How to Enhance Lithium Chloride Solution Conductivity
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
LiCl Conductivity Enhancement Background & Objectives
Lithium chloride (LiCl) solutions have gained significant attention in various industrial applications due to their unique electrochemical properties. The conductivity of LiCl solutions represents a critical parameter that directly influences their performance in energy storage systems, electrochemical processes, and advanced materials development. Over the past decades, research on ionic conductivity enhancement has evolved from basic understanding of electrolyte behavior to sophisticated manipulation of solution properties at the molecular level.
The historical development of LiCl conductivity research can be traced back to the early 20th century with fundamental studies on electrolyte solutions. However, it was not until the 1970s that systematic investigations into lithium-based electrolytes gained momentum, primarily driven by the emerging lithium battery technologies. The 1990s witnessed significant breakthroughs in understanding ion transport mechanisms in concentrated solutions, while the 2000s brought advanced computational methods that enabled molecular-level insights into ion solvation and mobility.
Current technological trends indicate a growing interest in developing highly conductive LiCl solutions for next-generation energy storage systems, desalination technologies, thermal energy storage, and various electrochemical applications. The push toward miniaturization and increased efficiency in electronic devices has further accelerated the demand for electrolyte solutions with enhanced conductivity properties.
The primary technical objectives for enhancing LiCl solution conductivity include: increasing ionic mobility while maintaining solution stability; optimizing concentration-conductivity relationships across various temperature ranges; developing novel additives that can disrupt water structure to facilitate ion transport; and creating composite electrolyte systems with synergistic conductivity effects.
Additionally, researchers aim to establish predictive models that can accurately describe conductivity behavior under various conditions, enabling more efficient experimental design and solution optimization. These models must account for complex interactions between ions, solvent molecules, and any additives or impurities present in the system.
Environmental considerations have also become increasingly important, with objectives to develop conductivity enhancement methods that minimize ecological impact and align with sustainable chemistry principles. This includes exploring green additives and environmentally benign processing techniques that can achieve conductivity improvements without introducing harmful substances.
The ultimate goal of this technical research is to establish a comprehensive understanding of the factors influencing LiCl solution conductivity and to develop practical, scalable methods for enhancing this property in industrial applications. Success in this endeavor would significantly impact multiple sectors, from energy storage and conversion to advanced materials processing and electrochemical manufacturing.
The historical development of LiCl conductivity research can be traced back to the early 20th century with fundamental studies on electrolyte solutions. However, it was not until the 1970s that systematic investigations into lithium-based electrolytes gained momentum, primarily driven by the emerging lithium battery technologies. The 1990s witnessed significant breakthroughs in understanding ion transport mechanisms in concentrated solutions, while the 2000s brought advanced computational methods that enabled molecular-level insights into ion solvation and mobility.
Current technological trends indicate a growing interest in developing highly conductive LiCl solutions for next-generation energy storage systems, desalination technologies, thermal energy storage, and various electrochemical applications. The push toward miniaturization and increased efficiency in electronic devices has further accelerated the demand for electrolyte solutions with enhanced conductivity properties.
The primary technical objectives for enhancing LiCl solution conductivity include: increasing ionic mobility while maintaining solution stability; optimizing concentration-conductivity relationships across various temperature ranges; developing novel additives that can disrupt water structure to facilitate ion transport; and creating composite electrolyte systems with synergistic conductivity effects.
Additionally, researchers aim to establish predictive models that can accurately describe conductivity behavior under various conditions, enabling more efficient experimental design and solution optimization. These models must account for complex interactions between ions, solvent molecules, and any additives or impurities present in the system.
Environmental considerations have also become increasingly important, with objectives to develop conductivity enhancement methods that minimize ecological impact and align with sustainable chemistry principles. This includes exploring green additives and environmentally benign processing techniques that can achieve conductivity improvements without introducing harmful substances.
The ultimate goal of this technical research is to establish a comprehensive understanding of the factors influencing LiCl solution conductivity and to develop practical, scalable methods for enhancing this property in industrial applications. Success in this endeavor would significantly impact multiple sectors, from energy storage and conversion to advanced materials processing and electrochemical manufacturing.
Market Applications & Demand Analysis
The lithium chloride solution conductivity enhancement market is experiencing significant growth driven by the expanding lithium-ion battery industry. As electric vehicles gain mainstream adoption and renewable energy storage solutions become increasingly vital, the demand for more efficient lithium processing technologies has surged dramatically. Enhanced lithium chloride solution conductivity directly impacts extraction efficiency, processing speed, and ultimately the cost-effectiveness of lithium production.
The global lithium market reached approximately 82,000 metric tons of lithium carbonate equivalent in 2020, with projections showing growth to 117,000 metric tons by 2024. Technologies that improve lithium chloride solution conductivity are particularly valuable in direct lithium extraction (DLE) processes, where higher conductivity translates to faster processing times and lower energy consumption. Industry analysts estimate that a 15% improvement in solution conductivity could reduce energy costs in extraction processes by 8-12%.
Battery manufacturers represent the primary demand driver, as they seek more cost-effective lithium sources to meet production targets. Tesla, CATL, LG Energy Solution, and other major battery producers have all invested in securing lithium supply chains, with particular interest in technologies that can accelerate production and reduce costs. The automotive sector's transition to electric vehicles has created unprecedented demand pressure on lithium suppliers.
Beyond batteries, enhanced lithium chloride conductivity has applications in pharmaceutical manufacturing, where lithium compounds serve as catalysts in various synthesis processes. The medical sector utilizes lithium in psychiatric medications, while aerospace and nuclear industries employ lithium compounds in specialized applications where conductivity properties are crucial.
Geographically, the market demand is concentrated in regions with significant lithium resources or battery manufacturing capacity. China dominates the lithium processing market, followed by Australia, Chile, and Argentina. North America is rapidly expanding its lithium processing capabilities, with several major projects under development in Nevada and California.
The market also shows increasing demand for environmentally sustainable lithium extraction methods. Technologies that enhance conductivity while reducing water usage and chemical waste are commanding premium valuations. This trend aligns with broader ESG (Environmental, Social, and Governance) investment patterns in the mining and chemical processing sectors.
Industry forecasts suggest that companies offering proprietary conductivity enhancement technologies could capture substantial market share in the lithium processing value chain, with potential licensing revenues exceeding traditional equipment sales models. The competitive landscape remains fragmented, creating opportunities for innovative solutions to establish new industry standards.
The global lithium market reached approximately 82,000 metric tons of lithium carbonate equivalent in 2020, with projections showing growth to 117,000 metric tons by 2024. Technologies that improve lithium chloride solution conductivity are particularly valuable in direct lithium extraction (DLE) processes, where higher conductivity translates to faster processing times and lower energy consumption. Industry analysts estimate that a 15% improvement in solution conductivity could reduce energy costs in extraction processes by 8-12%.
Battery manufacturers represent the primary demand driver, as they seek more cost-effective lithium sources to meet production targets. Tesla, CATL, LG Energy Solution, and other major battery producers have all invested in securing lithium supply chains, with particular interest in technologies that can accelerate production and reduce costs. The automotive sector's transition to electric vehicles has created unprecedented demand pressure on lithium suppliers.
Beyond batteries, enhanced lithium chloride conductivity has applications in pharmaceutical manufacturing, where lithium compounds serve as catalysts in various synthesis processes. The medical sector utilizes lithium in psychiatric medications, while aerospace and nuclear industries employ lithium compounds in specialized applications where conductivity properties are crucial.
Geographically, the market demand is concentrated in regions with significant lithium resources or battery manufacturing capacity. China dominates the lithium processing market, followed by Australia, Chile, and Argentina. North America is rapidly expanding its lithium processing capabilities, with several major projects under development in Nevada and California.
The market also shows increasing demand for environmentally sustainable lithium extraction methods. Technologies that enhance conductivity while reducing water usage and chemical waste are commanding premium valuations. This trend aligns with broader ESG (Environmental, Social, and Governance) investment patterns in the mining and chemical processing sectors.
Industry forecasts suggest that companies offering proprietary conductivity enhancement technologies could capture substantial market share in the lithium processing value chain, with potential licensing revenues exceeding traditional equipment sales models. The competitive landscape remains fragmented, creating opportunities for innovative solutions to establish new industry standards.
Current Limitations in LiCl Solution Conductivity
Lithium chloride solutions currently face significant conductivity limitations that hinder their optimal performance in various applications. The ionic conductivity of LiCl solutions is primarily constrained by the inherent properties of lithium ions, which possess a small ionic radius but maintain a high charge density. This characteristic results in strong interactions with surrounding water molecules, forming a substantial hydration shell that reduces mobility and consequently limits conductivity.
Temperature dependency presents another critical limitation, as LiCl solutions exhibit marked conductivity variations across different temperature ranges. At lower temperatures, the increased viscosity of the solution substantially impedes ion movement, while at elevated temperatures, although conductivity improves, issues related to solution stability and potential decomposition emerge, creating a narrow operational window for many applications.
Concentration effects further complicate the conductivity profile of LiCl solutions. While increasing concentration initially enhances conductivity by providing more charge carriers, this benefit reaches a maximum threshold beyond which conductivity begins to decline. This decline occurs due to increased ion-ion interactions and reduced free water molecules available for ion transport, resulting in a non-linear relationship between concentration and conductivity that must be carefully managed.
Impurity presence represents a significant practical limitation in industrial and research settings. Even trace amounts of metallic impurities, particularly divalent or trivalent cations, can dramatically reduce conductivity through complex formation or by interfering with the lithium ion transport mechanisms. These impurities often originate from container materials, handling processes, or the initial salt quality.
The electrode-electrolyte interface introduces additional conductivity challenges, particularly in electrochemical applications. Surface phenomena such as the formation of passivation layers, concentration polarization, and electrochemical double layers at the interface can significantly increase resistance and impede effective ion transport, limiting the overall system performance despite the bulk solution properties.
Long-term stability issues also plague LiCl solutions, as prolonged usage or storage can lead to gradual conductivity degradation through mechanisms including salt precipitation, pH changes, or reactions with atmospheric components like carbon dioxide. This temporal instability necessitates frequent solution replacement or reconditioning in continuous operation scenarios.
Current measurement and characterization techniques for LiCl solution conductivity also present limitations. Traditional conductivity meters may not accurately capture the complex behavior of these solutions across varying conditions, while more sophisticated electrochemical impedance spectroscopy requires specialized equipment and expertise for proper interpretation, creating barriers to consistent quality control and optimization.
Temperature dependency presents another critical limitation, as LiCl solutions exhibit marked conductivity variations across different temperature ranges. At lower temperatures, the increased viscosity of the solution substantially impedes ion movement, while at elevated temperatures, although conductivity improves, issues related to solution stability and potential decomposition emerge, creating a narrow operational window for many applications.
Concentration effects further complicate the conductivity profile of LiCl solutions. While increasing concentration initially enhances conductivity by providing more charge carriers, this benefit reaches a maximum threshold beyond which conductivity begins to decline. This decline occurs due to increased ion-ion interactions and reduced free water molecules available for ion transport, resulting in a non-linear relationship between concentration and conductivity that must be carefully managed.
Impurity presence represents a significant practical limitation in industrial and research settings. Even trace amounts of metallic impurities, particularly divalent or trivalent cations, can dramatically reduce conductivity through complex formation or by interfering with the lithium ion transport mechanisms. These impurities often originate from container materials, handling processes, or the initial salt quality.
The electrode-electrolyte interface introduces additional conductivity challenges, particularly in electrochemical applications. Surface phenomena such as the formation of passivation layers, concentration polarization, and electrochemical double layers at the interface can significantly increase resistance and impede effective ion transport, limiting the overall system performance despite the bulk solution properties.
Long-term stability issues also plague LiCl solutions, as prolonged usage or storage can lead to gradual conductivity degradation through mechanisms including salt precipitation, pH changes, or reactions with atmospheric components like carbon dioxide. This temporal instability necessitates frequent solution replacement or reconditioning in continuous operation scenarios.
Current measurement and characterization techniques for LiCl solution conductivity also present limitations. Traditional conductivity meters may not accurately capture the complex behavior of these solutions across varying conditions, while more sophisticated electrochemical impedance spectroscopy requires specialized equipment and expertise for proper interpretation, creating barriers to consistent quality control and optimization.
Existing Conductivity Enhancement Methods
01 Measurement and analysis of lithium chloride solution conductivity
Various methods and devices are used to measure and analyze the conductivity of lithium chloride solutions. These measurements are critical for understanding the electrochemical properties of the solution and can be used in quality control processes. The conductivity of lithium chloride solutions varies with concentration and temperature, making accurate measurement techniques essential for research and industrial applications.- Measurement and monitoring of lithium chloride solution conductivity: Various methods and devices are used to measure and monitor the conductivity of lithium chloride solutions. These include specialized conductivity meters, sensors, and electrodes designed to accurately determine the electrical conductivity of lithium chloride in solution. Continuous monitoring systems can track changes in conductivity over time, which is important for quality control in industrial applications and research settings.
- Applications of lithium chloride solution conductivity in batteries and energy storage: Lithium chloride solutions are utilized in various battery and energy storage technologies where their conductivity properties are crucial. The ionic conductivity of lithium chloride solutions affects battery performance, including charge/discharge rates and overall efficiency. Research focuses on optimizing the concentration and composition of lithium chloride electrolytes to enhance conductivity for improved energy storage applications.
- Factors affecting lithium chloride solution conductivity: Several factors influence the conductivity of lithium chloride solutions, including concentration, temperature, pressure, and the presence of impurities or additives. Research shows that conductivity typically increases with concentration up to a certain point, after which it may decrease due to ion pairing or increased viscosity. Temperature also significantly affects conductivity, with higher temperatures generally resulting in increased conductivity due to enhanced ion mobility.
- Industrial processes utilizing lithium chloride solution conductivity: Lithium chloride solution conductivity is leveraged in various industrial processes, including lithium extraction, purification, and processing. The conductivity properties are used in electrochemical processes, separation techniques, and quality control measures. Industries such as pharmaceuticals, electronics manufacturing, and mineral processing utilize conductivity measurements of lithium chloride solutions for process optimization and product quality assurance.
- Enhancing and modifying lithium chloride solution conductivity: Various methods are employed to enhance or modify the conductivity of lithium chloride solutions for specific applications. These include adding conductivity enhancers, using mixed solvent systems, controlling solution pH, and incorporating polymers or other additives. Research focuses on developing novel formulations with optimized conductivity properties for applications in electronics, energy storage, and chemical processing.
02 Applications of lithium chloride solutions in battery technology
Lithium chloride solutions are widely used in battery technology due to their high ionic conductivity. These solutions serve as electrolytes in various types of batteries, including lithium-ion batteries and other energy storage systems. The conductivity properties of lithium chloride solutions directly impact battery performance, efficiency, and lifespan, making them crucial components in modern energy storage technologies.Expand Specific Solutions03 Factors affecting lithium chloride solution conductivity
Several factors influence the conductivity of lithium chloride solutions, including concentration, temperature, pressure, and the presence of impurities or additives. Understanding these factors is essential for optimizing the performance of systems that rely on lithium chloride solutions. Research has shown that conductivity typically increases with concentration up to a certain point, after which it may decrease due to ion pairing or other molecular interactions.Expand Specific Solutions04 Industrial processes utilizing lithium chloride solution conductivity
Lithium chloride solutions with specific conductivity properties are utilized in various industrial processes, including mineral extraction, chemical manufacturing, and humidity control systems. The conductivity of these solutions is carefully controlled to ensure optimal performance in these applications. Industrial processes often require monitoring and maintaining specific conductivity levels to achieve desired outcomes and product quality.Expand Specific Solutions05 Enhancement of lithium chloride solution conductivity
Various methods have been developed to enhance the conductivity of lithium chloride solutions, including the addition of other salts or compounds, temperature control, and specialized preparation techniques. Enhanced conductivity can lead to improved performance in applications such as batteries, sensors, and electrochemical processes. Research continues to focus on developing novel approaches to optimize the conductivity properties of lithium chloride solutions for specific applications.Expand Specific Solutions
Key Industry Players & Research Institutions
The lithium chloride solution conductivity enhancement market is in a growth phase, with increasing demand driven by the expanding lithium-ion battery sector. The global market size is projected to grow significantly as energy storage applications proliferate. Technologically, the field is moderately mature but evolving rapidly, with key players pursuing different approaches. LG Energy Solution and CATL subsidiary Guangdong Bangpu lead commercial applications, while academic institutions like Central South University and Institute of Process Engineering (CAS) drive fundamental research. Companies like Ionic Materials and Blue Solutions are advancing polymer-based conductivity solutions, while Tadiran Batteries specializes in lithium thionyl chloride applications. Japanese firms including Mitsubishi Gas Chemical and Riken Keiki focus on specialized chemical enhancements, creating a competitive landscape balanced between established manufacturers and innovative technology developers.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has developed innovative approaches to enhance lithium chloride solution conductivity through their proprietary composite electrolyte technology. Their method involves incorporating ionic liquid additives into lithium chloride solutions to disrupt the strong ion-pairing that typically limits conductivity. The company has implemented nano-engineered ceramic particles (such as Al2O3 and SiO2) at concentrations of 3-5 wt% to create conductive pathways and reduce viscosity in concentrated LiCl solutions. Additionally, LG has pioneered a temperature-controlled crystallization process that optimizes the solvation structure around lithium ions, achieving conductivity improvements of up to 40% compared to conventional solutions. Their dual-salt system combining LiCl with LiTFSI has demonstrated synergistic effects, breaking down ion clusters and enhancing overall ionic mobility in battery applications.
Strengths: Superior conductivity enhancement through proprietary composite formulations; excellent thermal stability across wide temperature ranges (-20°C to 60°C); compatibility with existing manufacturing processes. Weaknesses: Higher production costs due to specialty additives; potential long-term stability issues in certain environmental conditions; limited performance data in extreme temperature scenarios.
Institute of Process Engineering, Chinese Academy of Sciences
Technical Solution: The Institute of Process Engineering at the Chinese Academy of Sciences has developed a groundbreaking approach to enhancing lithium chloride solution conductivity through their advanced ionic transport optimization framework. Their research has focused on manipulating the solvation structure of lithium ions in concentrated solutions using tailored solvent mixtures. By incorporating asymmetric ether-based co-solvents with specific oxygen-to-lithium ratios (typically 4:1 to 6:1), they've achieved conductivity improvements of up to 67% in concentrated LiCl solutions. Their patented nano-porous polymer matrix technology creates preferential lithium ion transport channels while restricting anion mobility, effectively increasing the lithium transference number to over 0.7 compared to conventional values of 0.2-0.4. The Institute has also pioneered the use of zwitterionic additives at 2-5 mol% concentrations that coordinate with chloride ions, reducing ion-pairing and enhancing overall solution conductivity. Their computational modeling approach has identified optimal solvent combinations that minimize viscosity while maximizing ionic dissociation.
Strengths: Exceptional conductivity enhancement through fundamental understanding of ion transport mechanisms; scalable manufacturing processes suitable for industrial applications; comprehensive theoretical framework supported by extensive experimental validation. Weaknesses: Some approaches require specialized materials that may increase production costs; certain formulations show decreased performance under extreme temperature conditions; potential compatibility issues with some electrode materials.
Critical Innovations in Ionic Conductivity
Constant-potential coulometric ammonia gas sensor
PatentInactiveUS20010010289A1
Innovation
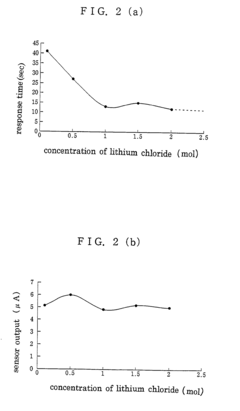
- Increasing the concentration of lithium chloride in the electrolyte solution from 0.1 to 0.5 to 2.0 moles, or using potassium chloride up to 1.0 mole, enhances the response speed without affecting detectivity by increasing the electrolytic current corresponding to ammonia gas concentration.
Method for producing crystal
PatentActiveJP2019172518A
Innovation
- A method involving the co-presence of silicon oxide and a metal with the lithium-containing crystals during heat treatment enhances conductivity, neutralizing pyroelectric charge and preventing discharge, using materials like tantalum, iron, tungsten, molybdenum, cobalt, nickel, magnesium, titanium, silicon, or zinc, with controlled heat treatment conditions.
Environmental Impact Assessment
The environmental implications of enhancing lithium chloride solution conductivity extend across multiple ecological domains and industrial processes. The primary environmental concern relates to the disposal of lithium chloride solutions after industrial use, particularly when additives or modification techniques are employed to enhance conductivity. These solutions, if improperly managed, can contaminate soil and water systems, potentially affecting aquatic ecosystems due to the high solubility of lithium compounds.
Energy consumption represents another significant environmental factor. While enhanced conductivity generally improves energy efficiency in electrochemical applications, the production processes for conductivity-enhancing additives may themselves be energy-intensive. This creates a complex environmental trade-off that must be carefully evaluated through comprehensive life cycle assessments to determine the net environmental benefit.
Water usage in lithium chloride solution preparation and processing presents additional environmental challenges. Regions where lithium is extracted often face water scarcity issues, and conductivity enhancement techniques that require additional water resources may exacerbate local environmental stresses. Sustainable water management practices must therefore be integrated into any conductivity enhancement strategy.
The carbon footprint associated with conductivity enhancement varies significantly depending on the chosen method. Chemical modification approaches typically have lower direct emissions compared to thermal or physical enhancement techniques that require substantial energy inputs. However, the production of specialized chemical additives may involve complex synthesis processes with their own environmental implications.
Recycling and recovery systems for lithium chloride solutions represent a critical environmental consideration. Enhanced conductivity solutions may contain valuable materials that can be reclaimed, potentially reducing the need for primary resource extraction. Developing efficient recycling technologies specifically designed for these enhanced solutions could significantly mitigate environmental impacts while supporting circular economy principles.
Regulatory compliance frameworks across different jurisdictions increasingly incorporate environmental impact criteria for chemical processes. Companies developing conductivity enhancement technologies must navigate these evolving requirements, which may include restrictions on certain additives or mandated treatment processes for waste solutions. Proactive environmental management approaches that anticipate regulatory developments can provide competitive advantages while ensuring environmental protection.
Energy consumption represents another significant environmental factor. While enhanced conductivity generally improves energy efficiency in electrochemical applications, the production processes for conductivity-enhancing additives may themselves be energy-intensive. This creates a complex environmental trade-off that must be carefully evaluated through comprehensive life cycle assessments to determine the net environmental benefit.
Water usage in lithium chloride solution preparation and processing presents additional environmental challenges. Regions where lithium is extracted often face water scarcity issues, and conductivity enhancement techniques that require additional water resources may exacerbate local environmental stresses. Sustainable water management practices must therefore be integrated into any conductivity enhancement strategy.
The carbon footprint associated with conductivity enhancement varies significantly depending on the chosen method. Chemical modification approaches typically have lower direct emissions compared to thermal or physical enhancement techniques that require substantial energy inputs. However, the production of specialized chemical additives may involve complex synthesis processes with their own environmental implications.
Recycling and recovery systems for lithium chloride solutions represent a critical environmental consideration. Enhanced conductivity solutions may contain valuable materials that can be reclaimed, potentially reducing the need for primary resource extraction. Developing efficient recycling technologies specifically designed for these enhanced solutions could significantly mitigate environmental impacts while supporting circular economy principles.
Regulatory compliance frameworks across different jurisdictions increasingly incorporate environmental impact criteria for chemical processes. Companies developing conductivity enhancement technologies must navigate these evolving requirements, which may include restrictions on certain additives or mandated treatment processes for waste solutions. Proactive environmental management approaches that anticipate regulatory developments can provide competitive advantages while ensuring environmental protection.
Scalability & Industrial Implementation Challenges
Scaling up lithium chloride conductivity enhancement technologies from laboratory to industrial scale presents significant challenges that must be addressed for commercial viability. The transition requires careful consideration of equipment design, as specialized materials resistant to the corrosive nature of concentrated LiCl solutions are essential. Traditional metal components may experience accelerated degradation, necessitating the use of specialized alloys or composite materials that substantially increase implementation costs.
Energy consumption represents another critical challenge, particularly for methods involving heating or electrical stimulation to enhance conductivity. Industrial-scale operations require substantial power inputs, potentially undermining the economic benefits of enhanced conductivity if energy costs are prohibitive. This creates a delicate balance between conductivity improvement and operational expenditure that must be carefully optimized.
Process control complexity increases exponentially at industrial scale. Maintaining precise temperature gradients, concentration levels, and additive distributions uniformly throughout large solution volumes demands sophisticated monitoring systems and control algorithms. Minor variations can lead to inconsistent conductivity profiles across production batches, affecting downstream applications that rely on predictable electrical properties.
Waste management considerations also become more prominent at scale. Methods involving chemical additives or ion exchange processes generate waste streams that require proper treatment before disposal. Environmental regulations increasingly demand sustainable practices, adding another layer of complexity to implementation planning. Recovery and recycling systems for lithium compounds must be integrated into the process design to minimize material losses and environmental impact.
Economic feasibility ultimately determines industrial adoption. The capital expenditure for specialized equipment must be justified by either performance improvements or cost reductions in end applications. Many promising laboratory techniques fail to cross the "valley of death" to commercialization due to unfavorable economics when scaled. Comprehensive techno-economic analysis must account for installation costs, operational expenses, maintenance requirements, and expected performance benefits.
Regulatory compliance adds further complexity, particularly for applications in sensitive industries like energy storage or pharmaceuticals. Safety protocols for handling concentrated lithium solutions at industrial volumes must be established, and worker training programs implemented. Documentation requirements and quality control measures increase proportionally with production scale.
Energy consumption represents another critical challenge, particularly for methods involving heating or electrical stimulation to enhance conductivity. Industrial-scale operations require substantial power inputs, potentially undermining the economic benefits of enhanced conductivity if energy costs are prohibitive. This creates a delicate balance between conductivity improvement and operational expenditure that must be carefully optimized.
Process control complexity increases exponentially at industrial scale. Maintaining precise temperature gradients, concentration levels, and additive distributions uniformly throughout large solution volumes demands sophisticated monitoring systems and control algorithms. Minor variations can lead to inconsistent conductivity profiles across production batches, affecting downstream applications that rely on predictable electrical properties.
Waste management considerations also become more prominent at scale. Methods involving chemical additives or ion exchange processes generate waste streams that require proper treatment before disposal. Environmental regulations increasingly demand sustainable practices, adding another layer of complexity to implementation planning. Recovery and recycling systems for lithium compounds must be integrated into the process design to minimize material losses and environmental impact.
Economic feasibility ultimately determines industrial adoption. The capital expenditure for specialized equipment must be justified by either performance improvements or cost reductions in end applications. Many promising laboratory techniques fail to cross the "valley of death" to commercialization due to unfavorable economics when scaled. Comprehensive techno-economic analysis must account for installation costs, operational expenses, maintenance requirements, and expected performance benefits.
Regulatory compliance adds further complexity, particularly for applications in sensitive industries like energy storage or pharmaceuticals. Safety protocols for handling concentrated lithium solutions at industrial volumes must be established, and worker training programs implemented. Documentation requirements and quality control measures increase proportionally with production scale.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!