Determining HPLC Solvent Purity: Ensuring Accurate Outcomes
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HPLC Solvent Purity Background and Objectives
High-Performance Liquid Chromatography (HPLC) has evolved significantly since its inception in the late 1960s, becoming a cornerstone analytical technique in pharmaceutical, environmental, food safety, and clinical research sectors. The evolution of HPLC technology has been marked by continuous improvements in column technology, detection methods, and automation capabilities, all contributing to enhanced separation efficiency and analytical precision.
Solvent purity represents a critical yet often overlooked factor in HPLC analysis. Historical data indicates that impurities in solvents can significantly compromise analytical results, with studies showing that up to 15% of chromatographic anomalies can be attributed to solvent-related issues. The technological trajectory in this domain has been directed toward developing more stringent purity standards and more sensitive detection methods for solvent contaminants.
The current industry standards for HPLC-grade solvents, established by organizations such as ASTM International and the American Chemical Society, specify maximum allowable levels for various impurities. However, these standards continue to evolve as analytical requirements become more demanding, particularly in fields like proteomics and metabolomics where trace-level detection is essential.
Recent technological advancements have introduced innovative approaches to solvent purification and quality assessment. These include real-time monitoring systems, advanced spectroscopic techniques for impurity profiling, and computational models that predict the impact of specific impurities on chromatographic performance. The integration of these technologies represents a significant step forward in ensuring solvent quality consistency.
The primary objective of this technical research is to comprehensively evaluate current methodologies for determining HPLC solvent purity and their impact on analytical outcomes. This includes assessing the sensitivity and reliability of various testing protocols, identifying critical impurities that most significantly affect chromatographic performance, and exploring emerging technologies that promise more efficient purity determination.
Additionally, this research aims to establish a correlation between specific solvent impurities and their effects on different types of HPLC analyses, providing a framework for selecting appropriate solvents based on specific analytical requirements. This objective addresses a significant gap in current literature, where such correlations are often empirically observed but not systematically documented.
The ultimate goal is to develop a standardized approach to solvent purity assessment that balances analytical rigor with practical feasibility in routine laboratory settings. This approach should be adaptable to various analytical contexts while maintaining sufficient sensitivity to detect impurities at levels that could potentially impact analytical results.
Solvent purity represents a critical yet often overlooked factor in HPLC analysis. Historical data indicates that impurities in solvents can significantly compromise analytical results, with studies showing that up to 15% of chromatographic anomalies can be attributed to solvent-related issues. The technological trajectory in this domain has been directed toward developing more stringent purity standards and more sensitive detection methods for solvent contaminants.
The current industry standards for HPLC-grade solvents, established by organizations such as ASTM International and the American Chemical Society, specify maximum allowable levels for various impurities. However, these standards continue to evolve as analytical requirements become more demanding, particularly in fields like proteomics and metabolomics where trace-level detection is essential.
Recent technological advancements have introduced innovative approaches to solvent purification and quality assessment. These include real-time monitoring systems, advanced spectroscopic techniques for impurity profiling, and computational models that predict the impact of specific impurities on chromatographic performance. The integration of these technologies represents a significant step forward in ensuring solvent quality consistency.
The primary objective of this technical research is to comprehensively evaluate current methodologies for determining HPLC solvent purity and their impact on analytical outcomes. This includes assessing the sensitivity and reliability of various testing protocols, identifying critical impurities that most significantly affect chromatographic performance, and exploring emerging technologies that promise more efficient purity determination.
Additionally, this research aims to establish a correlation between specific solvent impurities and their effects on different types of HPLC analyses, providing a framework for selecting appropriate solvents based on specific analytical requirements. This objective addresses a significant gap in current literature, where such correlations are often empirically observed but not systematically documented.
The ultimate goal is to develop a standardized approach to solvent purity assessment that balances analytical rigor with practical feasibility in routine laboratory settings. This approach should be adaptable to various analytical contexts while maintaining sufficient sensitivity to detect impurities at levels that could potentially impact analytical results.
Market Demand Analysis for High-Purity HPLC Solvents
The global market for high-purity HPLC solvents has experienced significant growth in recent years, driven primarily by expanding applications in pharmaceutical research, clinical diagnostics, and environmental testing. Current market valuations indicate that the HPLC solvent market reached approximately 3.5 billion USD in 2022, with projections suggesting a compound annual growth rate of 6.8% through 2028.
Pharmaceutical and biotechnology sectors represent the largest demand segment, accounting for nearly 45% of the total market share. This dominance stems from stringent regulatory requirements for drug development and quality control processes, where even minor impurities in solvents can compromise analytical results and potentially affect drug safety profiles.
Academic and research institutions constitute the second-largest market segment, driven by increasing research activities in proteomics, genomics, and metabolomics. These advanced research areas require ultra-high purity solvents to ensure reproducible and reliable analytical outcomes, particularly when dealing with trace-level analytes.
Regional analysis reveals North America as the leading market for high-purity HPLC solvents, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is demonstrating the fastest growth rate, attributed to expanding pharmaceutical manufacturing capabilities in China and India, coupled with increasing adoption of advanced analytical techniques across the region.
Customer demand patterns show a clear shift toward higher purity grades, with HPLC-grade (99.9%) and LC-MS grade (99.99%) solvents experiencing the strongest growth. This trend reflects the increasing sensitivity of modern analytical instruments and the growing need for lower detection limits in various applications.
Price sensitivity varies significantly across market segments. While large pharmaceutical companies prioritize consistency and reliability over cost, academic institutions and smaller laboratories often face budget constraints that influence purchasing decisions. This dichotomy has created a two-tiered market structure with premium and value-oriented product offerings.
Supply chain challenges, particularly those exposed during the COVID-19 pandemic, have heightened awareness about solvent quality consistency. End-users increasingly demand comprehensive certificates of analysis, batch-to-batch consistency, and improved packaging solutions that maintain solvent purity during storage and handling.
Environmental considerations are also shaping market dynamics, with growing demand for greener alternatives to traditional HPLC solvents. This trend is particularly evident in Europe, where regulatory pressures and corporate sustainability initiatives are driving interest in bio-based solvents and recycling programs for used HPLC solvents.
Pharmaceutical and biotechnology sectors represent the largest demand segment, accounting for nearly 45% of the total market share. This dominance stems from stringent regulatory requirements for drug development and quality control processes, where even minor impurities in solvents can compromise analytical results and potentially affect drug safety profiles.
Academic and research institutions constitute the second-largest market segment, driven by increasing research activities in proteomics, genomics, and metabolomics. These advanced research areas require ultra-high purity solvents to ensure reproducible and reliable analytical outcomes, particularly when dealing with trace-level analytes.
Regional analysis reveals North America as the leading market for high-purity HPLC solvents, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is demonstrating the fastest growth rate, attributed to expanding pharmaceutical manufacturing capabilities in China and India, coupled with increasing adoption of advanced analytical techniques across the region.
Customer demand patterns show a clear shift toward higher purity grades, with HPLC-grade (99.9%) and LC-MS grade (99.99%) solvents experiencing the strongest growth. This trend reflects the increasing sensitivity of modern analytical instruments and the growing need for lower detection limits in various applications.
Price sensitivity varies significantly across market segments. While large pharmaceutical companies prioritize consistency and reliability over cost, academic institutions and smaller laboratories often face budget constraints that influence purchasing decisions. This dichotomy has created a two-tiered market structure with premium and value-oriented product offerings.
Supply chain challenges, particularly those exposed during the COVID-19 pandemic, have heightened awareness about solvent quality consistency. End-users increasingly demand comprehensive certificates of analysis, batch-to-batch consistency, and improved packaging solutions that maintain solvent purity during storage and handling.
Environmental considerations are also shaping market dynamics, with growing demand for greener alternatives to traditional HPLC solvents. This trend is particularly evident in Europe, where regulatory pressures and corporate sustainability initiatives are driving interest in bio-based solvents and recycling programs for used HPLC solvents.
Current Challenges in HPLC Solvent Purity Assessment
The assessment of HPLC solvent purity faces significant challenges that impact analytical accuracy and reliability. Current methodologies often struggle with detecting trace impurities that can significantly affect chromatographic performance. Traditional quality control measures rely heavily on certificate of analysis (CoA) data provided by manufacturers, which may not always reflect the actual condition of solvents after storage or handling.
One major challenge is the detection of water contamination in organic solvents. Even minimal water content can alter retention times, peak shapes, and separation efficiency. Current Karl Fischer titration methods, while effective for water determination, require specialized equipment and expertise that may not be available in all laboratories.
Gradient HPLC applications face particular difficulties as trace impurities can accumulate on analytical columns during repeated injections, leading to baseline drift, ghost peaks, and reduced column lifetime. The industry lacks standardized protocols for evaluating how solvent impurities specifically impact gradient separations, creating inconsistencies across different laboratory environments.
Metal contamination presents another significant challenge. Trace metals can catalyze sample degradation, particularly with sensitive biomolecules and pharmaceuticals. Current techniques for metal detection in HPLC solvents often require separate analytical methods such as ICP-MS, adding complexity and cost to comprehensive purity assessment.
UV-absorbing impurities pose special problems for analyses conducted at low wavelengths (below 220 nm). These contaminants can create baseline disturbances that mask analyte signals or generate false positives. Current spectrophotometric screening methods lack sensitivity for detecting these impurities at concentrations relevant to high-sensitivity HPLC applications.
The stability of solvents during storage represents an ongoing challenge. Environmental factors such as light exposure, temperature fluctuations, and container interactions can introduce degradation products over time. Current practices for monitoring solvent stability during shelf-life are inconsistent and often reactive rather than preventative.
Automation and high-throughput screening methods for solvent purity are underdeveloped. While modern laboratories increasingly rely on automated systems, solvent quality assessment remains largely manual and time-consuming, creating bottlenecks in analytical workflows.
Regulatory guidance on HPLC solvent purity standards varies globally, creating compliance challenges for multinational organizations. The lack of harmonized specifications makes it difficult to establish universal quality control parameters that satisfy all regulatory requirements while maintaining operational efficiency.
One major challenge is the detection of water contamination in organic solvents. Even minimal water content can alter retention times, peak shapes, and separation efficiency. Current Karl Fischer titration methods, while effective for water determination, require specialized equipment and expertise that may not be available in all laboratories.
Gradient HPLC applications face particular difficulties as trace impurities can accumulate on analytical columns during repeated injections, leading to baseline drift, ghost peaks, and reduced column lifetime. The industry lacks standardized protocols for evaluating how solvent impurities specifically impact gradient separations, creating inconsistencies across different laboratory environments.
Metal contamination presents another significant challenge. Trace metals can catalyze sample degradation, particularly with sensitive biomolecules and pharmaceuticals. Current techniques for metal detection in HPLC solvents often require separate analytical methods such as ICP-MS, adding complexity and cost to comprehensive purity assessment.
UV-absorbing impurities pose special problems for analyses conducted at low wavelengths (below 220 nm). These contaminants can create baseline disturbances that mask analyte signals or generate false positives. Current spectrophotometric screening methods lack sensitivity for detecting these impurities at concentrations relevant to high-sensitivity HPLC applications.
The stability of solvents during storage represents an ongoing challenge. Environmental factors such as light exposure, temperature fluctuations, and container interactions can introduce degradation products over time. Current practices for monitoring solvent stability during shelf-life are inconsistent and often reactive rather than preventative.
Automation and high-throughput screening methods for solvent purity are underdeveloped. While modern laboratories increasingly rely on automated systems, solvent quality assessment remains largely manual and time-consuming, creating bottlenecks in analytical workflows.
Regulatory guidance on HPLC solvent purity standards varies globally, creating compliance challenges for multinational organizations. The lack of harmonized specifications makes it difficult to establish universal quality control parameters that satisfy all regulatory requirements while maintaining operational efficiency.
Established Methods for HPLC Solvent Purity Determination
01 Solvent purification methods for HPLC
Various methods are employed to purify solvents used in HPLC analysis to ensure accurate results. These methods include distillation, filtration, and specialized purification systems that remove impurities that could interfere with chromatographic separation. Advanced techniques such as molecular sieve treatment and activated carbon filtration are also utilized to achieve high purity levels required for sensitive HPLC applications.- Solvent purification methods for HPLC: Various methods are employed to purify solvents for HPLC applications, including distillation, filtration, and specialized purification systems. These methods aim to remove impurities that could interfere with chromatographic separation or damage HPLC equipment. Purification techniques often involve multiple steps to ensure the removal of particulates, organic contaminants, and water, resulting in high-purity solvents suitable for sensitive analytical applications.
- Purity standards and specifications for HPLC solvents: HPLC solvents must meet specific purity standards to ensure reliable analytical results. These standards typically specify maximum allowable levels of impurities such as water, particulates, UV-absorbing compounds, and other contaminants. HPLC-grade solvents generally require higher purity levels than reagent-grade chemicals, with specifications for parameters like UV absorbance, fluorescence background, and residue after evaporation. Manufacturers must perform rigorous testing to certify that solvents meet these specifications.
- Impact of solvent purity on HPLC performance: The purity of solvents significantly affects HPLC performance, including separation efficiency, baseline stability, and detection sensitivity. Impurities in solvents can cause baseline drift, ghost peaks, increased background noise, and reduced column lifetime. High-purity solvents are particularly critical for trace analysis, where even minor contaminants can interfere with the detection of analytes at low concentrations. Using ultra-pure solvents can improve chromatographic resolution and enable more accurate quantification of target compounds.
- On-line monitoring and quality control of HPLC solvent purity: Systems and methods for continuous monitoring of HPLC solvent purity help maintain consistent analytical performance. These include in-line sensors that detect impurities, automated quality control processes, and real-time monitoring of critical parameters such as conductivity, UV absorbance, and particulate content. On-line monitoring enables early detection of contamination issues before they affect analytical results, allowing for timely intervention and maintenance of high-quality chromatographic separations.
- Novel solvent formulations and additives for enhanced HPLC performance: Innovative solvent formulations and additives are developed to enhance HPLC performance while maintaining high purity standards. These include specialized solvent blends optimized for specific separation challenges, additives that improve chromatographic resolution, and formulations designed to reduce background noise. Some formulations incorporate stabilizers to prevent degradation during storage or use, while others are specifically designed for particular detection methods such as mass spectrometry or UV detection, ensuring compatibility and optimal analytical sensitivity.
02 Purity standards and quality control for HPLC solvents
Specific purity standards have been established for HPLC solvents to ensure consistent analytical results. These standards define acceptable levels of impurities such as water content, particulate matter, and UV-absorbing compounds. Quality control procedures include spectrophotometric analysis, conductivity measurements, and residue testing to verify that solvents meet the required specifications for chromatographic applications.Expand Specific Solutions03 Impact of solvent purity on HPLC performance
The purity of solvents significantly affects HPLC performance parameters including baseline stability, detection sensitivity, and peak resolution. Impurities in solvents can lead to ghost peaks, baseline drift, and reduced column lifetime. High-purity solvents enable more accurate quantification of analytes at low concentrations and improve the reproducibility of retention times, especially in gradient elution methods.Expand Specific Solutions04 Specialized HPLC solvent formulations
Specialized solvent formulations have been developed for specific HPLC applications. These include ultra-pure solvent blends for gradient HPLC, deuterated solvents for LC-MS applications, and custom buffer solutions with precise pH control. Some formulations incorporate additives that enhance separation efficiency or improve detector response while maintaining the required purity levels for reliable chromatographic analysis.Expand Specific Solutions05 On-line monitoring and maintenance of solvent purity
Systems for continuous monitoring and maintenance of solvent purity during HPLC operation have been developed. These include in-line degassing units, real-time purity sensors, and automated filtration systems that ensure consistent solvent quality throughout analytical runs. Some advanced systems incorporate feedback mechanisms that alert operators when solvent purity falls below acceptable thresholds, preventing compromised analytical results.Expand Specific Solutions
Key Manufacturers and Suppliers in HPLC Solvent Industry
The HPLC solvent purity market is in a mature growth phase, with an estimated global market size of $1.5-2 billion annually, driven by increasing demands for analytical precision in pharmaceutical, biotechnology, and chemical industries. The technology has reached high maturity, with established players like Merck, Thermo Fisher, and Agilent dominating the landscape. Among specialized pharmaceutical companies, Bristol Myers Squibb, Novartis, Takeda, and Daiichi Sankyo have developed proprietary methodologies for solvent purity assessment, while smaller players like Alembic Pharmaceuticals and Zydus Lifesciences are gaining market share through cost-effective solutions. Johnson Matthey and FUJIFILM Electronic Materials provide high-purity solvents for specialized applications, creating a competitive ecosystem balancing innovation with standardization requirements.
Takeda Pharmaceutical Co., Ltd.
Technical Solution: Takeda has pioneered an advanced HPLC solvent purity assessment framework called "PureTrace" that incorporates real-time monitoring capabilities. Their system utilizes conductivity sensors and UV absorbance detectors installed directly in solvent reservoirs to continuously monitor purity levels before and during analytical runs. Takeda's approach includes proprietary algorithms that can detect subtle changes in baseline characteristics indicative of solvent degradation or contamination. The company has developed specialized pre-column filters with molecular trapping technology that selectively remove common contaminants while preserving critical solvent properties. Their research facilities employ dedicated solvent purification systems that include multiple distillation steps and molecular sieve treatments customized for different solvent types. Takeda has implemented a barcode tracking system for all solvent containers that maintains complete chain-of-custody documentation and automatically flags containers approaching expiration dates. Their validation studies have shown that this comprehensive approach reduces method variability by up to 25% and extends column lifetimes by approximately 40%.
Strengths: Real-time monitoring prevents failed runs due to solvent issues; integrated tracking system ensures compliance with regulatory requirements; specialized filtration technology extends column life. Weaknesses: Significant initial capital investment required; system complexity necessitates dedicated technical support; potential for false positives in highly sensitive continuous monitoring.
Bristol Myers Squibb Co.
Technical Solution: Bristol Myers Squibb has developed a comprehensive HPLC solvent purity verification system that integrates multiple analytical techniques. Their approach combines traditional HPLC with mass spectrometry (LC-MS) to detect trace impurities down to parts per billion levels. The company employs a proprietary three-tier testing protocol that first screens solvents using UV detection, followed by refractive index analysis, and finally confirmation via mass spectrometry for ambiguous results. BMS has implemented automated solvent handling systems that minimize contamination during transfer and storage, utilizing specialized containers with inert linings to prevent leaching. Their quality control laboratories maintain rigorous documentation of solvent certificates of analysis and perform regular stability testing to monitor degradation over time. The company has published research demonstrating that their enhanced testing protocols have reduced chromatographic baseline noise by approximately 30% and improved retention time reproducibility by 15% compared to standard methods.
Strengths: Superior detection limits for trace impurities; integrated multi-technique approach provides redundant verification; automated handling reduces human error and contamination. Weaknesses: Higher implementation costs compared to standard methods; requires specialized training for laboratory personnel; longer processing time for complete three-tier analysis.
Critical Technologies in Solvent Purity Analysis
Method for determining the purity of a compound by high-performance liquid chromatography
PatentWO2025157997A1
Innovation
- A high-performance liquid chromatography (HPLC) method using a porous silica-based column with chemically bonded aryl groups and a mobile phase gradient, combined with a sandwich injection system and a Charged Aerosol Detector, to analyze DOTA-NHS purity and by-products, ensuring stability and separation by using water-free polar solvents and acids to dissolve the sample.
System for the Simultaneous Monitoring of Constituents of an Electroplating Bath
PatentPendingUS20240133074A1
Innovation
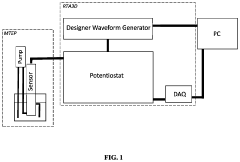
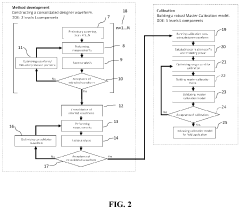
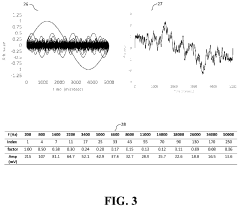
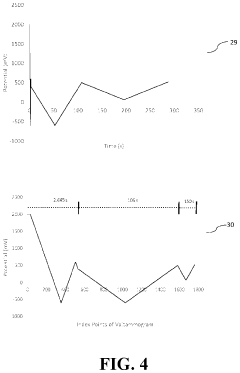
- The development of novel second-order, consolidated voltammetric waveforms combined with chemometric analysis and data compression techniques allows for the simultaneous measurement and analysis of all electroplating bath constituents without pretreatment, using a multi-frequency, variable amplitude waveform to generate a diagnostic voltammetric output that captures the synergistic interactions and maintains process control within the electroplating process.
Regulatory Compliance for Analytical Grade Solvents
Regulatory compliance for analytical grade solvents represents a critical framework governing the production, testing, and use of high-purity solvents in HPLC applications. These regulations ensure that solvents meet stringent quality standards necessary for accurate analytical results across pharmaceutical, environmental, and food safety testing sectors.
The global regulatory landscape for HPLC solvents is primarily shaped by pharmacopeial standards, including the United States Pharmacopeia (USP), European Pharmacopoeia (EP), and Japanese Pharmacopoeia (JP). These authorities establish specific purity criteria, acceptable impurity levels, and standardized testing methodologies that manufacturers must adhere to when producing analytical grade solvents.
ISO 9001 certification represents another crucial compliance requirement for solvent manufacturers, ensuring consistent quality management systems throughout production processes. Additionally, ISO/IEC 17025 accreditation for testing laboratories validates their technical competence in accurately determining solvent purity parameters.
For pharmaceutical applications, regulatory bodies like the FDA and EMA impose additional requirements through Good Manufacturing Practice (GMP) guidelines. These regulations mandate comprehensive documentation of solvent quality, including certificates of analysis (CoA) that verify batch-specific purity testing results and traceability throughout the supply chain.
Environmental considerations have also become increasingly important in regulatory frameworks. The Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) in Europe and similar programs globally require detailed safety assessments and environmental impact evaluations for chemical substances, including HPLC solvents.
Compliance verification typically involves rigorous documentation practices. Manufacturers must maintain detailed records of production parameters, quality control testing, and stability studies. End-users in regulated industries must implement incoming material verification protocols to confirm that received solvents meet specified purity requirements before use in analytical procedures.
Recent regulatory trends show increasing harmonization efforts between major pharmacopeias, aiming to establish globally consistent standards for analytical grade solvents. Simultaneously, regulations are evolving to address emerging concerns about ultra-trace contaminants that may impact sensitive analytical methods, particularly in bioanalytical applications and environmental testing.
For laboratories seeking to maintain regulatory compliance, implementing a robust supplier qualification program represents a best practice. This includes periodic audit of solvent manufacturers, review of their quality systems, and verification of their compliance with relevant regulatory standards to ensure consistent access to high-quality HPLC solvents that meet all applicable regulatory requirements.
The global regulatory landscape for HPLC solvents is primarily shaped by pharmacopeial standards, including the United States Pharmacopeia (USP), European Pharmacopoeia (EP), and Japanese Pharmacopoeia (JP). These authorities establish specific purity criteria, acceptable impurity levels, and standardized testing methodologies that manufacturers must adhere to when producing analytical grade solvents.
ISO 9001 certification represents another crucial compliance requirement for solvent manufacturers, ensuring consistent quality management systems throughout production processes. Additionally, ISO/IEC 17025 accreditation for testing laboratories validates their technical competence in accurately determining solvent purity parameters.
For pharmaceutical applications, regulatory bodies like the FDA and EMA impose additional requirements through Good Manufacturing Practice (GMP) guidelines. These regulations mandate comprehensive documentation of solvent quality, including certificates of analysis (CoA) that verify batch-specific purity testing results and traceability throughout the supply chain.
Environmental considerations have also become increasingly important in regulatory frameworks. The Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) in Europe and similar programs globally require detailed safety assessments and environmental impact evaluations for chemical substances, including HPLC solvents.
Compliance verification typically involves rigorous documentation practices. Manufacturers must maintain detailed records of production parameters, quality control testing, and stability studies. End-users in regulated industries must implement incoming material verification protocols to confirm that received solvents meet specified purity requirements before use in analytical procedures.
Recent regulatory trends show increasing harmonization efforts between major pharmacopeias, aiming to establish globally consistent standards for analytical grade solvents. Simultaneously, regulations are evolving to address emerging concerns about ultra-trace contaminants that may impact sensitive analytical methods, particularly in bioanalytical applications and environmental testing.
For laboratories seeking to maintain regulatory compliance, implementing a robust supplier qualification program represents a best practice. This includes periodic audit of solvent manufacturers, review of their quality systems, and verification of their compliance with relevant regulatory standards to ensure consistent access to high-quality HPLC solvents that meet all applicable regulatory requirements.
Environmental Impact of HPLC Solvent Production and Disposal
The production and disposal of HPLC solvents present significant environmental challenges that warrant careful consideration in analytical laboratories. The manufacturing processes for high-purity solvents typically involve energy-intensive distillation and purification steps, contributing substantially to carbon emissions. For instance, the production of one liter of HPLC-grade acetonitrile requires approximately 13-15 kWh of energy, resulting in 5-7 kg of CO2 emissions depending on the energy source.
Water consumption represents another critical environmental factor, with solvent manufacturing facilities utilizing between 20-50 liters of water per liter of high-purity solvent produced. This water usage extends beyond direct production to cooling systems and equipment cleaning processes, placing additional pressure on local water resources.
Chemical waste generation during solvent production includes byproducts and impurities removed during purification. These often contain toxic compounds that require specialized treatment before release into the environment. The purification process for HPLC-grade solvents typically generates 0.5-2 kg of chemical waste per liter of final product, creating a substantial waste management challenge.
The disposal phase presents equally concerning environmental implications. Laboratory practices often involve discarding used HPLC solvents as hazardous waste, with global analytical laboratories generating an estimated 5-7 million liters of waste solvents annually. Improper disposal can lead to soil contamination, groundwater pollution, and adverse effects on aquatic ecosystems, as many common HPLC solvents like acetonitrile and methanol exhibit high toxicity to aquatic organisms.
Regulatory frameworks governing solvent disposal vary significantly across regions, creating inconsistent environmental protection standards. While the European Union's REACH regulations and the United States EPA guidelines establish strict protocols for solvent handling and disposal, enforcement and compliance remain challenging in many regions, particularly in developing economies where analytical chemistry applications are rapidly expanding.
Recent sustainability initiatives have focused on developing greener alternatives to traditional HPLC solvents. Water-based mobile phases, bio-derived solvents, and supercritical CO2 systems represent promising approaches to reducing environmental impact. Additionally, solvent recycling technologies have advanced significantly, with modern distillation and filtration systems capable of recovering 70-85% of used HPLC solvents at sufficient purity for reuse in certain applications.
The environmental footprint of HPLC solvent usage ultimately necessitates a comprehensive lifecycle approach, considering both production impacts and end-of-life management strategies to ensure sustainable analytical practices.
Water consumption represents another critical environmental factor, with solvent manufacturing facilities utilizing between 20-50 liters of water per liter of high-purity solvent produced. This water usage extends beyond direct production to cooling systems and equipment cleaning processes, placing additional pressure on local water resources.
Chemical waste generation during solvent production includes byproducts and impurities removed during purification. These often contain toxic compounds that require specialized treatment before release into the environment. The purification process for HPLC-grade solvents typically generates 0.5-2 kg of chemical waste per liter of final product, creating a substantial waste management challenge.
The disposal phase presents equally concerning environmental implications. Laboratory practices often involve discarding used HPLC solvents as hazardous waste, with global analytical laboratories generating an estimated 5-7 million liters of waste solvents annually. Improper disposal can lead to soil contamination, groundwater pollution, and adverse effects on aquatic ecosystems, as many common HPLC solvents like acetonitrile and methanol exhibit high toxicity to aquatic organisms.
Regulatory frameworks governing solvent disposal vary significantly across regions, creating inconsistent environmental protection standards. While the European Union's REACH regulations and the United States EPA guidelines establish strict protocols for solvent handling and disposal, enforcement and compliance remain challenging in many regions, particularly in developing economies where analytical chemistry applications are rapidly expanding.
Recent sustainability initiatives have focused on developing greener alternatives to traditional HPLC solvents. Water-based mobile phases, bio-derived solvents, and supercritical CO2 systems represent promising approaches to reducing environmental impact. Additionally, solvent recycling technologies have advanced significantly, with modern distillation and filtration systems capable of recovering 70-85% of used HPLC solvents at sufficient purity for reuse in certain applications.
The environmental footprint of HPLC solvent usage ultimately necessitates a comprehensive lifecycle approach, considering both production impacts and end-of-life management strategies to ensure sustainable analytical practices.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!