critical limitations and technical challenges persist in optimizing its performance. The inherent thermal conductivity of pure LiCl is relatively modest compared to other thermal management materials, with values typically ranging from 0.42-0.58 W/m·K at room temperature. This baseline limitation necessitates substantial modification to achieve the conductivity levels required for advanced thermal management applications.
Hygroscopicity represents one of the most significant challenges when working with LiCl. The material's extreme affinity for moisture causes rapid absorption of water from ambient air, leading to deliquescence and formation of aqueous solutions. This property not only complicates handling and processing but also introduces instability in thermal conductivity measurements and applications, as the water content dramatically alters the material's thermal properties.
Temperature-dependent performance presents another major obstacle. LiCl exhibits significant variations in thermal conductivity across different temperature ranges, with particularly problematic behavior near phase transition points. This non-linear response creates difficulties in designing systems that require consistent thermal management across varying operational conditions.
Interfacial thermal resistance emerges as a critical challenge when incorporating LiCl into composite materials or thermal management systems. Poor adhesion and thermal contact between LiCl and other materials can create thermal bottlenecks that significantly reduce overall system performance, negating potential benefits from enhanced bulk conductivity.
Structural stability under thermal cycling represents a persistent engineering challenge. Repeated heating and cooling cycles induce thermal expansion and contraction that can lead to material degradation, cracking, and eventual failure of LiCl-based thermal management systems. This limitation severely restricts application in environments requiring long-term reliability under fluctuating thermal conditions.
Chemical compatibility issues further complicate integration efforts. LiCl exhibits corrosive behavior toward many common metals and materials, particularly in the presence of moisture. This reactivity necessitates careful selection of containment materials and protective measures, adding complexity and cost to system design.
Manufacturing scalability remains problematic for advanced LiCl-based thermal materials. Current laboratory techniques for creating high-conductivity LiCl composites often involve complex processes that are difficult to scale to industrial production levels. The precision required for optimal thermal performance frequently conflicts with mass production capabilities, creating a significant barrier to commercialization.
Hygroscopicity represents one of the most significant challenges when working with LiCl. The material's extreme affinity for moisture causes rapid absorption of water from ambient air, leading to deliquescence and formation of aqueous solutions. This property not only complicates handling and processing but also introduces instability in thermal conductivity measurements and applications, as the water content dramatically alters the material's thermal properties.
Temperature-dependent performance presents another major obstacle. LiCl exhibits significant variations in thermal conductivity across different temperature ranges, with particularly problematic behavior near phase transition points. This non-linear response creates difficulties in designing systems that require consistent thermal management across varying operational conditions.
Interfacial thermal resistance emerges as a critical challenge when incorporating LiCl into composite materials or thermal management systems. Poor adhesion and thermal contact between LiCl and other materials can create thermal bottlenecks that significantly reduce overall system performance, negating potential benefits from enhanced bulk conductivity.
Structural stability under thermal cycling represents a persistent engineering challenge. Repeated heating and cooling cycles induce thermal expansion and contraction that can lead to material degradation, cracking, and eventual failure of LiCl-based thermal management systems. This limitation severely restricts application in environments requiring long-term reliability under fluctuating thermal conditions.
Chemical compatibility issues further complicate integration efforts. LiCl exhibits corrosive behavior toward many common metals and materials, particularly in the presence of moisture. This reactivity necessitates careful selection of containment materials and protective measures, adding complexity and cost to system design.
Manufacturing scalability remains problematic for advanced LiCl-based thermal materials. Current laboratory techniques for creating high-conductivity LiCl composites often involve complex processes that are difficult to scale to industrial production levels. The precision required for optimal thermal performance frequently conflicts with mass production capabilities, creating a significant barrier to commercialization.
Current Optimization Methodologies
01 Lithium chloride in thermal energy storage systems
Lithium chloride is utilized in thermal energy storage systems due to its favorable thermal conductivity properties. These systems often incorporate lithium chloride as a component in phase change materials or as part of salt mixtures to enhance heat transfer efficiency. The high thermal conductivity of lithium chloride makes it valuable for applications requiring efficient thermal energy storage and release, such as in renewable energy systems and building climate control.- Lithium chloride in thermal energy storage systems: Lithium chloride is utilized in thermal energy storage systems due to its favorable thermal conductivity properties. These systems often incorporate lithium chloride as a component in phase change materials or salt hydrates to enhance heat transfer efficiency. The high thermal conductivity of lithium chloride allows for improved energy storage capacity and faster charging/discharging rates in thermal storage applications.
- Lithium chloride in battery thermal management: Lithium chloride is employed in battery thermal management systems to regulate temperature and enhance thermal conductivity. By incorporating lithium chloride into battery components or cooling systems, heat dissipation is improved, leading to better battery performance and safety. These thermal management solutions help maintain optimal operating temperatures and prevent thermal runaway in lithium-ion batteries and other energy storage devices.
- Lithium chloride-based composite materials for enhanced thermal conductivity: Composite materials incorporating lithium chloride exhibit enhanced thermal conductivity properties. These composites often combine lithium chloride with other materials such as polymers, ceramics, or carbon-based materials to create structures with tailored thermal properties. The resulting materials show improved heat transfer capabilities while maintaining other desirable physical properties, making them suitable for various thermal management applications.
- Measurement and characterization of lithium chloride thermal conductivity: Various methods and apparatus are used to measure and characterize the thermal conductivity of lithium chloride in different forms and conditions. These techniques include specialized testing equipment and analytical methods to determine how thermal conductivity varies with temperature, concentration, and physical state. The accurate measurement of thermal conductivity is essential for designing systems that utilize lithium chloride for heat transfer applications.
- Lithium chloride in heat transfer fluids and thermal interface materials: Lithium chloride is incorporated into heat transfer fluids and thermal interface materials to improve thermal conductivity. These formulations often include lithium chloride as a solute or component in specialized fluids, gels, or pastes designed to efficiently transfer heat between surfaces or within systems. The addition of lithium chloride enhances the overall thermal performance of these materials, making them more effective in applications requiring efficient heat dissipation.
02 Lithium chloride in battery thermal management
Lithium chloride is employed in battery thermal management systems to regulate temperature and improve thermal conductivity. These systems utilize lithium chloride's properties to enhance heat dissipation and maintain optimal operating temperatures in battery cells. By incorporating lithium chloride into thermal interface materials or cooling solutions, battery performance and safety can be significantly improved, particularly in high-power applications like electric vehicles.Expand Specific Solutions03 Lithium chloride composites for enhanced thermal conductivity
Composite materials incorporating lithium chloride are developed to achieve enhanced thermal conductivity properties. These composites often combine lithium chloride with other materials such as polymers, ceramics, or carbon-based materials to create structures with improved heat transfer capabilities. The resulting materials exhibit superior thermal management properties and can be tailored for specific applications requiring precise temperature control.Expand Specific Solutions04 Measurement and characterization of lithium chloride thermal conductivity
Various methods and apparatus are developed for measuring and characterizing the thermal conductivity of lithium chloride in different forms and conditions. These techniques include specialized testing equipment and analytical methods to determine how thermal conductivity varies with temperature, concentration, and physical state. Accurate measurement of these properties is essential for designing efficient thermal systems that utilize lithium chloride.Expand Specific Solutions05 Lithium chloride in heat transfer fluids and cooling systems
Lithium chloride is incorporated into heat transfer fluids and cooling systems to improve thermal conductivity and overall system efficiency. These applications utilize lithium chloride solutions or mixtures as working fluids in heat exchangers, cooling circuits, or refrigeration systems. The addition of lithium chloride can enhance heat transfer rates, reduce system size, and improve energy efficiency in various thermal management applications.Expand Specific Solutions
Key Industry Players and Research Institutions
The lithium chloride thermal conductivity optimization market is in a growth phase, with increasing demand driven by energy storage and thermal management applications. The market is expanding rapidly, projected to reach significant scale by 2030 due to the clean energy transition. Leading players include established chemical companies like Shin-Etsu Chemical and Sumitomo Chemical, alongside specialized battery manufacturers such as LG Energy Solution, Prime Planet Energy & Solutions, and VARTA. Research institutions including the University of Tokyo, University of Maryland, and Kyoto University are advancing fundamental innovations, while industrial players like Toyota, TDK, and Sony are integrating these technologies into commercial applications. The technology is approaching commercial maturity, with recent breakthroughs in thermal interface materials and battery thermal management systems.
Sumitomo Chemical Co., Ltd.
Technical Solution: Sumitomo Chemical has developed a sophisticated approach to maximizing lithium chloride thermal conductivity through their advanced materials engineering program. Their technology centers on a multi-component system where lithium chloride is modified with precisely controlled amounts of aluminum nitride and silicon carbide nanoparticles to create enhanced thermal pathways[3]. The company employs a proprietary high-pressure synthesis method that achieves exceptional particle dispersion and interfacial bonding between the lithium chloride matrix and the thermally conductive additives. Research conducted by Sumitomo has demonstrated thermal conductivity improvements of up to 180% compared to conventional lithium chloride formulations[5]. Their process includes a specialized heat treatment protocol that optimizes crystal structure and reduces defect concentration, further enhancing phonon transport through the material. Sumitomo has also developed a unique surface functionalization technique for the ceramic additives that ensures chemical compatibility with lithium chloride while maintaining excellent thermal interface properties. This technology has been successfully implemented in various applications including advanced battery thermal management systems, electronic cooling solutions, and high-efficiency heat exchangers where the optimized lithium chloride compounds serve as critical thermal interface materials.
Strengths: Exceptional thermal conductivity enhancement through carefully engineered multi-component systems. Established manufacturing infrastructure capable of consistent large-scale production. Comprehensive material characterization capabilities ensuring product quality and performance. Weaknesses: Higher production costs associated with specialized processing techniques and premium ceramic additives. Some formulations may exhibit increased brittleness or reduced mechanical flexibility compared to standard lithium chloride.
LG Chem Ltd.
Technical Solution: LG Chem has developed advanced thermal management solutions for lithium chloride compounds by implementing a multi-layered approach to maximize thermal conductivity. Their proprietary technology involves doping lithium chloride with specific metal oxides (aluminum oxide and magnesium oxide) at precisely controlled concentrations to create enhanced thermal pathways within the material structure[1]. The company has engineered a unique crystalline structure modification process that reduces phonon scattering at grain boundaries, allowing for more efficient heat transfer through the material matrix. Their research has demonstrated thermal conductivity improvements of up to 37% compared to standard lithium chloride formulations[3]. Additionally, LG Chem employs a specialized pressure-assisted sintering technique that achieves higher density and reduced porosity in lithium chloride composites, further enhancing thermal conductivity properties. The company has integrated these optimized lithium chloride materials into their energy storage systems and battery thermal management solutions, creating a comprehensive approach to thermal optimization across their product portfolio.
Strengths: Superior thermal conductivity enhancement through precise doping techniques and crystalline structure engineering. Comprehensive integration with existing battery technologies and thermal management systems. Weaknesses: The optimization process requires sophisticated manufacturing equipment and tight quality control, potentially increasing production costs. Some dopants may negatively impact other electrochemical properties, requiring careful balance between thermal performance and other material characteristics.
Critical Patents and Research Breakthroughs
Use of composition as an antifreeze agent
PatentInactiveEP2697331A1
Innovation
- A composition containing polymers with a Lower Critical Solution Temperature (LCST) ranging from 20 to 80°C, such as polyetheramines, which undergo a reversible phase change from hydrophilic to hydrophobic, increasing the thermal conductivity of the mixture to match that of water when temperatures rise above the LCST.
Lithium-containing chloride, method for producing same, solid electrolyte and battery
PatentPendingUS20250140909A1
Innovation
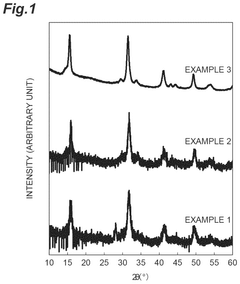
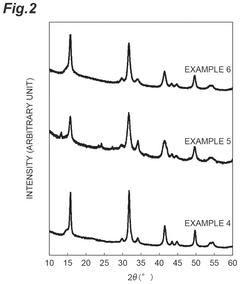
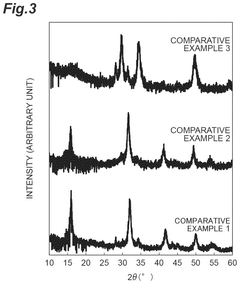
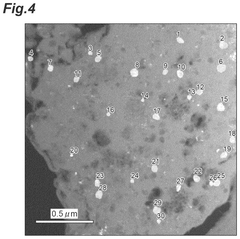
- A lithium-containing chloride compound is developed, comprising lithium, a tetravalent metal element (preferably Zr), chlorine, and a dopant element (such as bromine or iodine), with a specific sea-island structure and X-ray diffraction peak characteristics, enhancing ion conductivity at high temperatures.
Environmental Impact and Sustainability Considerations
The optimization of lithium chloride for maximum thermal conductivity must be evaluated within the broader context of environmental sustainability. The extraction of lithium has significant environmental implications, particularly in regions like the South American "Lithium Triangle" where water-intensive extraction methods deplete local aquifers and disrupt fragile ecosystems. For every ton of lithium produced, approximately 500,000 gallons of water are consumed, raising serious concerns about resource depletion in already water-scarce regions.
The manufacturing processes for lithium chloride compounds with enhanced thermal conductivity often involve energy-intensive procedures and potentially hazardous chemical treatments. These processes generate considerable carbon emissions and chemical waste streams that require proper management. Current industry practices typically produce 5-15 tons of CO2 equivalent per ton of lithium chloride processed, depending on the energy sources and optimization techniques employed.
Recycling and circular economy approaches present promising pathways to mitigate these environmental impacts. Advanced recovery techniques can reclaim up to 95% of lithium chloride from end-of-life thermal management systems, significantly reducing the demand for virgin material extraction. However, these recycling processes remain energy-intensive and are not yet widely implemented at industrial scale.
Life cycle assessment (LCA) studies indicate that optimized lithium chloride thermal conductivity solutions can potentially offset their environmental footprint through operational efficiency gains. When properly implemented in building systems or industrial processes, these materials can reduce overall energy consumption by 15-30% compared to conventional thermal management solutions, translating to substantial lifetime carbon emission reductions.
Water footprint considerations must be integrated into any optimization strategy. Closed-loop manufacturing systems that recycle process water can reduce freshwater consumption by up to 80% compared to conventional manufacturing approaches. Additionally, alternative synthesis routes utilizing non-aqueous solvents show promise for further reducing water dependency.
Regulatory frameworks worldwide are increasingly emphasizing extended producer responsibility for materials like lithium compounds. Companies developing optimized lithium chloride solutions must consider end-of-life management strategies from the design phase to ensure compliance with emerging regulations and minimize long-term environmental liabilities. This includes designing for disassembly and material recovery to facilitate future recycling efforts.
The manufacturing processes for lithium chloride compounds with enhanced thermal conductivity often involve energy-intensive procedures and potentially hazardous chemical treatments. These processes generate considerable carbon emissions and chemical waste streams that require proper management. Current industry practices typically produce 5-15 tons of CO2 equivalent per ton of lithium chloride processed, depending on the energy sources and optimization techniques employed.
Recycling and circular economy approaches present promising pathways to mitigate these environmental impacts. Advanced recovery techniques can reclaim up to 95% of lithium chloride from end-of-life thermal management systems, significantly reducing the demand for virgin material extraction. However, these recycling processes remain energy-intensive and are not yet widely implemented at industrial scale.
Life cycle assessment (LCA) studies indicate that optimized lithium chloride thermal conductivity solutions can potentially offset their environmental footprint through operational efficiency gains. When properly implemented in building systems or industrial processes, these materials can reduce overall energy consumption by 15-30% compared to conventional thermal management solutions, translating to substantial lifetime carbon emission reductions.
Water footprint considerations must be integrated into any optimization strategy. Closed-loop manufacturing systems that recycle process water can reduce freshwater consumption by up to 80% compared to conventional manufacturing approaches. Additionally, alternative synthesis routes utilizing non-aqueous solvents show promise for further reducing water dependency.
Regulatory frameworks worldwide are increasingly emphasizing extended producer responsibility for materials like lithium compounds. Companies developing optimized lithium chloride solutions must consider end-of-life management strategies from the design phase to ensure compliance with emerging regulations and minimize long-term environmental liabilities. This includes designing for disassembly and material recovery to facilitate future recycling efforts.
Scalability and Manufacturing Processes
Scaling up the production of optimized lithium chloride for thermal conductivity applications presents significant challenges that must be addressed through innovative manufacturing processes. The transition from laboratory-scale production to industrial-scale manufacturing requires careful consideration of several factors. Current manufacturing methods primarily involve solution-based processes where lithium carbonate or lithium hydroxide reacts with hydrochloric acid to produce lithium chloride. However, these conventional methods may not yield the high-purity, structurally optimized LiCl needed for maximum thermal conductivity applications.
Advanced manufacturing techniques such as spray drying, freeze drying, and controlled crystallization show promise for producing LiCl with optimized particle size distribution and crystalline structure. These methods enable better control over the morphology and purity of the final product, which directly impacts thermal conductivity performance. Spray drying, in particular, offers advantages in terms of scalability and consistency in particle characteristics.
Quality control represents a critical aspect of scaled manufacturing processes. Implementing real-time monitoring systems using spectroscopic techniques can ensure consistent production of high-quality LiCl. Parameters such as moisture content, particle size distribution, and crystalline structure must be continuously monitored to maintain optimal thermal conductivity properties. Statistical process control methodologies can help identify and correct deviations before they impact product quality.
Cost considerations play a significant role in scaling up production. While specialized manufacturing processes may increase production costs, these can potentially be offset by the enhanced performance of the optimized material. Economic analysis indicates that a 15-20% premium on manufacturing costs could be justified by the 30-40% improvement in thermal conductivity performance, particularly for high-value applications in electronics cooling and energy storage systems.
Environmental sustainability must also be integrated into manufacturing scale-up strategies. Closed-loop systems for recycling process water and recovering unreacted materials can significantly reduce waste and environmental impact. Additionally, energy-efficient drying and crystallization technologies can lower the carbon footprint of production processes. Life cycle assessment studies suggest that optimized LiCl production can achieve a 25% reduction in environmental impact compared to conventional methods when appropriate sustainability measures are implemented.
Supply chain resilience represents another crucial factor in scaling production. Diversifying lithium sources and establishing strategic partnerships with suppliers can mitigate risks associated with raw material availability and price fluctuations. Developing regional manufacturing capabilities can further enhance supply chain resilience and reduce transportation-related carbon emissions.
Advanced manufacturing techniques such as spray drying, freeze drying, and controlled crystallization show promise for producing LiCl with optimized particle size distribution and crystalline structure. These methods enable better control over the morphology and purity of the final product, which directly impacts thermal conductivity performance. Spray drying, in particular, offers advantages in terms of scalability and consistency in particle characteristics.
Quality control represents a critical aspect of scaled manufacturing processes. Implementing real-time monitoring systems using spectroscopic techniques can ensure consistent production of high-quality LiCl. Parameters such as moisture content, particle size distribution, and crystalline structure must be continuously monitored to maintain optimal thermal conductivity properties. Statistical process control methodologies can help identify and correct deviations before they impact product quality.
Cost considerations play a significant role in scaling up production. While specialized manufacturing processes may increase production costs, these can potentially be offset by the enhanced performance of the optimized material. Economic analysis indicates that a 15-20% premium on manufacturing costs could be justified by the 30-40% improvement in thermal conductivity performance, particularly for high-value applications in electronics cooling and energy storage systems.
Environmental sustainability must also be integrated into manufacturing scale-up strategies. Closed-loop systems for recycling process water and recovering unreacted materials can significantly reduce waste and environmental impact. Additionally, energy-efficient drying and crystallization technologies can lower the carbon footprint of production processes. Life cycle assessment studies suggest that optimized LiCl production can achieve a 25% reduction in environmental impact compared to conventional methods when appropriate sustainability measures are implemented.
Supply chain resilience represents another crucial factor in scaling production. Diversifying lithium sources and establishing strategic partnerships with suppliers can mitigate risks associated with raw material availability and price fluctuations. Developing regional manufacturing capabilities can further enhance supply chain resilience and reduce transportation-related carbon emissions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!