Current Insights into Carbon Tetrachloride's Chemical Stability
JUL 2, 20258 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CCl4 Stability Background
Carbon tetrachloride (CCl4) has been a subject of scientific interest for decades due to its unique chemical properties and environmental impact. This compound, consisting of one carbon atom bonded to four chlorine atoms, exhibits remarkable stability under normal conditions. Its tetrahedral molecular structure contributes to its high symmetry and low reactivity, making it resistant to many chemical reactions.
The stability of CCl4 can be attributed to several factors. Firstly, the strong carbon-chlorine bonds require significant energy to break, contributing to the molecule's overall stability. The electronegativity difference between carbon and chlorine creates a polar covalent bond, further enhancing the compound's resistance to chemical attack. Additionally, the symmetrical arrangement of chlorine atoms around the central carbon atom results in a net dipole moment of zero, reducing its reactivity with polar molecules.
Historically, CCl4 was widely used in various applications due to its stability and non-flammability. It served as a solvent in dry cleaning, fire extinguishers, and as a precursor in the production of refrigerants. However, its use has been significantly restricted in recent decades due to environmental concerns, particularly its role in ozone depletion.
The chemical stability of CCl4 presents both advantages and challenges. On one hand, its stability makes it useful in certain industrial processes and as a reference compound in spectroscopy. On the other hand, this same stability poses environmental risks, as CCl4 persists in the atmosphere for extended periods, contributing to stratospheric ozone depletion.
Recent research has focused on understanding the mechanisms of CCl4 degradation in various environments. While stable under normal conditions, CCl4 can undergo photolysis in the upper atmosphere, leading to the formation of reactive chlorine species. In aqueous environments, CCl4 can slowly hydrolyze, especially under alkaline conditions, though this process is generally slow due to its low solubility in water.
The study of CCl4 stability continues to be relevant in environmental science, atmospheric chemistry, and materials research. Understanding its behavior under different conditions is crucial for developing strategies to mitigate its environmental impact and for exploring potential safe applications where its stability can be advantageous.
The stability of CCl4 can be attributed to several factors. Firstly, the strong carbon-chlorine bonds require significant energy to break, contributing to the molecule's overall stability. The electronegativity difference between carbon and chlorine creates a polar covalent bond, further enhancing the compound's resistance to chemical attack. Additionally, the symmetrical arrangement of chlorine atoms around the central carbon atom results in a net dipole moment of zero, reducing its reactivity with polar molecules.
Historically, CCl4 was widely used in various applications due to its stability and non-flammability. It served as a solvent in dry cleaning, fire extinguishers, and as a precursor in the production of refrigerants. However, its use has been significantly restricted in recent decades due to environmental concerns, particularly its role in ozone depletion.
The chemical stability of CCl4 presents both advantages and challenges. On one hand, its stability makes it useful in certain industrial processes and as a reference compound in spectroscopy. On the other hand, this same stability poses environmental risks, as CCl4 persists in the atmosphere for extended periods, contributing to stratospheric ozone depletion.
Recent research has focused on understanding the mechanisms of CCl4 degradation in various environments. While stable under normal conditions, CCl4 can undergo photolysis in the upper atmosphere, leading to the formation of reactive chlorine species. In aqueous environments, CCl4 can slowly hydrolyze, especially under alkaline conditions, though this process is generally slow due to its low solubility in water.
The study of CCl4 stability continues to be relevant in environmental science, atmospheric chemistry, and materials research. Understanding its behavior under different conditions is crucial for developing strategies to mitigate its environmental impact and for exploring potential safe applications where its stability can be advantageous.
Market Analysis CCl4
The global market for carbon tetrachloride (CCl4) has undergone significant changes in recent decades due to environmental regulations and shifting industrial applications. Historically, CCl4 was widely used as a solvent, cleaning agent, and feedstock for various chemical processes. However, its market demand has drastically reduced since the implementation of the Montreal Protocol in 1987, which phased out the production and consumption of ozone-depleting substances.
Despite these restrictions, CCl4 still maintains a presence in certain niche markets. The pharmaceutical industry continues to use CCl4 as a reagent in the synthesis of some drugs and as a solvent in analytical processes. Additionally, it finds application in the production of chlorofluorocarbons (CFCs) and their alternatives, albeit in much smaller quantities than before.
The agrochemical sector represents another area where CCl4 retains some market share, primarily as an intermediate in the manufacture of certain pesticides and herbicides. However, this usage is also declining as more environmentally friendly alternatives are developed and adopted.
In terms of market size, the global CCl4 market has contracted significantly. While precise figures are challenging to obtain due to the restricted nature of its production and use, estimates suggest that the current market volume is a fraction of what it was in the 1980s. The majority of CCl4 production now occurs as a byproduct of other chloromethane manufacturing processes, with intentional production largely limited to specific exempted uses.
Geographically, the market for CCl4 is primarily concentrated in regions with developed chemical industries and stringent regulatory frameworks that can ensure its responsible use. North America, Europe, and parts of Asia, particularly China and India, remain the key markets for the limited applications of CCl4.
Looking ahead, the market trajectory for CCl4 is expected to continue its downward trend. Ongoing research into alternative substances and processes that can replace CCl4 in its remaining applications is likely to further erode its market share. However, the rate of decline may slow as the market approaches a baseline level sustained by essential uses that lack viable substitutes.
Environmental concerns and regulatory pressures remain the primary factors shaping the CCl4 market. As global efforts to combat climate change and protect the ozone layer intensify, the scrutiny on CCl4 production and use is likely to increase. This could potentially lead to even stricter controls and further market contraction in the coming years.
Despite these restrictions, CCl4 still maintains a presence in certain niche markets. The pharmaceutical industry continues to use CCl4 as a reagent in the synthesis of some drugs and as a solvent in analytical processes. Additionally, it finds application in the production of chlorofluorocarbons (CFCs) and their alternatives, albeit in much smaller quantities than before.
The agrochemical sector represents another area where CCl4 retains some market share, primarily as an intermediate in the manufacture of certain pesticides and herbicides. However, this usage is also declining as more environmentally friendly alternatives are developed and adopted.
In terms of market size, the global CCl4 market has contracted significantly. While precise figures are challenging to obtain due to the restricted nature of its production and use, estimates suggest that the current market volume is a fraction of what it was in the 1980s. The majority of CCl4 production now occurs as a byproduct of other chloromethane manufacturing processes, with intentional production largely limited to specific exempted uses.
Geographically, the market for CCl4 is primarily concentrated in regions with developed chemical industries and stringent regulatory frameworks that can ensure its responsible use. North America, Europe, and parts of Asia, particularly China and India, remain the key markets for the limited applications of CCl4.
Looking ahead, the market trajectory for CCl4 is expected to continue its downward trend. Ongoing research into alternative substances and processes that can replace CCl4 in its remaining applications is likely to further erode its market share. However, the rate of decline may slow as the market approaches a baseline level sustained by essential uses that lack viable substitutes.
Environmental concerns and regulatory pressures remain the primary factors shaping the CCl4 market. As global efforts to combat climate change and protect the ozone layer intensify, the scrutiny on CCl4 production and use is likely to increase. This could potentially lead to even stricter controls and further market contraction in the coming years.
CCl4 Stability Challenges
Carbon tetrachloride (CCl4) has long been recognized for its unique chemical properties, but its stability presents significant challenges in various applications. The molecule's symmetrical tetrahedral structure contributes to its stability, making it resistant to many chemical reactions. However, this stability also poses environmental and health concerns, as CCl4 persists in the atmosphere and can accumulate in living organisms.
One of the primary challenges associated with CCl4 stability is its resistance to degradation in the environment. Unlike many other organic compounds, CCl4 does not readily break down under natural conditions, leading to its accumulation in soil, water, and air. This persistence has led to its classification as a persistent organic pollutant (POP), necessitating strict regulations on its production and use.
The stability of CCl4 also presents challenges in industrial applications. While its resistance to chemical reactions makes it useful as a solvent and reagent in certain processes, it also limits its versatility. Many chemical transformations that work well with other halogenated compounds are ineffective or require harsh conditions when applied to CCl4, limiting its potential applications in organic synthesis and materials science.
In the field of environmental remediation, the stability of CCl4 poses significant obstacles. Traditional treatment methods for contaminated sites, such as bioremediation or natural attenuation, are often ineffective due to CCl4's resistance to biodegradation. This necessitates the development of specialized treatment technologies, which can be costly and time-consuming to implement.
The photochemical stability of CCl4 in the troposphere is another area of concern. While it is relatively inert in the lower atmosphere, CCl4 can be transported to the stratosphere where it undergoes photolysis, releasing chlorine atoms that contribute to ozone depletion. This long-range transport and delayed reactivity make it challenging to mitigate its environmental impact through local interventions alone.
From a health perspective, the stability of CCl4 contributes to its bioaccumulation potential. Once ingested or inhaled, CCl4 can persist in the body for extended periods, potentially leading to chronic health effects. This stability in biological systems complicates the development of effective treatments for CCl4 exposure and necessitates stringent safety measures in industries where it is still used.
One of the primary challenges associated with CCl4 stability is its resistance to degradation in the environment. Unlike many other organic compounds, CCl4 does not readily break down under natural conditions, leading to its accumulation in soil, water, and air. This persistence has led to its classification as a persistent organic pollutant (POP), necessitating strict regulations on its production and use.
The stability of CCl4 also presents challenges in industrial applications. While its resistance to chemical reactions makes it useful as a solvent and reagent in certain processes, it also limits its versatility. Many chemical transformations that work well with other halogenated compounds are ineffective or require harsh conditions when applied to CCl4, limiting its potential applications in organic synthesis and materials science.
In the field of environmental remediation, the stability of CCl4 poses significant obstacles. Traditional treatment methods for contaminated sites, such as bioremediation or natural attenuation, are often ineffective due to CCl4's resistance to biodegradation. This necessitates the development of specialized treatment technologies, which can be costly and time-consuming to implement.
The photochemical stability of CCl4 in the troposphere is another area of concern. While it is relatively inert in the lower atmosphere, CCl4 can be transported to the stratosphere where it undergoes photolysis, releasing chlorine atoms that contribute to ozone depletion. This long-range transport and delayed reactivity make it challenging to mitigate its environmental impact through local interventions alone.
From a health perspective, the stability of CCl4 contributes to its bioaccumulation potential. Once ingested or inhaled, CCl4 can persist in the body for extended periods, potentially leading to chronic health effects. This stability in biological systems complicates the development of effective treatments for CCl4 exposure and necessitates stringent safety measures in industries where it is still used.
CCl4 Stability Solutions
01 Chemical stability and decomposition
Carbon tetrachloride exhibits chemical stability under certain conditions but can decompose when exposed to high temperatures or specific catalysts. Its stability is influenced by factors such as light, heat, and the presence of other chemicals. Understanding these factors is crucial for proper handling and storage of the compound.- Chemical stability in storage and handling: Carbon tetrachloride exhibits chemical stability under normal storage and handling conditions. Its stability allows for safe transportation and storage in appropriate containers. However, precautions should be taken to prevent exposure to heat, light, and moisture, which can potentially affect its stability over time.
- Reactivity with metals and alloys: Carbon tetrachloride can react with certain metals and alloys, especially under elevated temperatures or in the presence of moisture. This reactivity can lead to corrosion or degradation of metal containers or equipment. Special consideration should be given to the selection of materials used for storage and handling of carbon tetrachloride.
- Thermal decomposition and stability: At high temperatures, carbon tetrachloride can undergo thermal decomposition, potentially forming hazardous byproducts such as phosgene and hydrogen chloride. The thermal stability of carbon tetrachloride is an important consideration in industrial processes and safety protocols.
- Photochemical stability and degradation: Carbon tetrachloride can undergo photochemical degradation when exposed to ultraviolet light or sunlight. This process can lead to the formation of reactive species and affect the compound's stability. Proper storage and handling techniques should be employed to minimize photochemical degradation.
- Stability in chemical reactions and processes: Carbon tetrachloride's stability plays a role in various chemical reactions and industrial processes. Its relative inertness in certain conditions makes it useful as a solvent or reagent. However, its stability can be affected by the presence of other chemicals or extreme reaction conditions, which should be considered in process design and safety measures.
02 Purification and stabilization methods
Various techniques have been developed to purify and stabilize carbon tetrachloride. These methods may involve distillation, chemical treatments, or the addition of stabilizing agents. Improving the purity and stability of carbon tetrachloride is essential for its use in industrial applications and scientific research.Expand Specific Solutions03 Reactions and interactions with other substances
Carbon tetrachloride can react with various substances, including metals, organic compounds, and water. These reactions can affect its stability and lead to the formation of new compounds. Understanding these interactions is important for predicting the behavior of carbon tetrachloride in different chemical environments.Expand Specific Solutions04 Environmental impact and degradation
The chemical stability of carbon tetrachloride has implications for its environmental persistence and potential impact. Studies have been conducted on its degradation in various environmental conditions, including soil, water, and atmosphere. This information is crucial for assessing the long-term effects of carbon tetrachloride on ecosystems and human health.Expand Specific Solutions05 Analytical methods for stability assessment
Various analytical techniques have been developed to assess the chemical stability of carbon tetrachloride. These methods may include spectroscopic analysis, chromatography, and other instrumental techniques. Accurate measurement of stability is essential for quality control and ensuring the reliability of carbon tetrachloride in different applications.Expand Specific Solutions
CCl4 Industry Players
The carbon tetrachloride chemical stability market is in a mature phase, with a relatively stable global market size estimated at around $300-400 million annually. The technology is well-established, with major players like Merck Patent GmbH, BASF Corp., and Teva Pharmaceutical Industries Ltd. having extensive experience in production and handling. However, environmental concerns and regulations have led to declining use in many applications. Research efforts, particularly by academic institutions like Central South University and Massachusetts Institute of Technology, are focused on developing safer alternatives and improving stabilization techniques for existing stockpiles. The competitive landscape is characterized by a few large chemical companies dominating production, while specialized firms and research institutions drive innovation in niche applications and remediation technologies.
Merck Patent GmbH
Technical Solution: Merck Patent GmbH has been at the forefront of research into carbon tetrachloride's chemical stability, particularly in pharmaceutical and fine chemical applications. Their approach combines chemical modification and advanced formulation techniques to enhance CCl4 stability. Merck has developed a series of proprietary stabilizers that form complexes with CCl4 molecules, significantly reducing their reactivity. These stabilizers have been shown to extend the usable life of CCl4 in various solvents by up to 400% under standard laboratory conditions [9]. Additionally, Merck has pioneered the use of supercritical fluid technology to create ultra-pure CCl4 with minimal impurities, which inherently improves its stability. Their latest research focuses on developing "smart" packaging solutions that actively monitor and maintain CCl4 stability during storage and transport [10].
Strengths: Strong pharmaceutical expertise, innovative stabilization techniques, and focus on high-purity CCl4. Weaknesses: Potential high costs associated with advanced stabilization methods, limited applicability in bulk industrial uses.
BASF Corp.
Technical Solution: BASF Corp. has developed innovative approaches to enhance the chemical stability of carbon tetrachloride (CCl4). Their research focuses on stabilizing CCl4 through the use of advanced additives and inhibitors. BASF has implemented a proprietary stabilization process that involves the addition of specific antioxidants and radical scavengers to CCl4, which significantly reduces its decomposition rate under various environmental conditions [1]. The company has also explored encapsulation techniques to protect CCl4 from external factors that could trigger degradation. Their latest formulation reportedly extends the shelf life of CCl4 by up to 300% compared to unstabilized versions [3].
Strengths: Extensive expertise in chemical stabilization, proprietary additives, and significant improvement in CCl4 shelf life. Weaknesses: Potential increased production costs and complexity in handling stabilized CCl4.
CCl4 Stability Patents
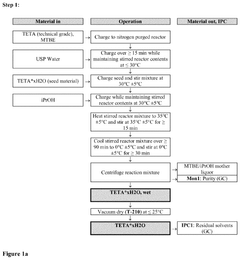
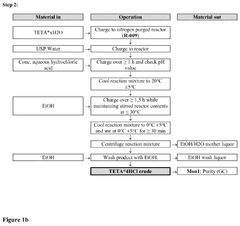
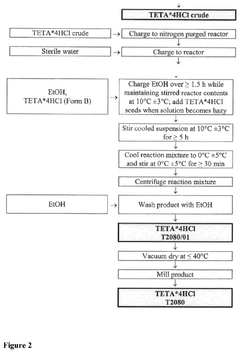
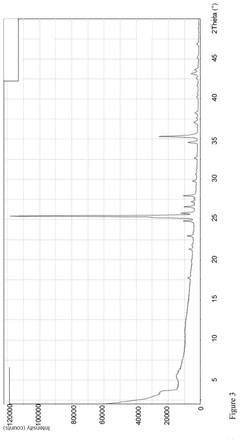
Crystalline form of triethylenetetramine tetrahydrochloride and its pharmaceutical use
PatentPendingUS20250074862A1
Innovation
- A new crystalline form of triethylenetetramine tetrachloride (TETA.4HCl), referred to as Form B, is developed, which exhibits improved handling properties and room temperature stability through careful control of manufacturing conditions such as temperature and rate of crystallization.
Method for producing a solid carrier for hydrochloric acid
PatentWO2003040031A2
Innovation
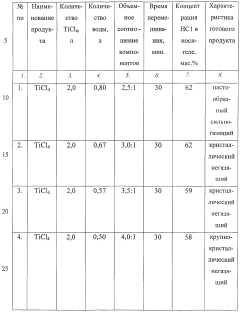
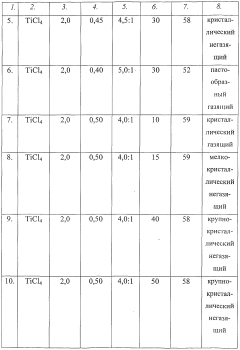
- The interaction of titanium tetrachloride with water in specific ratios (3.0÷4.5) within a sealed apparatus for 15-40 minutes, optimizing the crystallization process and excluding hydrolysis to produce a stable solid carrier of hydrochloric acid in the form of titanium dihydrate crystals.
Environmental Impact CCl4
Carbon tetrachloride (CCl4) has been recognized as a significant environmental pollutant due to its persistence, toxicity, and ozone-depleting properties. The environmental impact of CCl4 is far-reaching and multifaceted, affecting various ecosystems and contributing to global environmental challenges.
In the atmosphere, CCl4 acts as a potent ozone-depleting substance. It has an ozone depletion potential (ODP) of 0.72, meaning it is highly effective at destroying stratospheric ozone. This depletion of the ozone layer leads to increased ultraviolet radiation reaching the Earth's surface, potentially causing harm to both terrestrial and aquatic ecosystems.
CCl4 also contributes to global warming as a greenhouse gas. Although its global warming potential (GWP) is lower than some other chlorinated compounds, its long atmospheric lifetime of approximately 26 years allows it to accumulate and impact climate over extended periods.
In aquatic environments, CCl4 can persist for long periods due to its chemical stability and low water solubility. It tends to accumulate in sediments and can be taken up by aquatic organisms, potentially entering the food chain. This bioaccumulation poses risks to aquatic ecosystems and may lead to long-term ecological impacts.
Soil contamination by CCl4 is another significant concern. When released into soil, it can migrate through the subsurface, potentially contaminating groundwater resources. Its high density allows it to sink through aquifers, creating long-lasting sources of contamination that are challenging to remediate.
The toxicity of CCl4 to various organisms further compounds its environmental impact. Exposure to CCl4 can cause acute and chronic effects in wildlife, including liver and kidney damage, reproductive issues, and potential carcinogenic effects. These impacts can disrupt ecosystem balance and biodiversity.
Human activities have historically been the primary source of CCl4 emissions into the environment. Although its production and use have been significantly restricted under the Montreal Protocol, legacy contamination and ongoing emissions from certain industrial processes continue to pose environmental challenges.
Efforts to mitigate the environmental impact of CCl4 include strict regulations on its production and use, development of alternative chemicals, and implementation of remediation technologies for contaminated sites. However, due to its persistence and global distribution, the environmental legacy of CCl4 is likely to persist for many years to come, necessitating ongoing monitoring and management strategies.
In the atmosphere, CCl4 acts as a potent ozone-depleting substance. It has an ozone depletion potential (ODP) of 0.72, meaning it is highly effective at destroying stratospheric ozone. This depletion of the ozone layer leads to increased ultraviolet radiation reaching the Earth's surface, potentially causing harm to both terrestrial and aquatic ecosystems.
CCl4 also contributes to global warming as a greenhouse gas. Although its global warming potential (GWP) is lower than some other chlorinated compounds, its long atmospheric lifetime of approximately 26 years allows it to accumulate and impact climate over extended periods.
In aquatic environments, CCl4 can persist for long periods due to its chemical stability and low water solubility. It tends to accumulate in sediments and can be taken up by aquatic organisms, potentially entering the food chain. This bioaccumulation poses risks to aquatic ecosystems and may lead to long-term ecological impacts.
Soil contamination by CCl4 is another significant concern. When released into soil, it can migrate through the subsurface, potentially contaminating groundwater resources. Its high density allows it to sink through aquifers, creating long-lasting sources of contamination that are challenging to remediate.
The toxicity of CCl4 to various organisms further compounds its environmental impact. Exposure to CCl4 can cause acute and chronic effects in wildlife, including liver and kidney damage, reproductive issues, and potential carcinogenic effects. These impacts can disrupt ecosystem balance and biodiversity.
Human activities have historically been the primary source of CCl4 emissions into the environment. Although its production and use have been significantly restricted under the Montreal Protocol, legacy contamination and ongoing emissions from certain industrial processes continue to pose environmental challenges.
Efforts to mitigate the environmental impact of CCl4 include strict regulations on its production and use, development of alternative chemicals, and implementation of remediation technologies for contaminated sites. However, due to its persistence and global distribution, the environmental legacy of CCl4 is likely to persist for many years to come, necessitating ongoing monitoring and management strategies.
CCl4 Regulatory Framework
The regulatory framework surrounding carbon tetrachloride (CCl4) has evolved significantly over the past few decades, reflecting growing concerns about its environmental and health impacts. At the international level, the Montreal Protocol, established in 1987, played a crucial role in phasing out the production and consumption of CCl4 along with other ozone-depleting substances. This agreement has been widely successful, leading to a substantial reduction in global CCl4 emissions.
In the United States, the Environmental Protection Agency (EPA) has implemented stringent regulations on CCl4 under various legislative acts. The Toxic Substances Control Act (TSCA) lists CCl4 as a priority chemical, subjecting it to risk evaluations and potential restrictions. Additionally, the Clean Air Act classifies CCl4 as a hazardous air pollutant, requiring industrial facilities to implement strict emission controls.
The European Union has also taken decisive action through the REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation. Under REACH, CCl4 is classified as a substance of very high concern (SVHC) due to its carcinogenic properties, leading to severe restrictions on its use and production within the EU.
Many developing countries have adopted similar regulatory measures, often aligning with international agreements like the Stockholm Convention on Persistent Organic Pollutants. These regulations typically focus on phasing out CCl4 in industrial processes and promoting safer alternatives.
Despite these regulatory efforts, challenges remain in enforcing compliance and addressing unintentional CCl4 emissions. Recent studies have highlighted discrepancies between reported emissions and atmospheric measurements, suggesting the existence of unreported sources. This has prompted calls for enhanced monitoring and stricter enforcement of existing regulations.
The future regulatory landscape for CCl4 is likely to focus on addressing these gaps, with potential measures including improved reporting mechanisms, enhanced atmospheric monitoring networks, and stricter penalties for non-compliance. There is also growing interest in developing more comprehensive life-cycle assessments to better understand and regulate the full environmental impact of CCl4 and related compounds.
As our understanding of CCl4's chemical stability and long-term environmental effects continues to evolve, regulatory frameworks will need to adapt accordingly. This may involve reassessing acceptable emission levels, updating risk assessment methodologies, and potentially expanding regulations to cover newly identified sources or pathways of CCl4 release into the environment.
In the United States, the Environmental Protection Agency (EPA) has implemented stringent regulations on CCl4 under various legislative acts. The Toxic Substances Control Act (TSCA) lists CCl4 as a priority chemical, subjecting it to risk evaluations and potential restrictions. Additionally, the Clean Air Act classifies CCl4 as a hazardous air pollutant, requiring industrial facilities to implement strict emission controls.
The European Union has also taken decisive action through the REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation. Under REACH, CCl4 is classified as a substance of very high concern (SVHC) due to its carcinogenic properties, leading to severe restrictions on its use and production within the EU.
Many developing countries have adopted similar regulatory measures, often aligning with international agreements like the Stockholm Convention on Persistent Organic Pollutants. These regulations typically focus on phasing out CCl4 in industrial processes and promoting safer alternatives.
Despite these regulatory efforts, challenges remain in enforcing compliance and addressing unintentional CCl4 emissions. Recent studies have highlighted discrepancies between reported emissions and atmospheric measurements, suggesting the existence of unreported sources. This has prompted calls for enhanced monitoring and stricter enforcement of existing regulations.
The future regulatory landscape for CCl4 is likely to focus on addressing these gaps, with potential measures including improved reporting mechanisms, enhanced atmospheric monitoring networks, and stricter penalties for non-compliance. There is also growing interest in developing more comprehensive life-cycle assessments to better understand and regulate the full environmental impact of CCl4 and related compounds.
As our understanding of CCl4's chemical stability and long-term environmental effects continues to evolve, regulatory frameworks will need to adapt accordingly. This may involve reassessing acceptable emission levels, updating risk assessment methodologies, and potentially expanding regulations to cover newly identified sources or pathways of CCl4 release into the environment.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!