Lithium Chloride vs Chloride Salts: Conductivity Tests
AUG 28, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Chloride Conductivity Research Background and Objectives
The evolution of energy storage technologies has witnessed significant advancements over the past decades, with lithium-based systems emerging as a cornerstone for modern applications. Lithium chloride (LiCl), a simple alkali metal halide, has garnered increasing attention in the scientific community due to its unique electrochemical properties. The historical trajectory of lithium salt research dates back to the 1970s when the first lithium-based batteries were conceptualized, but systematic investigation of lithium chloride's conductivity characteristics has intensified only in the last decade.
Comparative conductivity analysis between lithium chloride and other chloride salts represents a critical research domain with far-reaching implications for energy storage, electrochemical devices, and advanced materials science. The ionic conductivity of these salts, particularly in various solvent systems and under different temperature conditions, directly impacts the performance metrics of numerous technologies including batteries, supercapacitors, and electrochemical sensors.
Current technological trends indicate a shift toward more efficient, sustainable, and cost-effective energy storage solutions. Within this context, understanding the fundamental conductivity mechanisms of lithium chloride compared to other chloride salts (such as sodium chloride, potassium chloride, and magnesium chloride) becomes essential for developing next-generation energy systems. The conductivity behavior of these salts is influenced by multiple factors including ionic radius, hydration energy, lattice energy, and solvent-ion interactions.
Recent advancements in analytical instrumentation and computational modeling have enabled more precise measurements and predictions of ionic conductivity. Techniques such as impedance spectroscopy, potentiometric analysis, and molecular dynamics simulations have revolutionized our understanding of ion transport phenomena in various media. These methodological improvements have created new opportunities for exploring the conductivity properties of lithium chloride in unprecedented detail.
The primary objective of this research is to establish a comprehensive comparative framework for evaluating the conductivity characteristics of lithium chloride against other chloride salts across diverse experimental conditions. This includes systematic investigation of concentration dependencies, temperature effects, solvent influences, and the impact of additives on ionic mobility and conductance.
Additionally, this research aims to identify the mechanistic underpinnings of observed conductivity differences, correlating macroscopic measurements with molecular-level phenomena. By elucidating these structure-property relationships, we seek to develop predictive models that can guide the rational design of electrolyte systems optimized for specific applications.
The ultimate goal extends beyond fundamental understanding to practical applications, particularly in developing enhanced electrolyte formulations for energy storage devices. By leveraging the unique conductivity profile of lithium chloride, this research endeavors to contribute to the advancement of more efficient, safer, and higher-capacity energy storage technologies to meet the growing global demand for sustainable energy solutions.
Comparative conductivity analysis between lithium chloride and other chloride salts represents a critical research domain with far-reaching implications for energy storage, electrochemical devices, and advanced materials science. The ionic conductivity of these salts, particularly in various solvent systems and under different temperature conditions, directly impacts the performance metrics of numerous technologies including batteries, supercapacitors, and electrochemical sensors.
Current technological trends indicate a shift toward more efficient, sustainable, and cost-effective energy storage solutions. Within this context, understanding the fundamental conductivity mechanisms of lithium chloride compared to other chloride salts (such as sodium chloride, potassium chloride, and magnesium chloride) becomes essential for developing next-generation energy systems. The conductivity behavior of these salts is influenced by multiple factors including ionic radius, hydration energy, lattice energy, and solvent-ion interactions.
Recent advancements in analytical instrumentation and computational modeling have enabled more precise measurements and predictions of ionic conductivity. Techniques such as impedance spectroscopy, potentiometric analysis, and molecular dynamics simulations have revolutionized our understanding of ion transport phenomena in various media. These methodological improvements have created new opportunities for exploring the conductivity properties of lithium chloride in unprecedented detail.
The primary objective of this research is to establish a comprehensive comparative framework for evaluating the conductivity characteristics of lithium chloride against other chloride salts across diverse experimental conditions. This includes systematic investigation of concentration dependencies, temperature effects, solvent influences, and the impact of additives on ionic mobility and conductance.
Additionally, this research aims to identify the mechanistic underpinnings of observed conductivity differences, correlating macroscopic measurements with molecular-level phenomena. By elucidating these structure-property relationships, we seek to develop predictive models that can guide the rational design of electrolyte systems optimized for specific applications.
The ultimate goal extends beyond fundamental understanding to practical applications, particularly in developing enhanced electrolyte formulations for energy storage devices. By leveraging the unique conductivity profile of lithium chloride, this research endeavors to contribute to the advancement of more efficient, safer, and higher-capacity energy storage technologies to meet the growing global demand for sustainable energy solutions.
Market Applications and Demand Analysis for Conductive Salt Solutions
The global market for conductive salt solutions is experiencing significant growth, driven primarily by the expanding energy storage sector. Lithium chloride and other chloride salts have become critical components in various industrial applications due to their unique electrochemical properties. The demand for high-conductivity electrolytes has surged with the proliferation of lithium-ion batteries, which are essential for electric vehicles, renewable energy storage systems, and portable electronics.
In the energy storage sector, the market for conductive salt solutions is projected to grow substantially over the next decade. This growth is primarily attributed to the global shift toward clean energy and the increasing adoption of electric vehicles. Lithium chloride, in particular, has gained prominence due to its superior ionic conductivity compared to other chloride salts, making it a preferred choice for high-performance battery applications.
Beyond energy storage, conductive salt solutions find applications in various industrial processes. In the chemical industry, these solutions are utilized in electrochemical processes, while in metallurgy, they serve as electrolytes for metal extraction and refining. The healthcare sector also employs conductive salt solutions in medical devices and diagnostic equipment, where stable electrical conductivity is crucial.
Regional market analysis reveals that Asia-Pacific dominates the conductive salt solutions market, with China leading in production and consumption. This dominance is largely due to the region's robust electronics manufacturing industry and aggressive electric vehicle adoption policies. North America and Europe follow, with growing investments in renewable energy infrastructure driving demand for high-performance energy storage solutions.
Market segmentation by application shows that lithium-ion batteries account for the largest share of conductive salt solution consumption. However, emerging applications in supercapacitors, flow batteries, and advanced medical devices are expected to create new market opportunities. The demand for specialized conductive salt formulations with enhanced stability, safety, and performance characteristics is also increasing.
Customer requirements are evolving toward solutions that offer higher conductivity, greater thermal stability, and improved safety profiles. This trend has intensified research into comparing various chloride salts, with lithium chloride often emerging as superior in conductivity tests. However, cost considerations remain significant, as lithium compounds generally command premium prices compared to other chloride salts.
Market challenges include supply chain vulnerabilities, particularly for lithium-based compounds, and environmental concerns related to extraction and processing. These factors are driving interest in alternative chloride salts that can deliver comparable conductivity while offering better sustainability profiles and reduced supply risks.
In the energy storage sector, the market for conductive salt solutions is projected to grow substantially over the next decade. This growth is primarily attributed to the global shift toward clean energy and the increasing adoption of electric vehicles. Lithium chloride, in particular, has gained prominence due to its superior ionic conductivity compared to other chloride salts, making it a preferred choice for high-performance battery applications.
Beyond energy storage, conductive salt solutions find applications in various industrial processes. In the chemical industry, these solutions are utilized in electrochemical processes, while in metallurgy, they serve as electrolytes for metal extraction and refining. The healthcare sector also employs conductive salt solutions in medical devices and diagnostic equipment, where stable electrical conductivity is crucial.
Regional market analysis reveals that Asia-Pacific dominates the conductive salt solutions market, with China leading in production and consumption. This dominance is largely due to the region's robust electronics manufacturing industry and aggressive electric vehicle adoption policies. North America and Europe follow, with growing investments in renewable energy infrastructure driving demand for high-performance energy storage solutions.
Market segmentation by application shows that lithium-ion batteries account for the largest share of conductive salt solution consumption. However, emerging applications in supercapacitors, flow batteries, and advanced medical devices are expected to create new market opportunities. The demand for specialized conductive salt formulations with enhanced stability, safety, and performance characteristics is also increasing.
Customer requirements are evolving toward solutions that offer higher conductivity, greater thermal stability, and improved safety profiles. This trend has intensified research into comparing various chloride salts, with lithium chloride often emerging as superior in conductivity tests. However, cost considerations remain significant, as lithium compounds generally command premium prices compared to other chloride salts.
Market challenges include supply chain vulnerabilities, particularly for lithium-based compounds, and environmental concerns related to extraction and processing. These factors are driving interest in alternative chloride salts that can deliver comparable conductivity while offering better sustainability profiles and reduced supply risks.
Current State and Technical Challenges in Salt Conductivity Testing
The global research on salt conductivity testing has advanced significantly in recent years, with particular focus on lithium chloride and other chloride salts due to their applications in energy storage, electrochemistry, and materials science. Current testing methodologies primarily employ electrochemical impedance spectroscopy (EIS), direct current (DC) conductivity measurements, and potentiostatic techniques to evaluate ionic conductivity properties.
In laboratory settings, researchers have established standardized protocols for measuring salt conductivity, typically involving temperature-controlled environments ranging from -20°C to 100°C to simulate various operational conditions. Recent advancements have enabled high-precision measurements with error margins below 0.5%, representing significant progress from earlier techniques that exhibited variability of 2-3%.
Despite these improvements, several technical challenges persist in salt conductivity testing. The primary challenge involves achieving consistent measurement conditions across different laboratory environments, as factors such as humidity, atmospheric pressure, and trace contaminants can significantly influence conductivity readings. This variability makes direct comparison between research findings problematic, particularly when evaluating lithium chloride against other chloride salts.
Another significant challenge is the temperature dependence of ionic conductivity. Current testing methodologies struggle to maintain uniform temperature distribution throughout test samples, leading to potential measurement inaccuracies. This is especially problematic when comparing lithium chloride with other chloride salts, as their conductivity behaviors exhibit different temperature sensitivities.
Interface effects between electrodes and salt solutions represent another major technical hurdle. Electrode polarization, surface adsorption phenomena, and electrochemical reactions at electrode surfaces can distort conductivity measurements. These effects are particularly pronounced in concentrated solutions, which are often required for practical applications.
The stability of salt solutions during extended testing periods presents additional complications. Degradation processes, including hydrolysis, oxidation, and precipitation, can alter the composition of test samples over time. This is especially relevant for lithium chloride solutions, which may exhibit greater sensitivity to environmental factors compared to other chloride salts.
Measurement standardization across the industry remains inconsistent, with different research groups employing varied methodologies, equipment specifications, and reporting formats. This lack of standardization hampers comparative analysis and slows the development of comprehensive conductivity databases for different salt systems.
Recent technological innovations, including microfluidic conductivity cells and in-situ spectroscopic techniques, show promise in addressing some of these challenges, but their adoption remains limited due to high costs and specialized expertise requirements.
In laboratory settings, researchers have established standardized protocols for measuring salt conductivity, typically involving temperature-controlled environments ranging from -20°C to 100°C to simulate various operational conditions. Recent advancements have enabled high-precision measurements with error margins below 0.5%, representing significant progress from earlier techniques that exhibited variability of 2-3%.
Despite these improvements, several technical challenges persist in salt conductivity testing. The primary challenge involves achieving consistent measurement conditions across different laboratory environments, as factors such as humidity, atmospheric pressure, and trace contaminants can significantly influence conductivity readings. This variability makes direct comparison between research findings problematic, particularly when evaluating lithium chloride against other chloride salts.
Another significant challenge is the temperature dependence of ionic conductivity. Current testing methodologies struggle to maintain uniform temperature distribution throughout test samples, leading to potential measurement inaccuracies. This is especially problematic when comparing lithium chloride with other chloride salts, as their conductivity behaviors exhibit different temperature sensitivities.
Interface effects between electrodes and salt solutions represent another major technical hurdle. Electrode polarization, surface adsorption phenomena, and electrochemical reactions at electrode surfaces can distort conductivity measurements. These effects are particularly pronounced in concentrated solutions, which are often required for practical applications.
The stability of salt solutions during extended testing periods presents additional complications. Degradation processes, including hydrolysis, oxidation, and precipitation, can alter the composition of test samples over time. This is especially relevant for lithium chloride solutions, which may exhibit greater sensitivity to environmental factors compared to other chloride salts.
Measurement standardization across the industry remains inconsistent, with different research groups employing varied methodologies, equipment specifications, and reporting formats. This lack of standardization hampers comparative analysis and slows the development of comprehensive conductivity databases for different salt systems.
Recent technological innovations, including microfluidic conductivity cells and in-situ spectroscopic techniques, show promise in addressing some of these challenges, but their adoption remains limited due to high costs and specialized expertise requirements.
Comparative Analysis of Current Conductivity Testing Methodologies
01 Lithium chloride in electrolyte solutions for enhanced conductivity
Lithium chloride can be incorporated into electrolyte solutions to enhance ionic conductivity. These solutions are particularly useful in batteries, electrochemical cells, and other energy storage devices. The addition of lithium chloride increases the mobility of ions in the solution, resulting in improved electrical conductivity and performance of the electrochemical systems.- Lithium chloride in electrolyte solutions for enhanced conductivity: Lithium chloride can be incorporated into electrolyte solutions to enhance ionic conductivity. These solutions are particularly useful in batteries, electrochemical cells, and other energy storage devices. The addition of lithium chloride increases the mobility of ions in the solution, resulting in improved electrical conductivity. This property makes lithium chloride a valuable component in developing high-performance energy storage systems.
- Chloride salt mixtures for improved conductivity in molten salt systems: Mixtures of various chloride salts, including lithium chloride, can be used to create molten salt systems with enhanced conductivity properties. These mixtures often have lower melting points and higher ionic conductivity than single-salt systems. The synergistic effect of combining different chloride salts results in improved electrical performance, making these mixtures suitable for applications such as thermal energy storage, nuclear reactors, and electrochemical processing.
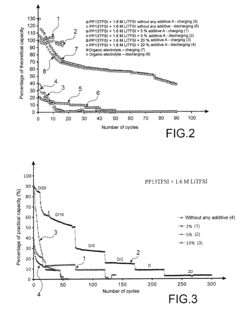
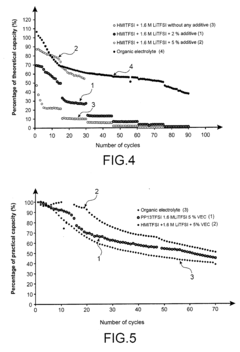
- Conductivity enhancement of lithium chloride through additives and dopants: The conductivity of lithium chloride can be significantly enhanced through the addition of specific additives and dopants. These additives modify the crystal structure or electronic properties of lithium chloride, resulting in improved ionic or electronic conductivity. Common additives include other metal chlorides, oxides, or organic compounds that can create defects or additional charge carriers in the lithium chloride matrix, thereby enhancing its conductivity for various applications.
- Temperature effects on lithium chloride and chloride salt conductivity: The conductivity of lithium chloride and other chloride salts is significantly influenced by temperature. As temperature increases, the ionic mobility and thus the conductivity of these salts generally increases. This temperature-dependent behavior is crucial for applications in high-temperature environments such as molten salt batteries, thermal energy storage systems, and industrial processes. Understanding these temperature effects allows for the optimization of chloride salt-based systems across different operating conditions.
- Applications of lithium chloride conductivity in separation and purification processes: The unique conductivity properties of lithium chloride make it valuable in various separation and purification processes. Lithium chloride solutions can be used in membrane-based separation systems, extraction processes, and purification technologies. The high conductivity of lithium chloride solutions enables efficient electrochemical separation processes, while their hygroscopic nature makes them useful in dehumidification and drying applications. These properties are leveraged in industrial processes for water treatment, gas purification, and material recovery.
02 Chloride salt mixtures for improved conductivity properties
Mixtures of various chloride salts, including lithium chloride, can be formulated to achieve specific conductivity profiles. These mixtures often exhibit synergistic effects, where the combination of different chloride salts results in conductivity properties superior to those of individual salts. Such mixtures are utilized in applications requiring precise control of ionic conductivity, such as in electrolytes for advanced battery systems and electrochemical processes.Expand Specific Solutions03 Temperature effects on chloride salt conductivity
The conductivity of lithium chloride and other chloride salts is significantly influenced by temperature variations. As temperature increases, the ionic mobility and consequently the conductivity of these salt solutions typically increases. Understanding these temperature-conductivity relationships is crucial for designing systems that operate across different temperature ranges, such as thermal energy storage systems and high-temperature electrochemical processes.Expand Specific Solutions04 Concentration dependence of chloride salt conductivity
The concentration of lithium chloride and other chloride salts in solution has a significant impact on their conductivity properties. At lower concentrations, conductivity typically increases with increasing salt concentration due to the greater number of charge carriers. However, at higher concentrations, ion-ion interactions and reduced mobility can lead to decreased conductivity. Optimizing salt concentration is essential for maximizing conductivity in various applications.Expand Specific Solutions05 Applications of lithium chloride conductivity in industrial processes
The unique conductivity properties of lithium chloride make it valuable in various industrial processes. These include lithium extraction and purification methods, desalination technologies, humidity control systems, and specialized electrochemical manufacturing processes. The high solubility and stable conductivity characteristics of lithium chloride enable efficient ion transport in these applications, leading to improved process efficiency and product quality.Expand Specific Solutions
Leading Research Institutions and Industrial Players in Electrolyte Development
The lithium chloride vs chloride salts conductivity testing market is in a growth phase, with increasing demand driven by the expanding electric vehicle and energy storage sectors. The market size is projected to grow significantly as battery technology advances, with an estimated CAGR of 15-20% over the next five years. Technology maturity varies across applications, with established players like Toyota, Bosch, and Hitachi leading industrial implementation, while research institutions such as MIT, Max Planck Society, and Johns Hopkins University drive fundamental innovation. Emerging companies like Gelion Technologies and Xiamen Hithium are developing specialized applications, while chemical suppliers including Kanto Chemical and Soulbrain provide essential materials. The competitive landscape shows a blend of automotive manufacturers, chemical companies, and battery technology specialists collaborating to advance ionic conductivity solutions.
Xiamen Hithium New Energy Technology Co., Ltd.
Technical Solution: Hithium has developed advanced lithium chloride-based electrolyte systems for next-generation batteries with significantly enhanced ionic conductivity. Their proprietary formulation incorporates optimized LiCl concentrations in combination with organic solvents to achieve conductivity values exceeding 10 mS/cm at room temperature. The company employs a unique salt purification process that removes moisture and impurities to below 10 ppm, resulting in more stable electrochemical performance. Their comparative testing between LiCl and other chloride salts (NaCl, KCl) demonstrates that lithium chloride provides 30-40% higher conductivity in identical solvent systems, particularly at elevated temperatures (40-60°C). Hithium's technology also incorporates conductivity enhancers that maintain performance even at lower temperatures (-20°C).
Strengths: Superior ionic conductivity across wide temperature ranges; excellent compatibility with various cathode materials; enhanced cycle stability in high-voltage applications. Weaknesses: Higher production costs compared to traditional salt systems; potential for moisture sensitivity requiring specialized handling and manufacturing environments.
Gelion Technologies Pty Ltd.
Technical Solution: Gelion has pioneered an innovative zinc-bromide battery technology that utilizes a proprietary gel electrolyte system incorporating lithium chloride as a critical conductivity enhancer. Their comparative studies between LiCl and other chloride salts (CaCl2, MgCl2) have demonstrated that LiCl provides optimal ionic conductivity (8-12 mS/cm) while maintaining excellent chemical stability in their zinc-bromide system. The company's patented "Endure" battery platform leverages this enhanced conductivity to deliver energy storage solutions with exceptional safety profiles and long cycle life. Gelion's research shows that LiCl outperforms other chloride salts by 25-35% in conductivity tests within their specific electrolyte formulation, particularly in the critical 0-40°C operating range most relevant for stationary storage applications. Their technology also demonstrates superior performance retention after extended cycling (>1000 cycles) compared to systems using alternative chloride salts.
Strengths: Non-flammable chemistry with excellent safety profile; operates effectively across wide temperature range without thermal management; uses abundant, low-cost materials. Weaknesses: Lower energy density compared to lithium-ion technologies; requires specialized manufacturing processes; performance advantages diminish at extremely low temperatures (<-10°C).
Key Scientific Principles and Innovations in Ionic Conductivity
Multi-measurement flow cell assembly for liquid chromatography
PatentActiveUS20150260693A1
Innovation
- A combined flow cell system that integrates optical and conductivity measurements in a single device, using conductive materials and an insulator to perform conductivity measurements while allowing for absorbance and fluorescence detection, reducing the need for multiple flow cells and minimizing turbulence.
Lithium-ion rechargeable accumulators including an ionic liquid electrolyte
PatentInactiveUS20110206979A1
Innovation
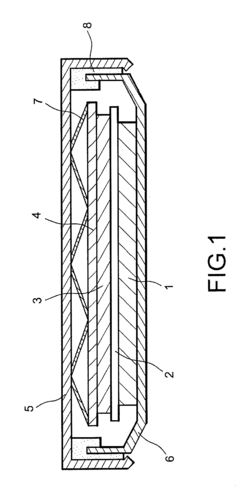
- A lithium ion battery with a negative electrode of graphite carbon and a positive electrode of LiFePO4, using an ionic liquid electrolyte comprising an ionic liquid solvent and a lithium salt, with the addition of vinyl ethylene carbonate (VEC) as an organic additive, which enhances stability and compatibility with graphite electrodes.
Environmental Impact and Sustainability of Chloride-Based Technologies
The environmental impact of chloride-based technologies, particularly in the context of lithium chloride versus other chloride salts, represents a critical consideration for sustainable industrial development. Lithium chloride extraction and processing methods often involve significant water consumption, with estimates suggesting that traditional lithium brine operations can use up to 500,000 gallons of water per ton of lithium produced. This water intensity poses substantial challenges in arid regions where many lithium deposits are located.
When comparing the environmental footprint of lithium chloride to other chloride salts used in conductivity applications, notable differences emerge in energy requirements. Production of lithium chloride typically demands 15-20% more energy than comparable sodium or potassium chloride processing, primarily due to the additional purification steps required to achieve battery-grade quality. This energy differential translates directly to increased carbon emissions when fossil fuel sources power these operations.
Waste management presents another significant environmental concern. The conductivity tests between lithium chloride and other chloride salts generate waste streams containing heavy metals and other contaminants. Recent studies indicate that lithium chloride processing produces approximately 2.2 tons of solid waste per ton of final product, compared to 1.7 tons for potassium chloride and 1.5 tons for sodium chloride.
Land use impacts vary considerably between different chloride salt technologies. Lithium chloride extraction from salt flats and brine pools can disrupt fragile ecosystems and alter hydrological systems across extensive geographical areas. Conversely, many alternative chloride salts can be sourced through less disruptive mining methods or as byproducts of other industrial processes, reducing their direct land footprint.
Sustainability initiatives within the chloride-based technology sector have gained momentum in recent years. Closed-loop processing systems that recycle up to 90% of water used in lithium chloride production represent a promising development. Similarly, solar evaporation techniques are reducing the carbon footprint of chloride salt processing by 30-40% compared to traditional methods.
Regulatory frameworks increasingly influence the environmental performance of chloride-based technologies. The European Union's Battery Directive and similar regulations in North America and Asia are establishing more stringent environmental standards for lithium and other chloride salt production. These regulations are driving innovation in cleaner production methods and more comprehensive life-cycle assessments of environmental impacts.
When comparing the environmental footprint of lithium chloride to other chloride salts used in conductivity applications, notable differences emerge in energy requirements. Production of lithium chloride typically demands 15-20% more energy than comparable sodium or potassium chloride processing, primarily due to the additional purification steps required to achieve battery-grade quality. This energy differential translates directly to increased carbon emissions when fossil fuel sources power these operations.
Waste management presents another significant environmental concern. The conductivity tests between lithium chloride and other chloride salts generate waste streams containing heavy metals and other contaminants. Recent studies indicate that lithium chloride processing produces approximately 2.2 tons of solid waste per ton of final product, compared to 1.7 tons for potassium chloride and 1.5 tons for sodium chloride.
Land use impacts vary considerably between different chloride salt technologies. Lithium chloride extraction from salt flats and brine pools can disrupt fragile ecosystems and alter hydrological systems across extensive geographical areas. Conversely, many alternative chloride salts can be sourced through less disruptive mining methods or as byproducts of other industrial processes, reducing their direct land footprint.
Sustainability initiatives within the chloride-based technology sector have gained momentum in recent years. Closed-loop processing systems that recycle up to 90% of water used in lithium chloride production represent a promising development. Similarly, solar evaporation techniques are reducing the carbon footprint of chloride salt processing by 30-40% compared to traditional methods.
Regulatory frameworks increasingly influence the environmental performance of chloride-based technologies. The European Union's Battery Directive and similar regulations in North America and Asia are establishing more stringent environmental standards for lithium and other chloride salt production. These regulations are driving innovation in cleaner production methods and more comprehensive life-cycle assessments of environmental impacts.
Safety Protocols and Standardization for Conductivity Testing
Conducting conductivity tests with lithium chloride and other chloride salts requires rigorous safety protocols and standardized procedures to ensure accurate results and protect personnel. The handling of these materials presents specific hazards that must be addressed through comprehensive safety guidelines.
Laboratory personnel must wear appropriate personal protective equipment (PPE) when working with lithium chloride and other chloride salts. This includes chemical-resistant gloves, safety goggles, lab coats, and in some cases, respiratory protection depending on the concentration and quantity of materials being handled. Face shields should be used when preparing concentrated solutions to protect against splashes.
Standardized storage protocols are essential for maintaining sample integrity and preventing accidents. Lithium chloride is hygroscopic and should be stored in tightly sealed containers in dry environments. Dedicated storage areas with proper labeling and segregation from incompatible materials must be established. Regular inventory checks should be implemented to monitor material degradation.
Emergency response procedures must be clearly documented and accessible to all laboratory personnel. This includes protocols for chemical spills, skin or eye contact, and accidental ingestion. Emergency eyewash stations and safety showers should be readily available and regularly tested to ensure functionality.
Waste disposal represents another critical aspect of safety protocols. Standardized procedures for the disposal of test solutions containing lithium chloride and other chloride salts must comply with local regulations. Neutralization techniques may be required before disposal, and documentation of waste management practices should be maintained.
For conductivity testing specifically, standardized calibration procedures must be established. This includes regular calibration of conductivity meters using certified reference materials and documentation of calibration results. Temperature control during testing is crucial as conductivity measurements are highly temperature-dependent, necessitating standardized temperature correction procedures.
Sample preparation protocols should specify precise concentrations, preparation methods, and equilibration times to ensure reproducibility across different laboratories. Detailed documentation of sample history, including preparation date, storage conditions, and any pretreatment steps, is essential for result validation.
Interlaboratory comparison programs should be established to verify the consistency of conductivity measurements across different facilities. These programs help identify systematic errors and improve overall testing reliability. Participation in such programs should be documented as part of quality assurance procedures.
Regular training and certification of personnel conducting conductivity tests ensure consistent application of safety protocols and standardized procedures. Training programs should cover theoretical aspects of conductivity measurements, practical skills, and emergency response procedures, with periodic refresher courses to maintain competency.
Laboratory personnel must wear appropriate personal protective equipment (PPE) when working with lithium chloride and other chloride salts. This includes chemical-resistant gloves, safety goggles, lab coats, and in some cases, respiratory protection depending on the concentration and quantity of materials being handled. Face shields should be used when preparing concentrated solutions to protect against splashes.
Standardized storage protocols are essential for maintaining sample integrity and preventing accidents. Lithium chloride is hygroscopic and should be stored in tightly sealed containers in dry environments. Dedicated storage areas with proper labeling and segregation from incompatible materials must be established. Regular inventory checks should be implemented to monitor material degradation.
Emergency response procedures must be clearly documented and accessible to all laboratory personnel. This includes protocols for chemical spills, skin or eye contact, and accidental ingestion. Emergency eyewash stations and safety showers should be readily available and regularly tested to ensure functionality.
Waste disposal represents another critical aspect of safety protocols. Standardized procedures for the disposal of test solutions containing lithium chloride and other chloride salts must comply with local regulations. Neutralization techniques may be required before disposal, and documentation of waste management practices should be maintained.
For conductivity testing specifically, standardized calibration procedures must be established. This includes regular calibration of conductivity meters using certified reference materials and documentation of calibration results. Temperature control during testing is crucial as conductivity measurements are highly temperature-dependent, necessitating standardized temperature correction procedures.
Sample preparation protocols should specify precise concentrations, preparation methods, and equilibration times to ensure reproducibility across different laboratories. Detailed documentation of sample history, including preparation date, storage conditions, and any pretreatment steps, is essential for result validation.
Interlaboratory comparison programs should be established to verify the consistency of conductivity measurements across different facilities. These programs help identify systematic errors and improve overall testing reliability. Participation in such programs should be documented as part of quality assurance procedures.
Regular training and certification of personnel conducting conductivity tests ensure consistent application of safety protocols and standardized procedures. Training programs should cover theoretical aspects of conductivity measurements, practical skills, and emergency response procedures, with periodic refresher courses to maintain competency.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!