How to Reduce HPLC Setup Time for Routine Assessments
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HPLC Technology Evolution and Efficiency Goals
High-Performance Liquid Chromatography (HPLC) has evolved significantly since its commercial introduction in the 1960s. Initially characterized by large, manually operated systems with limited automation, HPLC technology has undergone several transformative phases that have dramatically improved analytical capabilities while reducing operational complexity. The evolution trajectory shows a clear trend toward miniaturization, increased automation, and enhanced computational integration.
The 1980s marked the first major leap with the introduction of microprocessor-controlled systems, enabling basic sequence programming and data processing. By the 1990s, fully integrated software platforms emerged, allowing for comprehensive method development and validation protocols. The 2000s witnessed the rise of ultra-high-performance liquid chromatography (UHPLC), which leveraged smaller particle sizes and higher pressures to achieve faster separations with improved resolution.
Recent technological advancements have focused on intelligent system design, incorporating predictive maintenance algorithms, remote monitoring capabilities, and user-friendly interfaces that significantly reduce the learning curve for operators. Modern HPLC systems now feature plug-and-play components, automated calibration routines, and pre-configured method templates that streamline setup procedures for routine analyses.
The primary efficiency goal in contemporary HPLC development is to minimize non-productive time, particularly during system setup, method transfer, and column equilibration phases. Industry benchmarks suggest that traditional HPLC setup procedures for routine assessments can consume 15-30% of total analysis time, representing a substantial opportunity for operational optimization. Leading manufacturers have established targets to reduce setup times by 50-70% through technological innovation and workflow redesign.
Another critical objective is to enhance system reliability while decreasing the technical expertise required for operation. This democratization of HPLC technology aims to expand its accessibility across diverse laboratory environments, from sophisticated research facilities to quality control departments in manufacturing settings. The industry is moving toward "walk-up" systems that require minimal user intervention for routine analyses.
Future development trajectories indicate a convergence of HPLC technology with artificial intelligence and machine learning algorithms to create self-optimizing systems capable of adapting separation parameters in real-time based on sample characteristics. The ultimate goal is to achieve "one-touch" operation for standardized analyses, where sample loading becomes the only manual step in the analytical process.
These evolutionary trends and efficiency goals collectively point toward a future where HPLC setup time for routine assessments approaches the theoretical minimum, maximizing instrument utilization and laboratory throughput while maintaining analytical integrity and compliance with regulatory standards.
The 1980s marked the first major leap with the introduction of microprocessor-controlled systems, enabling basic sequence programming and data processing. By the 1990s, fully integrated software platforms emerged, allowing for comprehensive method development and validation protocols. The 2000s witnessed the rise of ultra-high-performance liquid chromatography (UHPLC), which leveraged smaller particle sizes and higher pressures to achieve faster separations with improved resolution.
Recent technological advancements have focused on intelligent system design, incorporating predictive maintenance algorithms, remote monitoring capabilities, and user-friendly interfaces that significantly reduce the learning curve for operators. Modern HPLC systems now feature plug-and-play components, automated calibration routines, and pre-configured method templates that streamline setup procedures for routine analyses.
The primary efficiency goal in contemporary HPLC development is to minimize non-productive time, particularly during system setup, method transfer, and column equilibration phases. Industry benchmarks suggest that traditional HPLC setup procedures for routine assessments can consume 15-30% of total analysis time, representing a substantial opportunity for operational optimization. Leading manufacturers have established targets to reduce setup times by 50-70% through technological innovation and workflow redesign.
Another critical objective is to enhance system reliability while decreasing the technical expertise required for operation. This democratization of HPLC technology aims to expand its accessibility across diverse laboratory environments, from sophisticated research facilities to quality control departments in manufacturing settings. The industry is moving toward "walk-up" systems that require minimal user intervention for routine analyses.
Future development trajectories indicate a convergence of HPLC technology with artificial intelligence and machine learning algorithms to create self-optimizing systems capable of adapting separation parameters in real-time based on sample characteristics. The ultimate goal is to achieve "one-touch" operation for standardized analyses, where sample loading becomes the only manual step in the analytical process.
These evolutionary trends and efficiency goals collectively point toward a future where HPLC setup time for routine assessments approaches the theoretical minimum, maximizing instrument utilization and laboratory throughput while maintaining analytical integrity and compliance with regulatory standards.
Market Demand for Rapid Analytical Methods
The analytical chemistry market has witnessed a significant shift towards rapid analytical methods, with HPLC (High-Performance Liquid Chromatography) remaining a cornerstone technology across multiple industries. The global HPLC market was valued at approximately 4.5 billion USD in 2022 and is projected to grow at a compound annual growth rate of 5.2% through 2028, driven largely by demand for faster analytical processes.
Pharmaceutical companies, which account for nearly 40% of the HPLC market, face increasing pressure to accelerate drug development timelines while maintaining rigorous quality standards. These organizations typically run hundreds of routine HPLC analyses daily, with setup times often consuming 20-30 minutes per analysis. This translates to thousands of labor hours annually dedicated solely to instrument preparation rather than actual analysis.
Clinical laboratories represent another significant market segment demanding rapid analytical methods. With the average hospital laboratory processing over 5,000 samples daily, even minor reductions in HPLC setup time can yield substantial operational benefits. Survey data indicates that laboratory managers rank "reduction in analysis preparation time" among their top three priorities when considering new equipment purchases.
Food safety testing laboratories face similar challenges, with regulatory requirements mandating frequent testing of products. The FDA's implementation of the Food Safety Modernization Act has increased testing frequency requirements by approximately 30%, creating significant backlogs in laboratories still using traditional HPLC setup procedures.
Environmental testing laboratories have experienced a 25% increase in sample volume over the past five years, primarily due to expanded regulatory monitoring requirements for emerging contaminants. These laboratories particularly value rapid analytical methods that can accommodate variable sample matrices while maintaining detection sensitivity.
Academic and research institutions, while less driven by throughput demands, increasingly seek efficient analytical methods to maximize research productivity within limited funding environments. A survey of university research laboratories revealed that approximately 60% of researchers consider instrument setup time a significant factor limiting experimental throughput.
The market demand for reduced HPLC setup time is further evidenced by recent purchasing trends, with systems advertising "quick-start" capabilities commanding premium prices of 15-20% above standard models. Vendors report that features reducing setup time consistently rank among the top selling points for new HPLC systems across all market segments.
Cross-industry analysis reveals that organizations achieving a 50% reduction in HPLC setup time typically realize productivity improvements of 15-20% in their analytical workflows, representing significant operational cost savings and competitive advantage in time-sensitive markets.
Pharmaceutical companies, which account for nearly 40% of the HPLC market, face increasing pressure to accelerate drug development timelines while maintaining rigorous quality standards. These organizations typically run hundreds of routine HPLC analyses daily, with setup times often consuming 20-30 minutes per analysis. This translates to thousands of labor hours annually dedicated solely to instrument preparation rather than actual analysis.
Clinical laboratories represent another significant market segment demanding rapid analytical methods. With the average hospital laboratory processing over 5,000 samples daily, even minor reductions in HPLC setup time can yield substantial operational benefits. Survey data indicates that laboratory managers rank "reduction in analysis preparation time" among their top three priorities when considering new equipment purchases.
Food safety testing laboratories face similar challenges, with regulatory requirements mandating frequent testing of products. The FDA's implementation of the Food Safety Modernization Act has increased testing frequency requirements by approximately 30%, creating significant backlogs in laboratories still using traditional HPLC setup procedures.
Environmental testing laboratories have experienced a 25% increase in sample volume over the past five years, primarily due to expanded regulatory monitoring requirements for emerging contaminants. These laboratories particularly value rapid analytical methods that can accommodate variable sample matrices while maintaining detection sensitivity.
Academic and research institutions, while less driven by throughput demands, increasingly seek efficient analytical methods to maximize research productivity within limited funding environments. A survey of university research laboratories revealed that approximately 60% of researchers consider instrument setup time a significant factor limiting experimental throughput.
The market demand for reduced HPLC setup time is further evidenced by recent purchasing trends, with systems advertising "quick-start" capabilities commanding premium prices of 15-20% above standard models. Vendors report that features reducing setup time consistently rank among the top selling points for new HPLC systems across all market segments.
Cross-industry analysis reveals that organizations achieving a 50% reduction in HPLC setup time typically realize productivity improvements of 15-20% in their analytical workflows, representing significant operational cost savings and competitive advantage in time-sensitive markets.
Current HPLC Setup Challenges and Limitations
High-Performance Liquid Chromatography (HPLC) setup for routine assessments faces numerous challenges that significantly impact laboratory efficiency and productivity. Current setup procedures typically require 30-60 minutes per analysis, creating substantial bottlenecks in high-throughput environments. This time consumption becomes particularly problematic in quality control laboratories, clinical settings, and pharmaceutical manufacturing where rapid turnaround times are essential.
A primary challenge lies in the manual nature of many HPLC setup processes. Technicians must physically install columns, connect tubing, prime pumps, and purge systems of air bubbles—all tasks requiring precision and experience. These manual interventions introduce variability and increase the risk of human error, potentially compromising analytical results and necessitating system restarts.
Method development and optimization represent another significant time sink. Analysts must determine appropriate mobile phase compositions, flow rates, column temperatures, and detection parameters for each new compound or sample matrix. This process often involves multiple iterations and can extend setup times by hours or even days for complex analyses.
System equilibration remains a persistent challenge, with columns requiring adequate conditioning time to reach stable baseline conditions. Insufficient equilibration leads to retention time drift, poor peak shapes, and unreliable quantification. Conversely, excessive equilibration wastes valuable instrument time and delays sample analysis.
Cross-contamination between analyses presents additional complications. Thorough system cleaning is necessary to prevent carryover, particularly when transitioning between different sample types or methods. These cleaning procedures add considerable time to the setup process but cannot be circumvented without risking data integrity.
Instrument calibration and system suitability testing further extend setup times. Regular performance verification is mandatory in regulated environments, requiring the analysis of standard solutions and system suitability samples before routine assessments can begin. These quality assurance measures, while essential, contribute significantly to overall setup duration.
Documentation requirements compound these challenges. In GMP-compliant laboratories, each setup step must be meticulously recorded, verified, and sometimes approved by supervisors. This administrative burden adds another layer of complexity and time to the HPLC setup process.
Resource constraints exacerbate these issues, with limited availability of trained personnel and equipment creating scheduling conflicts and workflow disruptions. Many laboratories operate with shared instruments, necessitating frequent method changes and reconfiguration, each requiring complete setup procedures.
The cumulative impact of these challenges is substantial, with some facilities reporting that HPLC setup activities consume up to 30% of total analytical time. This inefficiency translates directly to increased operational costs, delayed decision-making, and reduced laboratory throughput.
A primary challenge lies in the manual nature of many HPLC setup processes. Technicians must physically install columns, connect tubing, prime pumps, and purge systems of air bubbles—all tasks requiring precision and experience. These manual interventions introduce variability and increase the risk of human error, potentially compromising analytical results and necessitating system restarts.
Method development and optimization represent another significant time sink. Analysts must determine appropriate mobile phase compositions, flow rates, column temperatures, and detection parameters for each new compound or sample matrix. This process often involves multiple iterations and can extend setup times by hours or even days for complex analyses.
System equilibration remains a persistent challenge, with columns requiring adequate conditioning time to reach stable baseline conditions. Insufficient equilibration leads to retention time drift, poor peak shapes, and unreliable quantification. Conversely, excessive equilibration wastes valuable instrument time and delays sample analysis.
Cross-contamination between analyses presents additional complications. Thorough system cleaning is necessary to prevent carryover, particularly when transitioning between different sample types or methods. These cleaning procedures add considerable time to the setup process but cannot be circumvented without risking data integrity.
Instrument calibration and system suitability testing further extend setup times. Regular performance verification is mandatory in regulated environments, requiring the analysis of standard solutions and system suitability samples before routine assessments can begin. These quality assurance measures, while essential, contribute significantly to overall setup duration.
Documentation requirements compound these challenges. In GMP-compliant laboratories, each setup step must be meticulously recorded, verified, and sometimes approved by supervisors. This administrative burden adds another layer of complexity and time to the HPLC setup process.
Resource constraints exacerbate these issues, with limited availability of trained personnel and equipment creating scheduling conflicts and workflow disruptions. Many laboratories operate with shared instruments, necessitating frequent method changes and reconfiguration, each requiring complete setup procedures.
The cumulative impact of these challenges is substantial, with some facilities reporting that HPLC setup activities consume up to 30% of total analytical time. This inefficiency translates directly to increased operational costs, delayed decision-making, and reduced laboratory throughput.
Current Approaches to HPLC Setup Time Reduction
01 Automated HPLC setup systems
Automated systems for HPLC setup can significantly reduce preparation time by handling tasks such as mobile phase preparation, column equilibration, and system calibration automatically. These systems often include programmable interfaces that allow operators to pre-configure methods and sequences, enabling quick transitions between different analyses. The automation reduces human error and increases throughput by minimizing manual intervention during the setup process.- Automated HPLC system setup and calibration: Automated systems for HPLC setup reduce the time required for preparation and calibration. These systems can automatically configure parameters, prime columns, and perform system suitability tests. Automation technology helps eliminate manual steps in the setup process, reducing human error and ensuring consistent performance across multiple analyses. These innovations significantly decrease the overall setup time for HPLC systems in laboratory environments.
- Quick-connect components and modular HPLC systems: Modular HPLC systems with quick-connect components allow for rapid assembly and reconfiguration. These systems feature standardized interfaces for columns, detectors, and other components that can be swapped without extensive recalibration. The quick-connect technology reduces the time needed for changing between different analytical methods or sample types. This approach minimizes downtime between analyses and improves laboratory throughput.
- Pre-equilibration techniques for HPLC columns: Pre-equilibration techniques reduce the time required for HPLC column stabilization. These methods involve preparing columns with appropriate mobile phases before analysis begins, ensuring baseline stability is achieved more quickly. Some techniques include temperature-controlled pre-equilibration chambers and automated solvent conditioning systems. These approaches significantly reduce the waiting time between sample runs and improve overall analytical efficiency.
- Integrated software solutions for HPLC method development: Integrated software solutions streamline HPLC method development and setup processes. These platforms provide automated method creation, optimization tools, and predictive algorithms to determine optimal conditions. The software can simulate chromatographic separations, reducing the need for multiple trial runs. By automating parameter selection and method transfer, these systems significantly reduce the time required to establish reliable analytical methods.
- Rapid mobile phase preparation systems: Systems for rapid mobile phase preparation reduce HPLC setup time by automating solvent mixing and degassing. These technologies include on-demand solvent blending, in-line degassing, and quality verification of mobile phases. Some systems incorporate pre-packaged, ready-to-use mobile phase kits that eliminate manual preparation steps. By reducing the time required for mobile phase preparation, these innovations significantly decrease overall HPLC setup time.
02 Quick-connect column technologies
Advanced column connection technologies enable rapid installation and replacement of HPLC columns without compromising system integrity. These quick-connect systems feature tool-free mechanisms that maintain zero dead volume while allowing operators to change columns in seconds rather than minutes. Some designs incorporate pre-heating elements to reduce equilibration time when switching between methods requiring different temperature conditions.Expand Specific Solutions03 Rapid mobile phase preparation methods
Innovative approaches to mobile phase preparation can substantially reduce HPLC setup time. These include pre-mixed solvent systems, concentrated buffer solutions that can be quickly diluted to working concentrations, and in-line degassing technologies that eliminate the need for time-consuming vacuum degassing procedures. Some systems incorporate automatic solvent blending capabilities that prepare mobile phases on-demand according to pre-programmed recipes.Expand Specific Solutions04 Accelerated system equilibration techniques
Methods to reduce HPLC system equilibration time include optimized flow path designs, advanced temperature control systems, and gradient pre-conditioning protocols. These techniques ensure rapid stabilization of baseline signals and detector response. Some approaches utilize predictive algorithms to determine the minimum equilibration time needed based on method parameters, while others employ parallel column equilibration where one column is being prepared while another is in use.Expand Specific Solutions05 Integrated method development and validation tools
Software tools that integrate method development with system setup can reduce overall HPLC preparation time. These platforms provide template-based method creation, automated system suitability testing, and real-time method optimization capabilities. By streamlining the transition from method development to routine analysis, these tools eliminate redundant setup steps and provide guided workflows that ensure consistent system preparation across different operators and instruments.Expand Specific Solutions
Leading Manufacturers and Research Institutions in HPLC
The HPLC setup time reduction market is in a growth phase, driven by increasing demand for efficiency in pharmaceutical and biotechnology laboratories. The global market for HPLC optimization solutions is estimated at $1.2-1.5 billion, expanding at 6-8% annually as routine assessments become more prevalent. Technologically, the field shows varying maturity levels across companies. Industry leaders like Vertex Pharmaceuticals, Novo Nordisk, and Bristol Myers Squibb have developed advanced automated setup protocols, while Siemens and 3M Innovative Properties offer sophisticated hardware solutions. Emerging players such as Sunshine Lake Pharma and Merlin Biomedical are introducing innovative software-based approaches. Chinese companies including Hainan Chang'an and Guizhou Yibai are rapidly advancing with cost-effective alternatives, narrowing the technological gap with established Western counterparts.
Vertex Pharmaceuticals, Inc.
Technical Solution: Vertex Pharmaceuticals has developed an automated HPLC method development platform that significantly reduces setup time for routine assessments. Their approach incorporates pre-configured method templates with standardized mobile phase compositions and gradient profiles specifically optimized for different drug classes. The system employs intelligent column selection algorithms that recommend the most suitable stationary phases based on the analyte's chemical properties. Additionally, Vertex has implemented parallel sample preparation workflows using robotic liquid handling systems that can simultaneously prepare multiple samples while the HPLC system is running previous analyses. Their platform includes automated system suitability testing that runs overnight, ensuring the instrument is calibrated and ready for immediate use during working hours. The company has also developed specialized software that predicts optimal separation conditions based on molecular structure, reducing the need for extensive method development experiments.
Strengths: Highly automated workflow reduces hands-on time by approximately 60%; integrated data management system ensures compliance with regulatory requirements; scalable for both small and large molecule analyses. Weaknesses: Requires significant initial investment in automation equipment; system optimization needs specialized training; may be overly complex for simple routine analyses.
Bristol Myers Squibb Co.
Technical Solution: Bristol Myers Squibb has pioneered a comprehensive HPLC efficiency program called "Rapid Chromatography Excellence" (RCE) to minimize setup time for routine assessments. The RCE system features standardized instrument configurations across their global research sites, with pre-qualified column inventories and mobile phase preparation protocols. Their approach includes the implementation of quick-connect fittings and tool-free column couplings that reduce manual handling time by approximately 75% compared to traditional setups. BMS has developed specialized equilibration protocols that utilize higher flow rates during system preparation, followed by automatic adjustment to analytical conditions, cutting equilibration times by up to 40%. The company employs multi-channel HPLC systems with automated column and solvent selection capabilities, allowing analysts to queue multiple methods without manual intervention. Their platform incorporates predictive maintenance algorithms that schedule system checks during non-operational hours, minimizing unexpected downtime during critical analysis periods.
Strengths: Standardized global approach ensures method transferability between sites; quick-connect technology significantly reduces manual setup time; predictive maintenance minimizes unexpected instrument failures. Weaknesses: Requires organization-wide implementation to realize full benefits; specialized fittings may increase consumable costs; system complexity may require dedicated support personnel.
Key Innovations in Quick-Connect and Automated Systems
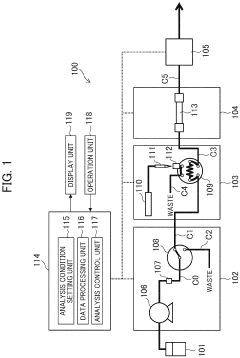
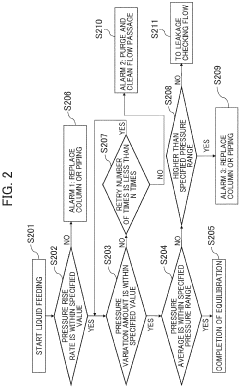
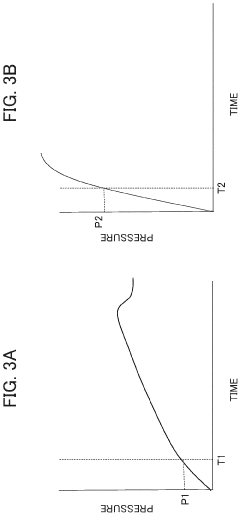
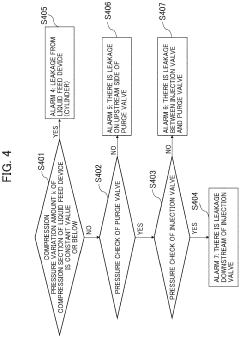
Automatic analysis device having HPLC, and method for controlling said automatic analysis device
PatentPendingEP4325218A1
Innovation
- An automatic analyzer with a pressure sensor and control unit that calculates pressure rise rate and compression pressure variation to automatically determine column equilibration completion and identify error positions, minimizing user intervention and preventing equipment damage.
High-performance liquid chromatography with a controllable transverse flow inducer
PatentActiveEP3322978A1
Innovation
- The use of a controllable transverse flow inducer, which generates micro-scale vortices through alternating current electrokinetics, allowing for orthogonal flow induction independent of axial velocity, reducing dispersion by combining pressure and electro-osmotic flow, and enabling retention modulation without permanent surface charges.
Method Transfer and Validation Considerations
Method transfer and validation represent critical processes when implementing time-saving HPLC protocols across different laboratories or instruments. Successful method transfer ensures that analytical procedures developed in one laboratory can be reliably reproduced in another with minimal setup time while maintaining data integrity. This requires careful consideration of several key factors to ensure seamless implementation.
When transferring HPLC methods between laboratories or instruments, it is essential to establish acceptance criteria that define successful transfer. These criteria typically include system suitability parameters, precision limits, and accuracy requirements. Laboratories should develop comprehensive transfer protocols that specify the number of replicates, sample types, and statistical analyses required to demonstrate equivalence between the sending and receiving laboratories.
Robustness testing plays a vital role in method transfer by identifying critical parameters that may affect method performance. By understanding how small variations in mobile phase composition, column temperature, or flow rate impact results, analysts can establish appropriate operating ranges that minimize setup time while ensuring consistent performance. This knowledge allows for faster method implementation with fewer troubleshooting iterations.
Analytical method validation should be approached strategically to reduce routine setup time. While ICH guidelines specify validation parameters such as specificity, linearity, accuracy, precision, range, and robustness, the extent of validation can be tailored based on the method's intended use. For routine quality control applications, a streamlined validation approach focusing on critical parameters can significantly reduce implementation time without compromising quality.
Life cycle management of analytical methods represents another important consideration. Implementing continuous method monitoring and periodic verification rather than complete revalidation can substantially reduce setup time for routine assessments. This approach involves establishing system suitability criteria and control charts to monitor method performance over time, allowing for quick identification of trends or shifts that might require attention.
Cross-training laboratory personnel on standardized method setup procedures ensures consistent implementation across different analysts. Developing detailed standard operating procedures (SOPs) with clear instructions for column conditioning, mobile phase preparation, and system equilibration can significantly reduce setup variability and associated time costs. These SOPs should incorporate best practices identified during method development and validation phases.
When transferring HPLC methods between laboratories or instruments, it is essential to establish acceptance criteria that define successful transfer. These criteria typically include system suitability parameters, precision limits, and accuracy requirements. Laboratories should develop comprehensive transfer protocols that specify the number of replicates, sample types, and statistical analyses required to demonstrate equivalence between the sending and receiving laboratories.
Robustness testing plays a vital role in method transfer by identifying critical parameters that may affect method performance. By understanding how small variations in mobile phase composition, column temperature, or flow rate impact results, analysts can establish appropriate operating ranges that minimize setup time while ensuring consistent performance. This knowledge allows for faster method implementation with fewer troubleshooting iterations.
Analytical method validation should be approached strategically to reduce routine setup time. While ICH guidelines specify validation parameters such as specificity, linearity, accuracy, precision, range, and robustness, the extent of validation can be tailored based on the method's intended use. For routine quality control applications, a streamlined validation approach focusing on critical parameters can significantly reduce implementation time without compromising quality.
Life cycle management of analytical methods represents another important consideration. Implementing continuous method monitoring and periodic verification rather than complete revalidation can substantially reduce setup time for routine assessments. This approach involves establishing system suitability criteria and control charts to monitor method performance over time, allowing for quick identification of trends or shifts that might require attention.
Cross-training laboratory personnel on standardized method setup procedures ensures consistent implementation across different analysts. Developing detailed standard operating procedures (SOPs) with clear instructions for column conditioning, mobile phase preparation, and system equilibration can significantly reduce setup variability and associated time costs. These SOPs should incorporate best practices identified during method development and validation phases.
Cost-Benefit Analysis of HPLC Time Reduction Solutions
When evaluating solutions for reducing HPLC setup time, a comprehensive cost-benefit analysis is essential to determine the most economically viable approaches. Initial investment costs for automated systems, such as autosamplers and column switching technologies, typically range from $15,000 to $100,000 depending on sophistication level. However, these systems can reduce setup times by 40-70%, translating to significant labor savings over time.
Labor cost calculations reveal that a typical analytical laboratory spends approximately 25-30% of technician time on HPLC setup procedures. With an average analytical chemist salary of $70,000-$90,000 annually, reducing setup time by 50% could save $8,750-$13,500 per technician per year. For laboratories with multiple HPLC systems, these savings multiply accordingly.
Consumable reduction represents another significant benefit. Implementing modern quick-connect fittings and pre-assembled column cartridges can reduce solvent waste by 15-25% during setup procedures. With specialty HPLC solvents costing $50-200 per liter, a medium-sized laboratory could save $3,000-$7,500 annually on solvent expenses alone.
Throughput improvements deliver perhaps the most substantial economic benefit. Reducing setup time by 60% allows laboratories to increase analytical capacity by 15-25% without additional equipment purchases. For contract research organizations or quality control laboratories charging $150-300 per sample analysis, this throughput increase could generate additional revenue of $75,000-$225,000 annually per instrument.
Return on investment calculations indicate that most automated solutions achieve payback within 12-24 months, with more sophisticated systems requiring longer periods but delivering greater long-term benefits. Cloud-based method transfer solutions typically show the fastest ROI at 6-12 months due to their lower initial investment requirements.
Opportunity cost considerations must also factor into the analysis. Delayed results from inefficient HPLC setups can postpone critical business decisions, potentially costing organizations substantially more than the direct laboratory expenses. For pharmaceutical companies, a one-day delay in product release can represent $50,000-$500,000 in lost revenue depending on the product's market value.
Risk mitigation benefits, while harder to quantify, include reduced human error rates, improved compliance with regulatory standards, and enhanced data integrity. These factors can prevent costly investigations, remediation efforts, and potential regulatory penalties that might otherwise result from manual setup errors.
Labor cost calculations reveal that a typical analytical laboratory spends approximately 25-30% of technician time on HPLC setup procedures. With an average analytical chemist salary of $70,000-$90,000 annually, reducing setup time by 50% could save $8,750-$13,500 per technician per year. For laboratories with multiple HPLC systems, these savings multiply accordingly.
Consumable reduction represents another significant benefit. Implementing modern quick-connect fittings and pre-assembled column cartridges can reduce solvent waste by 15-25% during setup procedures. With specialty HPLC solvents costing $50-200 per liter, a medium-sized laboratory could save $3,000-$7,500 annually on solvent expenses alone.
Throughput improvements deliver perhaps the most substantial economic benefit. Reducing setup time by 60% allows laboratories to increase analytical capacity by 15-25% without additional equipment purchases. For contract research organizations or quality control laboratories charging $150-300 per sample analysis, this throughput increase could generate additional revenue of $75,000-$225,000 annually per instrument.
Return on investment calculations indicate that most automated solutions achieve payback within 12-24 months, with more sophisticated systems requiring longer periods but delivering greater long-term benefits. Cloud-based method transfer solutions typically show the fastest ROI at 6-12 months due to their lower initial investment requirements.
Opportunity cost considerations must also factor into the analysis. Delayed results from inefficient HPLC setups can postpone critical business decisions, potentially costing organizations substantially more than the direct laboratory expenses. For pharmaceutical companies, a one-day delay in product release can represent $50,000-$500,000 in lost revenue depending on the product's market value.
Risk mitigation benefits, while harder to quantify, include reduced human error rates, improved compliance with regulatory standards, and enhanced data integrity. These factors can prevent costly investigations, remediation efforts, and potential regulatory penalties that might otherwise result from manual setup errors.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!