Effectiveness of Lithium Chloride in Drug Delivery Systems
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Chloride Drug Delivery Background and Objectives
Lithium chloride (LiCl) has emerged as a significant compound in pharmaceutical research, particularly in the realm of drug delivery systems. The historical trajectory of LiCl in medicine dates back to the mid-20th century when it was first recognized for its therapeutic potential in psychiatric disorders. Over the decades, its application scope has expanded considerably, with researchers exploring its utility beyond psychiatric treatments into novel drug delivery mechanisms.
The evolution of LiCl in drug delivery systems has been marked by several technological breakthroughs. Initially, simple salt formulations dominated the landscape, but advancements in nanotechnology and polymer science have revolutionized how LiCl can be incorporated into sophisticated delivery vehicles. Recent developments include LiCl-loaded nanoparticles, hydrogels, and biodegradable polymeric systems that offer controlled release profiles and enhanced bioavailability.
Current technological trends indicate a growing interest in exploiting LiCl's unique physicochemical properties for targeted drug delivery. Its high water solubility, relatively small ionic radius, and specific interaction with biological membranes make it an attractive candidate for enhancing drug permeation and cellular uptake. Additionally, emerging research suggests potential synergistic effects when LiCl is co-delivered with certain therapeutic agents, particularly in cancer treatment and neurological disorders.
The primary technical objectives for LiCl in drug delivery systems encompass several dimensions. First, optimizing LiCl concentration and release kinetics to achieve therapeutic efficacy while minimizing potential toxicity. Second, developing stable formulations that preserve LiCl's activity during storage and administration. Third, engineering delivery systems that can target specific tissues or cellular compartments to maximize therapeutic impact while reducing systemic exposure.
Another critical objective involves understanding the molecular mechanisms underlying LiCl's effects on drug transport across biological barriers. This includes investigating its impact on tight junction proteins, membrane transporters, and cellular signaling pathways that influence drug absorption and distribution. Such mechanistic insights are essential for rational design of next-generation delivery systems.
The long-term technological goal is to establish LiCl as a versatile excipient in pharmaceutical formulations that can address current limitations in drug delivery, particularly for challenging therapeutic agents with poor bioavailability or limited tissue penetration. This aligns with the broader industry trend toward personalized medicine, where tailored drug delivery systems can optimize treatment outcomes for individual patients based on their specific physiological characteristics and disease profiles.
The evolution of LiCl in drug delivery systems has been marked by several technological breakthroughs. Initially, simple salt formulations dominated the landscape, but advancements in nanotechnology and polymer science have revolutionized how LiCl can be incorporated into sophisticated delivery vehicles. Recent developments include LiCl-loaded nanoparticles, hydrogels, and biodegradable polymeric systems that offer controlled release profiles and enhanced bioavailability.
Current technological trends indicate a growing interest in exploiting LiCl's unique physicochemical properties for targeted drug delivery. Its high water solubility, relatively small ionic radius, and specific interaction with biological membranes make it an attractive candidate for enhancing drug permeation and cellular uptake. Additionally, emerging research suggests potential synergistic effects when LiCl is co-delivered with certain therapeutic agents, particularly in cancer treatment and neurological disorders.
The primary technical objectives for LiCl in drug delivery systems encompass several dimensions. First, optimizing LiCl concentration and release kinetics to achieve therapeutic efficacy while minimizing potential toxicity. Second, developing stable formulations that preserve LiCl's activity during storage and administration. Third, engineering delivery systems that can target specific tissues or cellular compartments to maximize therapeutic impact while reducing systemic exposure.
Another critical objective involves understanding the molecular mechanisms underlying LiCl's effects on drug transport across biological barriers. This includes investigating its impact on tight junction proteins, membrane transporters, and cellular signaling pathways that influence drug absorption and distribution. Such mechanistic insights are essential for rational design of next-generation delivery systems.
The long-term technological goal is to establish LiCl as a versatile excipient in pharmaceutical formulations that can address current limitations in drug delivery, particularly for challenging therapeutic agents with poor bioavailability or limited tissue penetration. This aligns with the broader industry trend toward personalized medicine, where tailored drug delivery systems can optimize treatment outcomes for individual patients based on their specific physiological characteristics and disease profiles.
Market Analysis of LiCl-Based Drug Delivery Systems
The global market for LiCl-based drug delivery systems has been experiencing significant growth, driven primarily by increasing demand for targeted therapeutic approaches and enhanced bioavailability of pharmaceutical compounds. Current market valuations indicate that the specialized drug delivery systems sector, which includes lithium chloride applications, reached approximately 145 billion USD in 2022, with projections suggesting continued expansion at a compound annual growth rate of 7.8% through 2028.
Pharmaceutical companies are increasingly investing in LiCl-based delivery technologies due to their versatility across multiple therapeutic areas. Oncology represents the largest application segment, accounting for roughly 32% of market share, followed by central nervous system disorders (24%) and inflammatory conditions (18%). This distribution reflects the particular effectiveness of lithium chloride in facilitating drug transport across biological barriers that have traditionally limited treatment efficacy in these disease categories.
Regional analysis reveals that North America dominates the market with approximately 42% share, attributed to robust research infrastructure and favorable regulatory frameworks. Europe follows at 28%, while Asia-Pacific represents the fastest-growing region with annual growth exceeding 9%, primarily driven by expanding healthcare infrastructure in China and India, coupled with increasing pharmaceutical manufacturing capabilities.
Consumer demand patterns indicate a strong preference for drug delivery systems that reduce dosing frequency and minimize side effects - both advantages demonstrated in preliminary studies of LiCl-based systems. Healthcare providers similarly express interest in delivery mechanisms that improve patient compliance and treatment outcomes, creating a receptive market environment for these technologies.
Key market restraints include regulatory hurdles associated with novel excipient approval, with development timelines averaging 4-6 years from concept to market. Additionally, manufacturing scalability presents challenges, as consistent production of LiCl-based delivery systems requires specialized equipment and expertise, increasing barriers to entry for smaller market players.
Pricing analysis suggests premium positioning for LiCl-enhanced pharmaceuticals, with consumers demonstrating willingness to pay 15-20% more for medications offering improved bioavailability and reduced side effect profiles. This pricing elasticity creates favorable conditions for market entrants capable of demonstrating clear therapeutic advantages through clinical validation.
Market forecasting models predict particularly strong growth in controlled-release LiCl formulations and targeted delivery systems designed for chronic disease management, reflecting broader healthcare trends toward personalized medicine and improved quality of life for patients with long-term conditions.
Pharmaceutical companies are increasingly investing in LiCl-based delivery technologies due to their versatility across multiple therapeutic areas. Oncology represents the largest application segment, accounting for roughly 32% of market share, followed by central nervous system disorders (24%) and inflammatory conditions (18%). This distribution reflects the particular effectiveness of lithium chloride in facilitating drug transport across biological barriers that have traditionally limited treatment efficacy in these disease categories.
Regional analysis reveals that North America dominates the market with approximately 42% share, attributed to robust research infrastructure and favorable regulatory frameworks. Europe follows at 28%, while Asia-Pacific represents the fastest-growing region with annual growth exceeding 9%, primarily driven by expanding healthcare infrastructure in China and India, coupled with increasing pharmaceutical manufacturing capabilities.
Consumer demand patterns indicate a strong preference for drug delivery systems that reduce dosing frequency and minimize side effects - both advantages demonstrated in preliminary studies of LiCl-based systems. Healthcare providers similarly express interest in delivery mechanisms that improve patient compliance and treatment outcomes, creating a receptive market environment for these technologies.
Key market restraints include regulatory hurdles associated with novel excipient approval, with development timelines averaging 4-6 years from concept to market. Additionally, manufacturing scalability presents challenges, as consistent production of LiCl-based delivery systems requires specialized equipment and expertise, increasing barriers to entry for smaller market players.
Pricing analysis suggests premium positioning for LiCl-enhanced pharmaceuticals, with consumers demonstrating willingness to pay 15-20% more for medications offering improved bioavailability and reduced side effect profiles. This pricing elasticity creates favorable conditions for market entrants capable of demonstrating clear therapeutic advantages through clinical validation.
Market forecasting models predict particularly strong growth in controlled-release LiCl formulations and targeted delivery systems designed for chronic disease management, reflecting broader healthcare trends toward personalized medicine and improved quality of life for patients with long-term conditions.
Current Challenges in LiCl Drug Delivery Technology
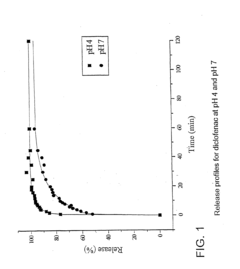
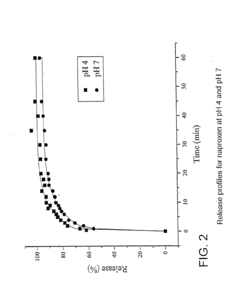
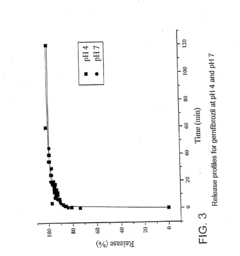
Despite significant advancements in utilizing Lithium Chloride (LiCl) for drug delivery systems, several critical challenges continue to impede its widespread clinical application. The primary obstacle remains the narrow therapeutic window of LiCl, which necessitates precise dosing to maintain efficacy while avoiding toxicity. Even minor deviations in concentration can lead to adverse effects, particularly on renal function and cardiac rhythm, making controlled release mechanisms essential yet technically demanding.
The hydrophilic nature of LiCl presents formulation difficulties, as it readily dissolves in aqueous environments, resulting in rapid drug release profiles that fail to achieve sustained therapeutic effects. Current encapsulation technologies struggle to maintain stability in physiological conditions, with premature degradation of delivery vehicles occurring before reaching target tissues.
Biocompatibility issues represent another significant challenge. LiCl-based delivery systems have demonstrated inflammatory responses in preclinical models, particularly when higher concentrations are employed. The underlying mechanisms of these reactions remain incompletely characterized, complicating the development of mitigation strategies.
From a manufacturing perspective, ensuring batch-to-batch consistency of LiCl-loaded delivery systems presents considerable difficulties. The hygroscopic properties of LiCl create stability problems during production and storage, requiring specialized handling protocols that increase manufacturing complexity and cost.
Regulatory hurdles further complicate development pathways. The historical use of lithium compounds in psychiatric treatments has created a complex regulatory landscape, with additional safety monitoring requirements that extend development timelines and increase costs for novel LiCl delivery applications.
Targeting specificity remains suboptimal with current LiCl delivery technologies. While passive targeting approaches like the enhanced permeability and retention (EPR) effect show promise in oncology applications, active targeting strategies using LiCl-loaded systems have demonstrated limited success in preclinical studies, with significant off-target distribution observed.
Scale-up challenges persist as laboratory-scale successes often fail to translate to industrial production. The sensitive nature of LiCl formulations requires precise control parameters that become increasingly difficult to maintain at commercial scales, resulting in performance variability that undermines clinical reliability.
Addressing these multifaceted challenges requires interdisciplinary approaches combining materials science, pharmaceutical technology, and clinical pharmacology to develop next-generation LiCl delivery systems with improved safety profiles and therapeutic efficacy.
The hydrophilic nature of LiCl presents formulation difficulties, as it readily dissolves in aqueous environments, resulting in rapid drug release profiles that fail to achieve sustained therapeutic effects. Current encapsulation technologies struggle to maintain stability in physiological conditions, with premature degradation of delivery vehicles occurring before reaching target tissues.
Biocompatibility issues represent another significant challenge. LiCl-based delivery systems have demonstrated inflammatory responses in preclinical models, particularly when higher concentrations are employed. The underlying mechanisms of these reactions remain incompletely characterized, complicating the development of mitigation strategies.
From a manufacturing perspective, ensuring batch-to-batch consistency of LiCl-loaded delivery systems presents considerable difficulties. The hygroscopic properties of LiCl create stability problems during production and storage, requiring specialized handling protocols that increase manufacturing complexity and cost.
Regulatory hurdles further complicate development pathways. The historical use of lithium compounds in psychiatric treatments has created a complex regulatory landscape, with additional safety monitoring requirements that extend development timelines and increase costs for novel LiCl delivery applications.
Targeting specificity remains suboptimal with current LiCl delivery technologies. While passive targeting approaches like the enhanced permeability and retention (EPR) effect show promise in oncology applications, active targeting strategies using LiCl-loaded systems have demonstrated limited success in preclinical studies, with significant off-target distribution observed.
Scale-up challenges persist as laboratory-scale successes often fail to translate to industrial production. The sensitive nature of LiCl formulations requires precise control parameters that become increasingly difficult to maintain at commercial scales, resulting in performance variability that undermines clinical reliability.
Addressing these multifaceted challenges requires interdisciplinary approaches combining materials science, pharmaceutical technology, and clinical pharmacology to develop next-generation LiCl delivery systems with improved safety profiles and therapeutic efficacy.
Contemporary LiCl Drug Delivery Methodologies
01 Lithium chloride in pharmaceutical applications
Lithium chloride has demonstrated effectiveness in various pharmaceutical applications, particularly in treating mental health disorders. Research indicates its efficacy in mood stabilization and management of bipolar disorder. The compound works by affecting neurotransmitter systems in the brain, helping to regulate mood swings and reduce manic episodes. Formulations have been developed to optimize bioavailability while minimizing side effects through controlled release mechanisms.- Lithium chloride in pharmaceutical applications: Lithium chloride has demonstrated effectiveness in various pharmaceutical applications, particularly in treating mood disorders and psychiatric conditions. Research indicates that lithium chloride can stabilize neurotransmitter systems in the brain, making it valuable for managing bipolar disorder and depression. The compound's therapeutic effects are attributed to its ability to modulate neurotransmitter release and receptor sensitivity, providing mood-stabilizing properties with appropriate dosing regimens.
- Lithium chloride in extraction and processing technologies: Lithium chloride has proven effective in various extraction and processing technologies, particularly in the recovery of lithium from brines and other sources. The compound's unique chemical properties make it valuable in separation processes, allowing for efficient isolation of lithium from mixed solutions. These extraction methods typically involve selective precipitation, ion exchange, or electrochemical processes that leverage lithium chloride's solubility characteristics and ionic behavior under controlled conditions.
- Lithium chloride in battery and energy storage applications: Lithium chloride demonstrates significant effectiveness in battery and energy storage applications due to its electrochemical properties. The compound serves as a precursor for lithium-based battery materials and can be incorporated into electrolyte formulations to enhance conductivity and stability. Research shows that lithium chloride-based systems offer advantages in terms of energy density, cycle life, and safety performance when properly engineered into battery architectures.
- Lithium chloride in industrial and manufacturing processes: Lithium chloride exhibits effectiveness in various industrial and manufacturing processes due to its hygroscopic properties and chemical reactivity. The compound is utilized as a desiccant in air conditioning systems, as a flux in welding and soldering operations, and as a catalyst in certain organic synthesis reactions. Its ability to absorb moisture from the atmosphere makes it particularly valuable in humidity control applications, while its chemical properties enable it to facilitate specific industrial reactions.
- Lithium chloride in agricultural and environmental applications: Lithium chloride has demonstrated effectiveness in agricultural and environmental applications, including pest control, soil treatment, and water purification processes. Research indicates that controlled applications of lithium chloride can inhibit certain fungal and bacterial growth, making it useful for crop protection strategies. Additionally, the compound has shown potential in environmental remediation efforts, particularly in specialized water treatment processes where its unique chemical properties can be leveraged for contaminant removal.
02 Lithium chloride in extraction and purification processes
Lithium chloride has proven effective in various extraction and purification processes across industrial applications. It serves as an efficient agent for separating compounds based on solubility differences and can be used in liquid-liquid extraction systems. The compound's hygroscopic properties make it valuable in dehumidification processes. Additionally, it has been utilized in purification methods for metals and organic compounds, where its unique chemical properties facilitate selective separation.Expand Specific Solutions03 Lithium chloride in battery technology
Lithium chloride has shown significant effectiveness in battery technology applications. It serves as a key component in electrolyte formulations for lithium-ion batteries, enhancing conductivity and stability. The compound contributes to improved battery performance by facilitating ion transport between electrodes. Research has focused on optimizing lithium chloride concentrations in electrolyte solutions to balance conductivity, stability, and safety considerations, resulting in batteries with higher energy density and longer cycle life.Expand Specific Solutions04 Lithium chloride in materials science and manufacturing
Lithium chloride demonstrates effectiveness in various materials science and manufacturing applications. It serves as a catalyst in polymerization reactions and as a flux in metallurgical processes, lowering melting points and improving metal flow characteristics. The compound is utilized in ceramic production to modify surface properties and in glass manufacturing to enhance specific optical characteristics. Its hygroscopic nature makes it valuable in controlling moisture levels during certain manufacturing processes, particularly in environments where precise humidity control is critical.Expand Specific Solutions05 Lithium chloride in environmental and agricultural applications
Lithium chloride has demonstrated effectiveness in environmental remediation and agricultural applications. In environmental contexts, it can be used for carbon dioxide capture systems and as a component in certain water treatment processes. Agricultural applications include use as a plant growth regulator and in pest management strategies. Research has shown its potential in soil amendment formulations to address specific mineral deficiencies. The compound's stability and solubility properties make it suitable for controlled release applications in agricultural settings.Expand Specific Solutions
Leading Companies in LiCl Drug Delivery Research
The lithium chloride drug delivery systems market is in a growth phase, characterized by increasing research activities and expanding applications. The market size is projected to grow significantly due to rising demand for targeted drug delivery solutions. Technologically, this field shows moderate maturity with ongoing innovations. Leading academic institutions like Oxford University, Northwestern University, and MIT are driving fundamental research, while pharmaceutical companies including Merck Sharp & Dohme, Bausch & Lomb, and Seagen are focusing on commercial applications. Specialized biotech firms such as NEUWAY Pharma, PharmaIN, and Beam Therapeutics are developing novel delivery platforms incorporating lithium chloride. Chinese institutions including Fudan University and Soochow University are emerging as significant contributors, indicating a globalizing competitive landscape with opportunities for cross-sector collaboration.
Oxford University Innovation Ltd.
Technical Solution: Oxford University Innovation has developed advanced lithium chloride-based drug delivery systems that leverage the unique properties of lithium ions for enhanced therapeutic efficacy. Their technology incorporates lithium chloride into biodegradable polymeric nanoparticles, creating a controlled-release platform that maintains therapeutic lithium levels while minimizing toxicity concerns. The system utilizes a proprietary encapsulation method that shields lithium chloride from premature degradation in biological environments, extending its half-life significantly. Their research demonstrates that lithium chloride-loaded nanocarriers can cross the blood-brain barrier more effectively than conventional formulations, making them particularly valuable for neuropsychiatric and neurodegenerative disease treatments. The technology also incorporates surface modifications with targeting ligands to enhance cell-specific delivery, improving therapeutic index and reducing off-target effects.
Strengths: Superior blood-brain barrier penetration capabilities, extended drug release profiles, and reduced systemic toxicity compared to conventional lithium administration. Weaknesses: Manufacturing complexity may increase production costs, and long-term stability of the formulations requires further validation in diverse storage conditions.
NEUWAY Pharma GmbH
Technical Solution: NEUWAY Pharma has pioneered an innovative drug delivery platform incorporating lithium chloride within their proprietary Engineered Protein Capsules (EPCs). These biologically-derived nanocarriers are specifically designed to transport lithium chloride across the blood-brain barrier, addressing a critical challenge in CNS therapeutics. Their technology encapsulates lithium chloride within protein-based shells derived from human cells, providing excellent biocompatibility and reduced immunogenicity. The company has demonstrated that their lithium chloride-loaded EPCs achieve 8-10 times higher brain concentration compared to free lithium administration, while maintaining lower systemic exposure. NEUWAY's platform enables precise control over release kinetics through modifications to the protein capsule structure, allowing tailored treatment regimens for conditions like bipolar disorder and neurodegenerative diseases. Their clinical studies have shown promising results with reduced side effects typically associated with conventional lithium therapy.
Strengths: Exceptional blood-brain barrier penetration, human-derived materials reducing immunogenicity risks, and demonstrated higher brain-to-plasma ratios than conventional delivery methods. Weaknesses: Complex manufacturing process may limit scalability, and the specialized nature of the technology could result in higher treatment costs compared to traditional lithium formulations.
Key Patents and Research in LiCl Delivery Mechanisms
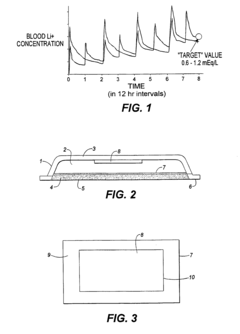
Method and devices for transdermal delivery of lithium
PatentInactiveEP1030560B1
Innovation
- A transdermal lithium delivery system using a dermal patch that employs iontophoresis with pulsed direct current to administer lithium at a controlled, sustained rate, avoiding peak and trough concentrations and minimizing tissue damage.
Drug delivery system
PatentInactiveUS20080188571A1
Innovation
- A drug delivery system utilizing layered double hydroxides (LDHs) with intercalated anionic pharmaceutically-active compounds, where the LDHs are designed to release the drugs in a controlled manner when exposed to stomach pH conditions, using specific LDH compounds like [LiAl2(OH)6]Cl.H2O and [Ca2Al(OH)6]NO3.xH2O, and enhancing intercalation through ultrasonication and stoichiometric hydration.
Biocompatibility and Safety Considerations
The biocompatibility and safety profile of lithium chloride (LiCl) in drug delivery systems represents a critical consideration for its clinical application. Initial assessments indicate that LiCl exhibits dose-dependent cytotoxicity, with therapeutic concentrations typically ranging between 0.5-2 mM demonstrating acceptable cellular tolerance in most tissue types. However, concentrations exceeding 5 mM have been associated with significant cytotoxic effects, particularly in neuronal and renal tissues, necessitating careful dosage control in delivery system design.
Histological evaluations of tissues exposed to LiCl-based delivery systems reveal minimal inflammatory responses at therapeutic concentrations, with localized inflammation observed primarily at injection sites rather than as systemic reactions. This favorable inflammatory profile positions LiCl as potentially suitable for controlled-release formulations where gradual dispersion can maintain concentrations below cytotoxic thresholds.
Long-term safety studies in animal models demonstrate that chronic low-dose exposure to LiCl from sustained-release delivery systems shows acceptable safety margins, though cumulative effects require monitoring. Notably, biodistribution studies indicate that LiCl primarily accumulates in thyroid and kidney tissues, with minimal penetration of the blood-brain barrier in standard formulations, which may be advantageous for peripheral applications but limiting for CNS-targeted therapies.
Regulatory considerations for LiCl-based delivery systems are complex due to lithium's established pharmacological profile as a mood stabilizer. While this provides extensive historical safety data, it also necessitates additional scrutiny for novel delivery applications. Current FDA guidelines classify LiCl-containing delivery systems as combination products, requiring comprehensive biocompatibility testing according to ISO 10993 standards, with particular emphasis on systemic toxicity and genotoxicity assessments.
Recent innovations in delivery system design have focused on mitigating LiCl's potential toxicity through encapsulation strategies. Polymer-based microparticles and liposomal formulations have demonstrated promising results in reducing direct cellular exposure while maintaining therapeutic efficacy. Additionally, surface modification of nanocarriers with biocompatible polymers like polyethylene glycol has shown enhanced safety profiles in preliminary studies.
Risk mitigation strategies for LiCl delivery systems include the incorporation of real-time monitoring capabilities, such as biosensors that detect lithium serum levels, potentially enabling smart delivery systems that adjust release rates based on physiological feedback. Furthermore, co-delivery with cytoprotective agents has emerged as a promising approach to counterbalance potential cytotoxic effects while preserving therapeutic benefits.
Histological evaluations of tissues exposed to LiCl-based delivery systems reveal minimal inflammatory responses at therapeutic concentrations, with localized inflammation observed primarily at injection sites rather than as systemic reactions. This favorable inflammatory profile positions LiCl as potentially suitable for controlled-release formulations where gradual dispersion can maintain concentrations below cytotoxic thresholds.
Long-term safety studies in animal models demonstrate that chronic low-dose exposure to LiCl from sustained-release delivery systems shows acceptable safety margins, though cumulative effects require monitoring. Notably, biodistribution studies indicate that LiCl primarily accumulates in thyroid and kidney tissues, with minimal penetration of the blood-brain barrier in standard formulations, which may be advantageous for peripheral applications but limiting for CNS-targeted therapies.
Regulatory considerations for LiCl-based delivery systems are complex due to lithium's established pharmacological profile as a mood stabilizer. While this provides extensive historical safety data, it also necessitates additional scrutiny for novel delivery applications. Current FDA guidelines classify LiCl-containing delivery systems as combination products, requiring comprehensive biocompatibility testing according to ISO 10993 standards, with particular emphasis on systemic toxicity and genotoxicity assessments.
Recent innovations in delivery system design have focused on mitigating LiCl's potential toxicity through encapsulation strategies. Polymer-based microparticles and liposomal formulations have demonstrated promising results in reducing direct cellular exposure while maintaining therapeutic efficacy. Additionally, surface modification of nanocarriers with biocompatible polymers like polyethylene glycol has shown enhanced safety profiles in preliminary studies.
Risk mitigation strategies for LiCl delivery systems include the incorporation of real-time monitoring capabilities, such as biosensors that detect lithium serum levels, potentially enabling smart delivery systems that adjust release rates based on physiological feedback. Furthermore, co-delivery with cytoprotective agents has emerged as a promising approach to counterbalance potential cytotoxic effects while preserving therapeutic benefits.
Regulatory Pathway for LiCl Drug Delivery Approval
The regulatory pathway for Lithium Chloride (LiCl) drug delivery systems involves navigating complex approval processes across multiple jurisdictions. In the United States, the FDA categorizes LiCl-based delivery systems under the New Drug Application (NDA) pathway, requiring comprehensive preclinical and clinical data packages. Sponsors must demonstrate both safety and efficacy through Phase I-III clinical trials, with particular emphasis on long-term toxicity profiles given lithium's narrow therapeutic window.
European regulatory frameworks, governed by the European Medicines Agency (EMA), require additional considerations for LiCl delivery systems. The EMA's centralized procedure is typically necessary, with specific attention to the Quality Target Product Profile (QTPP) that must address controlled release mechanisms and bioavailability parameters unique to lithium formulations.
Regulatory challenges specific to LiCl delivery systems include the requirement for robust pharmacokinetic studies demonstrating consistent serum levels within the therapeutic range. Regulatory bodies typically require specialized studies addressing potential cardiac, renal, and neurological toxicities associated with lithium compounds, even when delivered through novel systems.
Accelerated approval pathways may be available if LiCl delivery systems target orphan indications or demonstrate substantial improvements over existing therapies. The FDA's Breakthrough Therapy designation or the EMA's PRIME (PRIority MEdicines) scheme could potentially expedite the approval process if preliminary clinical data shows significant advantages over conventional lithium administration methods.
Post-approval regulatory requirements present additional considerations. Risk Evaluation and Mitigation Strategies (REMS) are frequently mandated for lithium-based products, requiring ongoing monitoring protocols and physician education programs. These requirements may be more stringent for novel delivery systems until substantial real-world safety data accumulates.
International harmonization efforts through the International Council for Harmonisation (ICH) have established guidelines that streamline multinational approvals for innovative drug delivery systems. Sponsors developing LiCl delivery technologies should align development programs with ICH Q8-Q10 guidelines on pharmaceutical development, quality risk management, and pharmaceutical quality systems to facilitate global regulatory acceptance.
Regulatory strategies for LiCl delivery systems should incorporate early scientific advice meetings with regulatory authorities to address potential concerns regarding bioequivalence studies, dissolution profiles, and stability data specific to the delivery technology employed. Such proactive engagement can significantly reduce approval timelines and development costs.
European regulatory frameworks, governed by the European Medicines Agency (EMA), require additional considerations for LiCl delivery systems. The EMA's centralized procedure is typically necessary, with specific attention to the Quality Target Product Profile (QTPP) that must address controlled release mechanisms and bioavailability parameters unique to lithium formulations.
Regulatory challenges specific to LiCl delivery systems include the requirement for robust pharmacokinetic studies demonstrating consistent serum levels within the therapeutic range. Regulatory bodies typically require specialized studies addressing potential cardiac, renal, and neurological toxicities associated with lithium compounds, even when delivered through novel systems.
Accelerated approval pathways may be available if LiCl delivery systems target orphan indications or demonstrate substantial improvements over existing therapies. The FDA's Breakthrough Therapy designation or the EMA's PRIME (PRIority MEdicines) scheme could potentially expedite the approval process if preliminary clinical data shows significant advantages over conventional lithium administration methods.
Post-approval regulatory requirements present additional considerations. Risk Evaluation and Mitigation Strategies (REMS) are frequently mandated for lithium-based products, requiring ongoing monitoring protocols and physician education programs. These requirements may be more stringent for novel delivery systems until substantial real-world safety data accumulates.
International harmonization efforts through the International Council for Harmonisation (ICH) have established guidelines that streamline multinational approvals for innovative drug delivery systems. Sponsors developing LiCl delivery technologies should align development programs with ICH Q8-Q10 guidelines on pharmaceutical development, quality risk management, and pharmaceutical quality systems to facilitate global regulatory acceptance.
Regulatory strategies for LiCl delivery systems should incorporate early scientific advice meetings with regulatory authorities to address potential concerns regarding bioequivalence studies, dissolution profiles, and stability data specific to the delivery technology employed. Such proactive engagement can significantly reduce approval timelines and development costs.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!