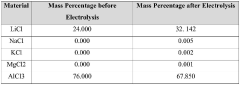
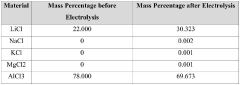
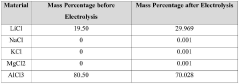
Optimize Synthesis of Anhydrous Lithium Chloride
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Anhydrous LiCl Synthesis Background and Objectives
Anhydrous lithium chloride (LiCl) represents a critical compound in various industrial applications, with its synthesis methods evolving significantly over the past several decades. The compound's importance has grown exponentially with the rise of lithium-based technologies, particularly in energy storage systems, pharmaceuticals, and advanced materials manufacturing. Historically, anhydrous LiCl production has transitioned from rudimentary thermal dehydration methods to more sophisticated chemical processes that aim to enhance purity while reducing energy consumption.
The technical evolution trajectory shows a clear shift from batch processing toward continuous flow methodologies, with significant breakthroughs occurring in the early 2000s when vacuum dehydration techniques were refined for industrial-scale implementation. Recent advancements have focused on addressing the hygroscopic nature of LiCl, which presents persistent challenges in maintaining anhydrous conditions throughout synthesis and storage phases.
Current research indicates that optimization efforts are primarily concentrated on three fronts: energy efficiency in dehydration processes, purity enhancement through novel filtration techniques, and stability improvement via innovative packaging and handling protocols. The technical community has established benchmark standards for anhydrous LiCl, typically requiring water content below 0.005% for high-purity applications.
The primary objective of optimizing anhydrous LiCl synthesis is to develop scalable, energy-efficient processes that consistently yield high-purity product while minimizing environmental impact. Specific technical goals include reducing energy consumption by at least 30% compared to conventional methods, achieving 99.99% purity levels consistently, and extending shelf-life stability under standard industrial storage conditions.
Secondary objectives encompass the development of in-line monitoring systems for real-time quality control, exploration of alternative precursor materials to reduce production costs, and implementation of closed-loop recycling systems for process waste streams. These objectives align with broader industry trends toward sustainable manufacturing practices and circular economy principles.
The technical landscape suggests promising opportunities for cross-disciplinary approaches, particularly at the intersection of materials science, chemical engineering, and process automation. Emerging technologies in microwave-assisted dehydration and supercritical fluid processing represent potential paradigm shifts in anhydrous LiCl synthesis, though these approaches remain in early development stages.
As global demand for lithium compounds continues to accelerate, driven largely by electric vehicle battery production and grid-scale energy storage systems, optimizing anhydrous LiCl synthesis has become increasingly strategic from both economic and sustainability perspectives. This technical investigation aims to comprehensively map the current state of the art while identifying high-potential pathways for meaningful advancement.
The technical evolution trajectory shows a clear shift from batch processing toward continuous flow methodologies, with significant breakthroughs occurring in the early 2000s when vacuum dehydration techniques were refined for industrial-scale implementation. Recent advancements have focused on addressing the hygroscopic nature of LiCl, which presents persistent challenges in maintaining anhydrous conditions throughout synthesis and storage phases.
Current research indicates that optimization efforts are primarily concentrated on three fronts: energy efficiency in dehydration processes, purity enhancement through novel filtration techniques, and stability improvement via innovative packaging and handling protocols. The technical community has established benchmark standards for anhydrous LiCl, typically requiring water content below 0.005% for high-purity applications.
The primary objective of optimizing anhydrous LiCl synthesis is to develop scalable, energy-efficient processes that consistently yield high-purity product while minimizing environmental impact. Specific technical goals include reducing energy consumption by at least 30% compared to conventional methods, achieving 99.99% purity levels consistently, and extending shelf-life stability under standard industrial storage conditions.
Secondary objectives encompass the development of in-line monitoring systems for real-time quality control, exploration of alternative precursor materials to reduce production costs, and implementation of closed-loop recycling systems for process waste streams. These objectives align with broader industry trends toward sustainable manufacturing practices and circular economy principles.
The technical landscape suggests promising opportunities for cross-disciplinary approaches, particularly at the intersection of materials science, chemical engineering, and process automation. Emerging technologies in microwave-assisted dehydration and supercritical fluid processing represent potential paradigm shifts in anhydrous LiCl synthesis, though these approaches remain in early development stages.
As global demand for lithium compounds continues to accelerate, driven largely by electric vehicle battery production and grid-scale energy storage systems, optimizing anhydrous LiCl synthesis has become increasingly strategic from both economic and sustainability perspectives. This technical investigation aims to comprehensively map the current state of the art while identifying high-potential pathways for meaningful advancement.
Market Demand Analysis for High-Purity Anhydrous LiCl
The global market for high-purity anhydrous lithium chloride (LiCl) has experienced significant growth in recent years, driven primarily by expanding applications in battery technologies, pharmaceuticals, and advanced materials. The lithium-ion battery sector represents the largest demand segment, with anhydrous LiCl serving as a critical precursor in electrolyte formulations and cathode material synthesis. Market research indicates that this segment alone has grown at a compound annual rate of 18% over the past five years.
In the pharmaceutical industry, high-purity anhydrous LiCl is increasingly utilized in organic synthesis processes and as a component in certain psychiatric medications. This sector accounts for approximately 22% of the total market demand, with projections suggesting continued steady growth as new therapeutic applications emerge.
The electronics industry represents another substantial market for anhydrous LiCl, particularly in applications requiring ultra-dry conditions such as semiconductor manufacturing and specialized soldering processes. This segment has shown consistent demand growth of 12-15% annually, reflecting the broader expansion of advanced electronics manufacturing globally.
Regional analysis reveals that Asia-Pacific dominates the consumption landscape, accounting for over 60% of global demand, with China, South Korea, and Japan being the primary markets. This concentration aligns with the regional distribution of lithium battery manufacturing facilities and electronics production hubs. North America and Europe follow with approximately 20% and 15% market share respectively, primarily driven by pharmaceutical applications and emerging energy storage initiatives.
Price sensitivity analysis indicates that high-purity anhydrous LiCl commands a significant premium over standard grades, with prices typically 3-4 times higher for material exceeding 99.99% purity. This price differential has remained relatively stable despite increasing production volumes, suggesting robust demand fundamentals for premium-grade material.
Supply chain assessment reveals potential vulnerabilities, as production remains concentrated among a limited number of specialized chemical manufacturers. Recent market disruptions have highlighted the strategic importance of securing reliable sources of high-purity anhydrous LiCl, prompting many end-users to pursue supply diversification strategies and long-term procurement agreements.
Future market projections indicate continued strong growth, with the global high-purity anhydrous LiCl market expected to expand at 14-16% annually through 2028. This growth trajectory is supported by accelerating adoption of lithium battery technologies across automotive, grid storage, and consumer electronics applications, as well as emerging uses in next-generation pharmaceutical manufacturing processes.
In the pharmaceutical industry, high-purity anhydrous LiCl is increasingly utilized in organic synthesis processes and as a component in certain psychiatric medications. This sector accounts for approximately 22% of the total market demand, with projections suggesting continued steady growth as new therapeutic applications emerge.
The electronics industry represents another substantial market for anhydrous LiCl, particularly in applications requiring ultra-dry conditions such as semiconductor manufacturing and specialized soldering processes. This segment has shown consistent demand growth of 12-15% annually, reflecting the broader expansion of advanced electronics manufacturing globally.
Regional analysis reveals that Asia-Pacific dominates the consumption landscape, accounting for over 60% of global demand, with China, South Korea, and Japan being the primary markets. This concentration aligns with the regional distribution of lithium battery manufacturing facilities and electronics production hubs. North America and Europe follow with approximately 20% and 15% market share respectively, primarily driven by pharmaceutical applications and emerging energy storage initiatives.
Price sensitivity analysis indicates that high-purity anhydrous LiCl commands a significant premium over standard grades, with prices typically 3-4 times higher for material exceeding 99.99% purity. This price differential has remained relatively stable despite increasing production volumes, suggesting robust demand fundamentals for premium-grade material.
Supply chain assessment reveals potential vulnerabilities, as production remains concentrated among a limited number of specialized chemical manufacturers. Recent market disruptions have highlighted the strategic importance of securing reliable sources of high-purity anhydrous LiCl, prompting many end-users to pursue supply diversification strategies and long-term procurement agreements.
Future market projections indicate continued strong growth, with the global high-purity anhydrous LiCl market expected to expand at 14-16% annually through 2028. This growth trajectory is supported by accelerating adoption of lithium battery technologies across automotive, grid storage, and consumer electronics applications, as well as emerging uses in next-generation pharmaceutical manufacturing processes.
Technical Challenges in Anhydrous LiCl Synthesis
The synthesis of anhydrous lithium chloride presents significant technical challenges due to its hygroscopic nature and the complexity of achieving high purity levels required for industrial applications. The conventional methods for producing anhydrous LiCl involve dehydration of hydrated lithium chloride, which is inherently problematic as water molecules are strongly coordinated to lithium ions through ion-dipole interactions, making complete removal difficult.
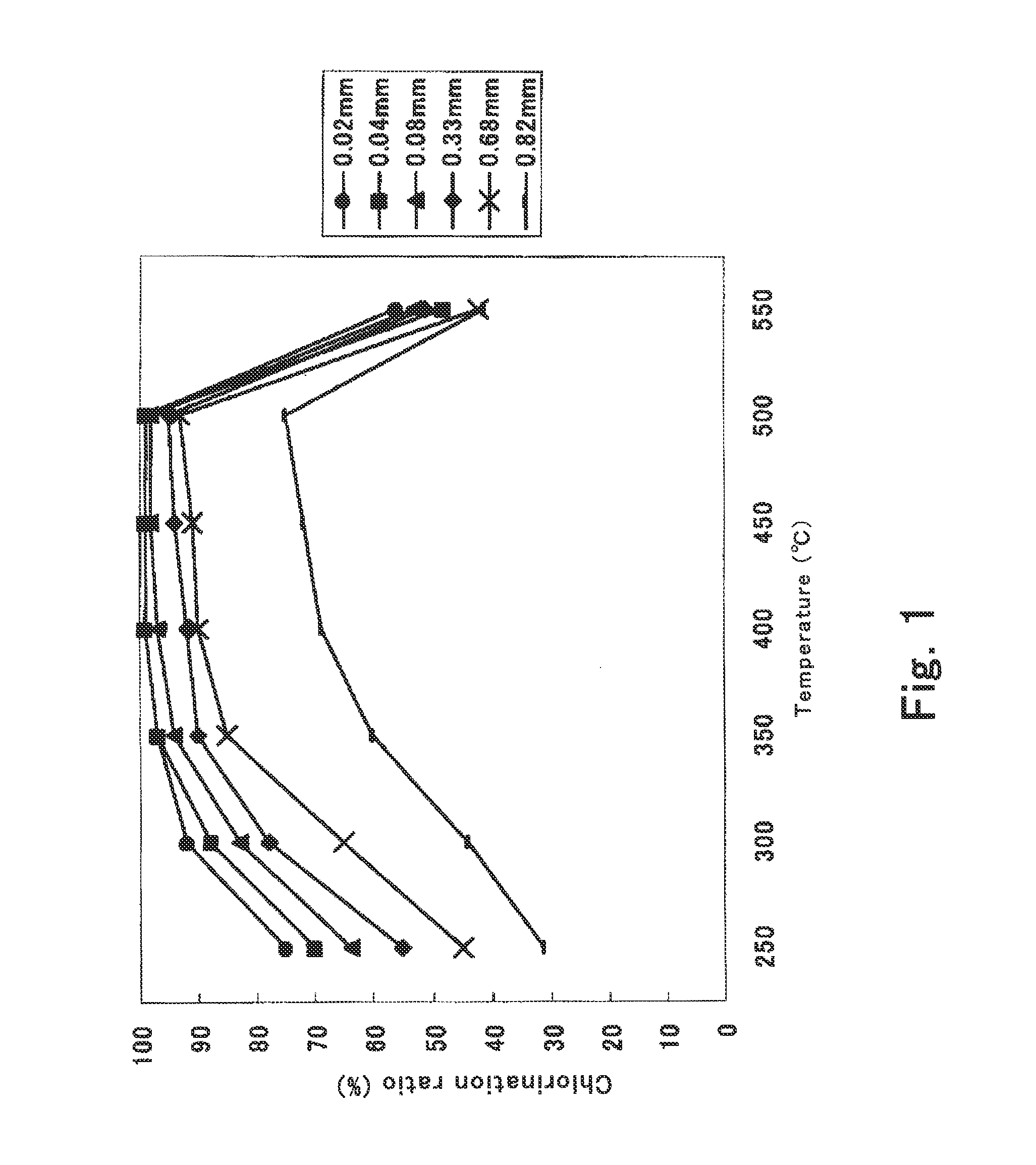
Temperature control represents a critical challenge in the synthesis process. While higher temperatures accelerate dehydration, they also increase the risk of thermal decomposition and potential contamination from reaction vessels. The optimal temperature range (typically 350-450°C) must be precisely maintained to ensure complete dehydration without degrading product quality or introducing impurities.
Atmospheric control presents another significant hurdle. Even trace amounts of moisture in the processing environment can compromise the anhydrous state of the final product. Current industrial processes require sophisticated dry rooms or inert gas environments, substantially increasing production costs and operational complexity. The development of cost-effective moisture exclusion systems remains an ongoing challenge.
Scaling production from laboratory to industrial levels introduces additional complications. Heat and mass transfer limitations become more pronounced at larger scales, leading to potential inconsistencies in product quality. The design of reactors that can maintain uniform conditions throughout larger batch volumes represents a significant engineering challenge.
Energy efficiency concerns are increasingly prominent in anhydrous LiCl synthesis. Traditional thermal dehydration methods consume substantial energy, contributing to high production costs and environmental impact. The industry faces pressure to develop lower-energy alternatives while maintaining product quality and production rates.
Purification techniques present further challenges, as conventional methods like recrystallization are complicated by LiCl's hygroscopic properties. Impurities such as sodium, calcium, and magnesium ions can significantly impact the performance of LiCl in downstream applications, particularly in battery technologies where high purity is essential.
Material compatibility issues arise during processing as hot, anhydrous LiCl can be corrosive to many common reactor materials. This necessitates the use of specialized, often expensive, materials of construction that can withstand the harsh processing conditions without contaminating the product.
Analytical challenges also exist in accurately determining the residual water content in the final product. Traditional Karl Fischer titration methods require careful sample handling to prevent moisture absorption during analysis, while newer spectroscopic techniques are still being optimized for sensitivity and reliability in production environments.
Temperature control represents a critical challenge in the synthesis process. While higher temperatures accelerate dehydration, they also increase the risk of thermal decomposition and potential contamination from reaction vessels. The optimal temperature range (typically 350-450°C) must be precisely maintained to ensure complete dehydration without degrading product quality or introducing impurities.
Atmospheric control presents another significant hurdle. Even trace amounts of moisture in the processing environment can compromise the anhydrous state of the final product. Current industrial processes require sophisticated dry rooms or inert gas environments, substantially increasing production costs and operational complexity. The development of cost-effective moisture exclusion systems remains an ongoing challenge.
Scaling production from laboratory to industrial levels introduces additional complications. Heat and mass transfer limitations become more pronounced at larger scales, leading to potential inconsistencies in product quality. The design of reactors that can maintain uniform conditions throughout larger batch volumes represents a significant engineering challenge.
Energy efficiency concerns are increasingly prominent in anhydrous LiCl synthesis. Traditional thermal dehydration methods consume substantial energy, contributing to high production costs and environmental impact. The industry faces pressure to develop lower-energy alternatives while maintaining product quality and production rates.
Purification techniques present further challenges, as conventional methods like recrystallization are complicated by LiCl's hygroscopic properties. Impurities such as sodium, calcium, and magnesium ions can significantly impact the performance of LiCl in downstream applications, particularly in battery technologies where high purity is essential.
Material compatibility issues arise during processing as hot, anhydrous LiCl can be corrosive to many common reactor materials. This necessitates the use of specialized, often expensive, materials of construction that can withstand the harsh processing conditions without contaminating the product.
Analytical challenges also exist in accurately determining the residual water content in the final product. Traditional Karl Fischer titration methods require careful sample handling to prevent moisture absorption during analysis, while newer spectroscopic techniques are still being optimized for sensitivity and reliability in production environments.
Current Synthesis Approaches for Anhydrous LiCl
01 Chemical reaction methods for anhydrous lithium chloride synthesis
Various chemical reaction methods can be employed to synthesize anhydrous lithium chloride. These include the reaction of lithium hydroxide with hydrochloric acid followed by dehydration, direct reaction of lithium metal with chlorine gas, and the conversion of lithium carbonate using hydrochloric acid. These methods focus on creating pure anhydrous lithium chloride through controlled chemical reactions that minimize water content in the final product.- Chemical reaction methods for anhydrous lithium chloride synthesis: Various chemical reaction methods can be employed to synthesize anhydrous lithium chloride. These include the reaction of lithium hydroxide with hydrochloric acid followed by dehydration, direct reaction of lithium metal with chlorine gas, and the conversion of lithium carbonate to lithium chloride using hydrochloric acid. These methods focus on achieving high purity anhydrous lithium chloride through controlled reaction conditions and subsequent dehydration steps.
- Dehydration techniques for lithium chloride: Effective dehydration is crucial for producing anhydrous lithium chloride. Techniques include vacuum drying, heating in inert gas atmosphere, azeotropic distillation, and the use of desiccants. The optimization of temperature, pressure, and duration parameters during the dehydration process significantly impacts the final product quality. Advanced dehydration methods can reduce energy consumption while ensuring complete removal of water molecules from the lithium chloride crystal structure.
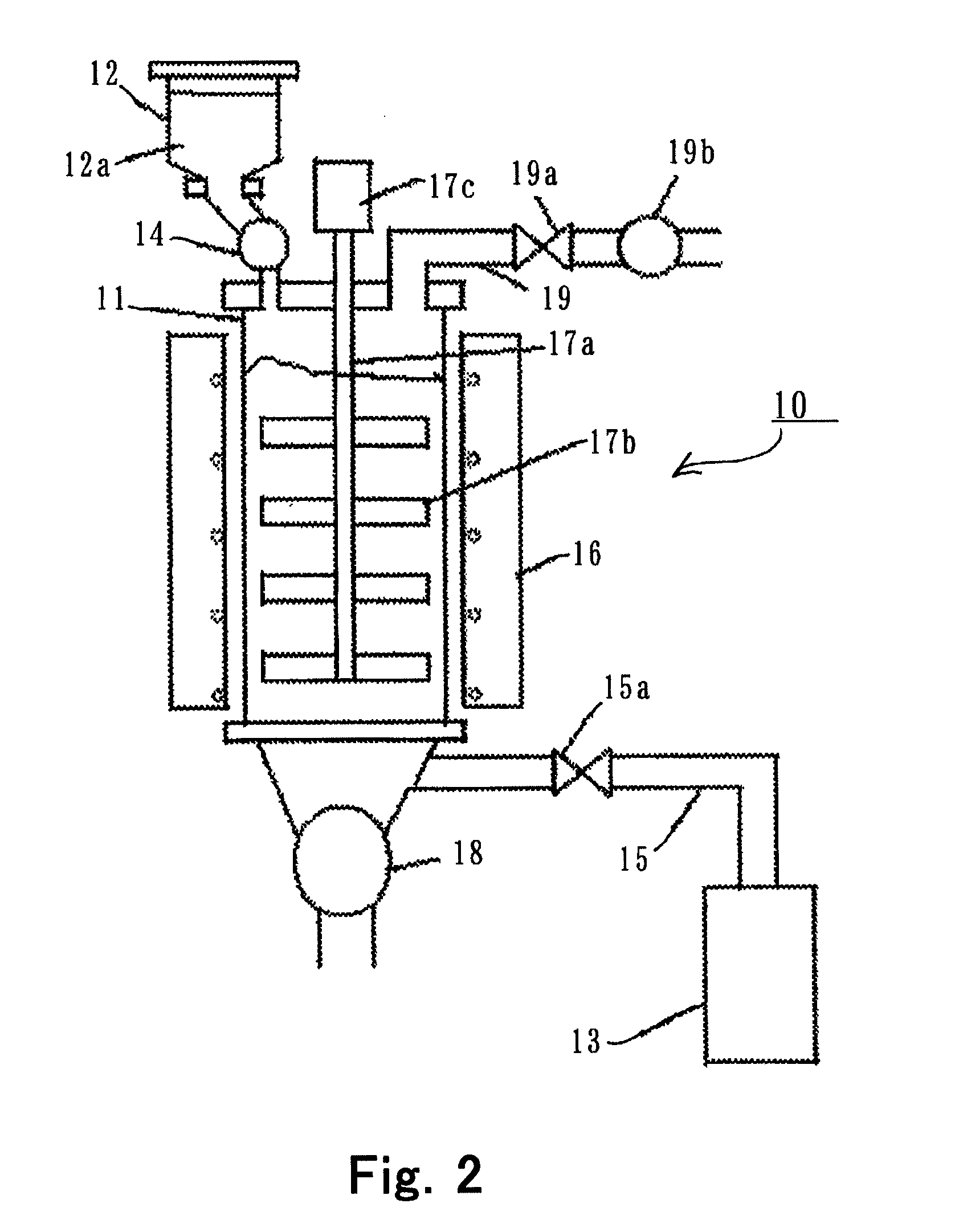
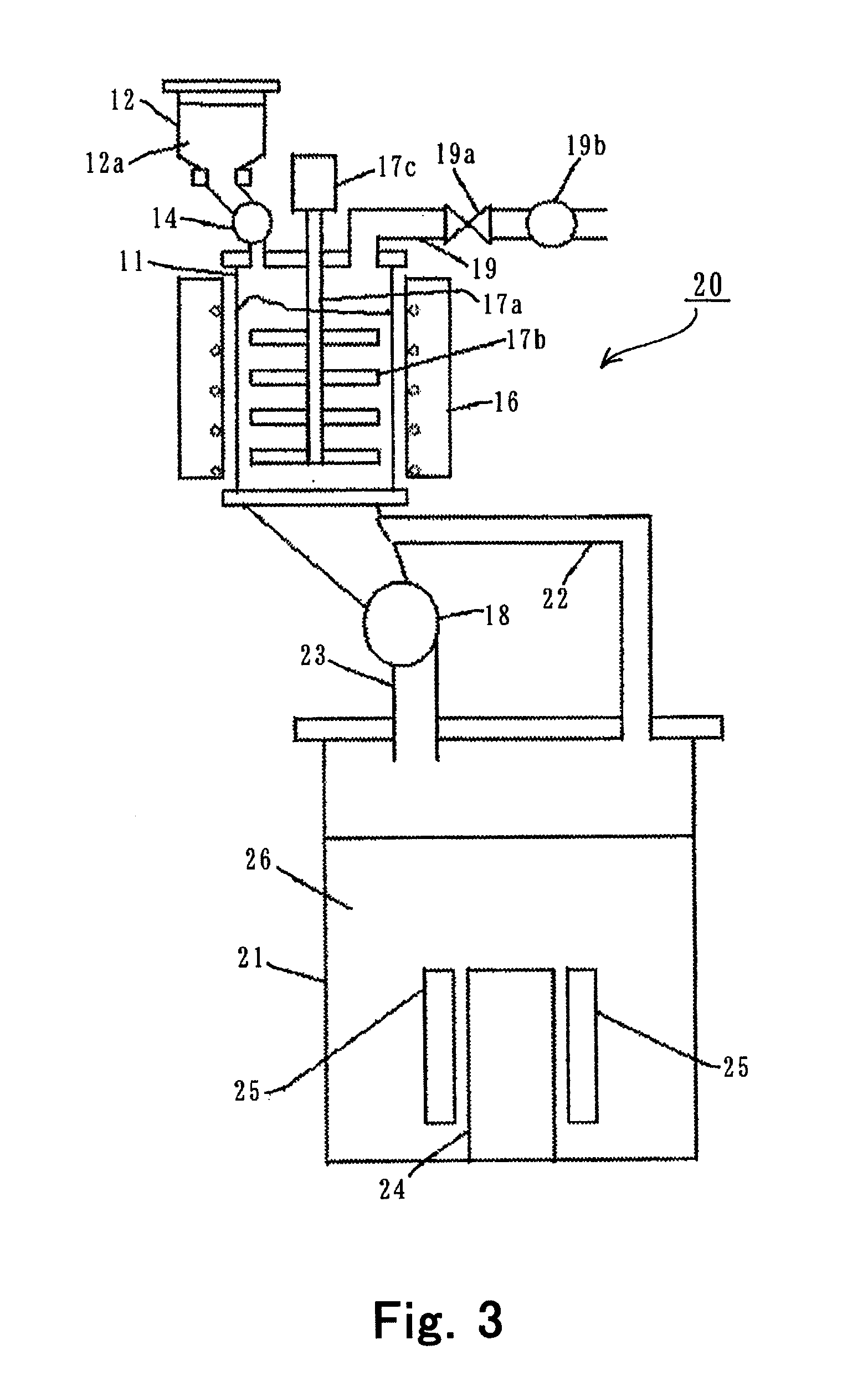
- Equipment and apparatus design for synthesis: Specialized equipment designs enhance the efficiency of anhydrous lithium chloride production. These include reactor vessels with precise temperature control, vacuum systems for moisture removal, specialized crystallization equipment, and continuous flow reactors. The equipment often incorporates corrosion-resistant materials due to the aggressive nature of the chemicals involved. Innovations in apparatus design focus on improving heat transfer, reducing contamination, and enabling better process control.
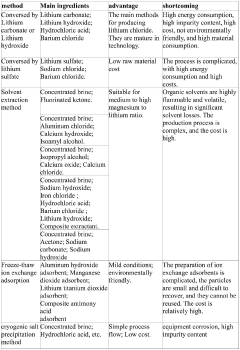
- Purification methods for high-purity anhydrous lithium chloride: Achieving high-purity anhydrous lithium chloride requires sophisticated purification techniques. These include recrystallization from anhydrous solvents, zone refining, ion exchange methods, and selective precipitation of impurities. The purification process often involves multiple steps to progressively remove different types of contaminants. Advanced analytical techniques are employed to monitor purity levels throughout the process, ensuring the final product meets stringent quality standards for specialized applications.
- Energy-efficient and sustainable production processes: Modern approaches to anhydrous lithium chloride synthesis focus on energy efficiency and sustainability. These include the development of low-temperature synthesis routes, recovery and recycling of reagents, utilization of renewable energy sources for process heating, and implementation of continuous manufacturing techniques. Process intensification strategies reduce waste generation and energy consumption while maintaining product quality. These sustainable methods are becoming increasingly important as demand for lithium compounds grows in various industries.
02 Dehydration techniques for lithium chloride
Effective dehydration is crucial for producing anhydrous lithium chloride. Techniques include vacuum drying at controlled temperatures, using desiccants like phosphorus pentoxide, heating in inert gas atmospheres, and multi-stage dehydration processes. These methods aim to remove water molecules from lithium chloride hydrates while preventing decomposition or contamination of the final product.Expand Specific Solutions03 Equipment and apparatus optimization for anhydrous production
Specialized equipment designs enhance the efficiency of anhydrous lithium chloride production. These include customized reaction vessels with precise temperature control, vacuum systems with moisture traps, continuous flow reactors for consistent quality, and sealed processing environments to prevent moisture reabsorption. The equipment often incorporates corrosion-resistant materials due to the aggressive nature of the chemicals involved in the synthesis process.Expand Specific Solutions04 Process optimization and quality control methods
Optimizing the synthesis process involves careful control of reaction parameters such as temperature, pressure, and reactant ratios. Quality control methods include X-ray diffraction analysis to confirm crystal structure, Karl Fischer titration to verify water content, and spectroscopic techniques to detect impurities. Advanced monitoring systems allow for real-time adjustments during production to maintain anhydrous conditions and product purity.Expand Specific Solutions05 Novel applications driving synthesis improvements
Emerging applications for anhydrous lithium chloride are driving improvements in synthesis methods. These applications include use as electrolytes in advanced batteries, as drying agents in industrial processes, as catalysts in organic synthesis, and as components in specialized ceramics. The specific requirements of these applications, such as ultra-high purity or particular physical properties, have led to tailored synthesis approaches that optimize relevant characteristics of the anhydrous lithium chloride.Expand Specific Solutions
Key Industrial Players in Lithium Compound Manufacturing
The lithium chloride synthesis optimization market is in a growth phase, characterized by increasing demand driven by lithium-ion battery applications. The global market size is expanding rapidly as electric vehicle adoption accelerates, with projections exceeding $2 billion by 2025. Technologically, the field shows moderate maturity with established processes, but significant innovation opportunities remain. Leading players include Tianqi Lithium and Ganfeng Lithium, who dominate raw material processing, while Saft Groupe and LG Chem focus on advanced applications. Research institutions like AIST and ETRI are developing next-generation synthesis methods, while specialized companies like Nanoscale Components are creating innovative prelithiation technologies that could disrupt traditional anhydrous lithium chloride production methods.
Tianqi Lithium Corp.
Technical Solution: Tianqi Lithium has pioneered an innovative anhydrous lithium chloride synthesis method utilizing azeotropic distillation with organic solvents. Their process begins with a high-purity lithium chloride solution that undergoes treatment with carefully selected water-scavenging solvents such as isopropanol or n-butanol to form azeotropic mixtures. These mixtures effectively remove water at temperatures significantly lower than traditional thermal dehydration (typically 80-100°C), reducing energy consumption by approximately 30%. The company employs specialized molecular sieves in the final purification stage to achieve anhydrous conditions with moisture levels below 50ppm. Their technology incorporates a closed-loop solvent recovery system with over 95% recycling efficiency, minimizing environmental impact. Tianqi has also developed proprietary catalysts that accelerate the dehydration process while preventing unwanted side reactions that could introduce impurities into the final product.
Strengths: Lower energy requirements compared to conventional thermal methods; achieves extremely high purity suitable for electronic applications; environmentally friendly with high solvent recovery rates. Weaknesses: Requires handling of flammable organic solvents with associated safety protocols; process complexity increases capital equipment costs; trace solvent contamination may require additional purification steps for certain applications.
Ganfeng Lithium Group Co., Ltd.
Technical Solution: Ganfeng Lithium has developed a proprietary process for anhydrous lithium chloride synthesis that employs a vacuum dehydration technique combined with controlled heating protocols. Their method utilizes a multi-stage dehydration process where lithium chloride hydrate is first subjected to preliminary drying at 120°C, followed by progressive temperature increases under vacuum conditions (10-2 to 10-3 Pa) up to 180-200°C. This approach prevents lithium chloride hydrolysis while achieving moisture content below 100ppm. The company has also implemented continuous flow reactors with specialized ceramic materials resistant to chloride corrosion, allowing for industrial-scale production with consistent quality. Their process incorporates real-time moisture monitoring using specialized hygrometers calibrated specifically for lithium salt environments, enabling precise endpoint determination during the dehydration process.
Strengths: Achieves extremely low moisture content (<100ppm) suitable for battery-grade applications; energy-efficient vacuum process reduces production costs; continuous flow system enables large-scale production. Weaknesses: Requires sophisticated vacuum equipment and precise temperature control; ceramic materials for reactors are expensive and may require periodic replacement due to chloride-induced degradation.
Critical Patents and Innovations in Dehydration Techniques
Process for producing metallic lithium
PatentActiveUS20130001097A1
Innovation
- A method involving the reaction of lithium carbonate with chlorine gas in a dry process to produce anhydrous lithium chloride, which is then used in molten salt electrolysis, with the generated chlorine gas reused within the system to continuously produce lithium metal, avoiding external discharge and minimizing corrosion.
A method and device for preparing high-purity lithium chloride based on lithium-ion solid electrolyte
PatentWO2024061312A1
Innovation
- Multi-stage countercurrent centrifugal extraction process using amide-complexing extractants for efficient separation of lithium from brine.
- pH control strategy at multiple stages (acidification in pretreatment and pH adjustment to 2-3 after extraction) to enhance lithium recovery and reduce impurities.
- Integration of chelating agents specifically targeting calcium and magnesium ions to achieve high-purity lithium chloride suitable for solid electrolyte applications.
Environmental Impact Assessment of LiCl Production
The production of anhydrous lithium chloride presents significant environmental challenges that require careful assessment and mitigation strategies. The conventional synthesis methods involve energy-intensive processes and potentially hazardous chemicals, resulting in considerable environmental footprints. Primary concerns include greenhouse gas emissions from high-temperature operations, which typically require temperatures exceeding 600°C for complete dehydration of lithium chloride hydrates. These thermal processes contribute substantially to the carbon footprint of LiCl production facilities.
Water consumption represents another critical environmental factor, particularly in regions facing water scarcity. Traditional synthesis routes may require 15-20 liters of water per kilogram of anhydrous LiCl produced, creating pressure on local water resources. Additionally, wastewater from production processes often contains elevated concentrations of lithium, chloride ions, and other process chemicals that can disrupt aquatic ecosystems if not properly treated.
Chemical waste management poses ongoing challenges, as the synthesis typically generates byproducts including metal hydroxides, sulfates, and other salts. These waste streams require specialized handling and disposal protocols to prevent soil contamination and groundwater pollution. Studies indicate that for every ton of anhydrous LiCl produced, approximately 0.3-0.5 tons of solid waste materials may be generated, depending on the specific synthesis route employed.
Air quality impacts extend beyond greenhouse gas emissions to include potential releases of chlorine gas, hydrogen chloride, and particulate matter. These emissions can contribute to local air pollution and pose occupational health risks for facility workers. Modern production facilities have implemented scrubber systems that can reduce these emissions by up to 95%, though implementation remains inconsistent across global production sites.
Land use considerations must also be evaluated, as LiCl production facilities typically require substantial industrial footprints. The extraction of raw materials for lithium compounds often involves mining operations that can lead to habitat disruption and landscape alteration. Rehabilitation efforts for mining sites have shown variable success rates, with complete ecosystem restoration requiring decades in many cases.
Recent innovations in green chemistry approaches have demonstrated potential for reducing environmental impacts through lower-temperature synthesis routes, closed-loop water systems, and improved catalyst efficiency. Life cycle assessments indicate that optimized synthesis methods could reduce the overall environmental footprint of anhydrous LiCl production by 30-40% compared to conventional approaches, highlighting the importance of continued research and development in this area.
Water consumption represents another critical environmental factor, particularly in regions facing water scarcity. Traditional synthesis routes may require 15-20 liters of water per kilogram of anhydrous LiCl produced, creating pressure on local water resources. Additionally, wastewater from production processes often contains elevated concentrations of lithium, chloride ions, and other process chemicals that can disrupt aquatic ecosystems if not properly treated.
Chemical waste management poses ongoing challenges, as the synthesis typically generates byproducts including metal hydroxides, sulfates, and other salts. These waste streams require specialized handling and disposal protocols to prevent soil contamination and groundwater pollution. Studies indicate that for every ton of anhydrous LiCl produced, approximately 0.3-0.5 tons of solid waste materials may be generated, depending on the specific synthesis route employed.
Air quality impacts extend beyond greenhouse gas emissions to include potential releases of chlorine gas, hydrogen chloride, and particulate matter. These emissions can contribute to local air pollution and pose occupational health risks for facility workers. Modern production facilities have implemented scrubber systems that can reduce these emissions by up to 95%, though implementation remains inconsistent across global production sites.
Land use considerations must also be evaluated, as LiCl production facilities typically require substantial industrial footprints. The extraction of raw materials for lithium compounds often involves mining operations that can lead to habitat disruption and landscape alteration. Rehabilitation efforts for mining sites have shown variable success rates, with complete ecosystem restoration requiring decades in many cases.
Recent innovations in green chemistry approaches have demonstrated potential for reducing environmental impacts through lower-temperature synthesis routes, closed-loop water systems, and improved catalyst efficiency. Life cycle assessments indicate that optimized synthesis methods could reduce the overall environmental footprint of anhydrous LiCl production by 30-40% compared to conventional approaches, highlighting the importance of continued research and development in this area.
Quality Control Standards for High-Purity Lithium Compounds
Quality control standards for high-purity lithium compounds represent a critical aspect in the optimization of anhydrous lithium chloride synthesis. These standards must address multiple parameters to ensure consistent product quality that meets stringent industrial requirements. The purity level for anhydrous lithium chloride typically demands 99.9% or higher, with specific limits on moisture content below 0.01% to maintain its anhydrous properties.
Analytical techniques employed for quality verification include inductively coupled plasma mass spectrometry (ICP-MS) for trace metal analysis, with detection limits in the parts-per-billion range. X-ray diffraction (XRD) provides crystallographic confirmation of the anhydrous form, while Karl Fischer titration specifically quantifies residual moisture content. These methods collectively ensure comprehensive characterization of the final product.
Contamination thresholds must be precisely defined, particularly for elements that impact downstream applications. For battery-grade lithium compounds, transition metal impurities such as iron, nickel, and copper must remain below 10 ppm, while sodium and potassium typically require limits under 20 ppm. These specifications directly influence electrochemical performance in lithium-ion battery applications.
Process validation protocols constitute another essential component of quality standards. Statistical process control (SPC) methodologies monitor critical process parameters during synthesis, establishing control limits that trigger corrective actions when deviations occur. Batch-to-batch consistency requires documented procedures for sampling frequency, with statistical evaluation of results to identify trends before they become problematic.
Certification requirements often follow international standards such as ISO 9001 for quality management systems and ISO 17025 for testing laboratories. Documentation must include certificates of analysis (CoA) detailing actual measured values for all specified parameters, not merely pass/fail results. Traceability systems linking final products to raw material batches facilitate rapid response to quality issues.
Environmental considerations have increasingly become integrated into quality standards, with requirements for monitoring and limiting process-related contaminants. Sustainable production practices now feature in advanced quality frameworks, addressing both environmental impact and regulatory compliance across global markets.
Implementation of these quality control standards requires sophisticated instrumentation and trained personnel. Modern facilities employ automated in-line monitoring systems that provide real-time data on critical parameters, enabling immediate process adjustments to maintain quality specifications throughout production cycles.
Analytical techniques employed for quality verification include inductively coupled plasma mass spectrometry (ICP-MS) for trace metal analysis, with detection limits in the parts-per-billion range. X-ray diffraction (XRD) provides crystallographic confirmation of the anhydrous form, while Karl Fischer titration specifically quantifies residual moisture content. These methods collectively ensure comprehensive characterization of the final product.
Contamination thresholds must be precisely defined, particularly for elements that impact downstream applications. For battery-grade lithium compounds, transition metal impurities such as iron, nickel, and copper must remain below 10 ppm, while sodium and potassium typically require limits under 20 ppm. These specifications directly influence electrochemical performance in lithium-ion battery applications.
Process validation protocols constitute another essential component of quality standards. Statistical process control (SPC) methodologies monitor critical process parameters during synthesis, establishing control limits that trigger corrective actions when deviations occur. Batch-to-batch consistency requires documented procedures for sampling frequency, with statistical evaluation of results to identify trends before they become problematic.
Certification requirements often follow international standards such as ISO 9001 for quality management systems and ISO 17025 for testing laboratories. Documentation must include certificates of analysis (CoA) detailing actual measured values for all specified parameters, not merely pass/fail results. Traceability systems linking final products to raw material batches facilitate rapid response to quality issues.
Environmental considerations have increasingly become integrated into quality standards, with requirements for monitoring and limiting process-related contaminants. Sustainable production practices now feature in advanced quality frameworks, addressing both environmental impact and regulatory compliance across global markets.
Implementation of these quality control standards requires sophisticated instrumentation and trained personnel. Modern facilities employ automated in-line monitoring systems that provide real-time data on critical parameters, enabling immediate process adjustments to maintain quality specifications throughout production cycles.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!