HPLC vs CE: Efficiency in Protein Separation
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Protein Separation Technology Evolution and Objectives
Protein separation techniques have evolved significantly over the past century, with major advancements occurring in the last few decades. The journey began with simple precipitation methods in the early 20th century, followed by the development of chromatography techniques in the 1940s. High-Performance Liquid Chromatography (HPLC) emerged in the 1970s as a revolutionary approach, offering improved resolution and efficiency for protein separation compared to traditional column chromatography.
Capillary Electrophoresis (CE) entered the scene in the 1980s, presenting an alternative methodology based on different separation principles. While HPLC relies on interactions between proteins and stationary phases under pressure, CE separates proteins based on their charge-to-mass ratio in an electric field. This fundamental difference has created two parallel technological paths in protein separation science, each with distinct advantages and limitations.
The evolution of these technologies has been driven by increasing demands in biopharmaceutical production, proteomics research, and clinical diagnostics. Modern protein separation objectives focus on achieving higher resolution, increased sensitivity, improved reproducibility, and greater throughput while maintaining protein integrity. Additionally, there is growing emphasis on miniaturization, automation, and integration with downstream analytical techniques.
Recent technological innovations have further enhanced both HPLC and CE capabilities. HPLC has seen advancements in column technology, including sub-2-μm particles, monolithic columns, and superficially porous particles, all contributing to improved separation efficiency. Meanwhile, CE has benefited from developments in capillary coating technologies, detection methods, and microchip implementations that have expanded its application range.
The current technological landscape shows a trend toward hybrid approaches that combine the strengths of both HPLC and CE. These hybrid systems aim to overcome the limitations of individual techniques while capitalizing on their respective advantages. Furthermore, computational modeling and machine learning algorithms are increasingly being employed to optimize separation parameters and predict protein behavior.
Looking forward, the objectives for protein separation technology development include achieving single-molecule resolution, developing truly orthogonal separation mechanisms, reducing sample volume requirements to nanoliter levels, and creating fully automated, integrated analytical platforms. There is also significant interest in developing sustainable, environmentally friendly separation methods that reduce solvent consumption and waste generation while maintaining or improving separation performance.
Capillary Electrophoresis (CE) entered the scene in the 1980s, presenting an alternative methodology based on different separation principles. While HPLC relies on interactions between proteins and stationary phases under pressure, CE separates proteins based on their charge-to-mass ratio in an electric field. This fundamental difference has created two parallel technological paths in protein separation science, each with distinct advantages and limitations.
The evolution of these technologies has been driven by increasing demands in biopharmaceutical production, proteomics research, and clinical diagnostics. Modern protein separation objectives focus on achieving higher resolution, increased sensitivity, improved reproducibility, and greater throughput while maintaining protein integrity. Additionally, there is growing emphasis on miniaturization, automation, and integration with downstream analytical techniques.
Recent technological innovations have further enhanced both HPLC and CE capabilities. HPLC has seen advancements in column technology, including sub-2-μm particles, monolithic columns, and superficially porous particles, all contributing to improved separation efficiency. Meanwhile, CE has benefited from developments in capillary coating technologies, detection methods, and microchip implementations that have expanded its application range.
The current technological landscape shows a trend toward hybrid approaches that combine the strengths of both HPLC and CE. These hybrid systems aim to overcome the limitations of individual techniques while capitalizing on their respective advantages. Furthermore, computational modeling and machine learning algorithms are increasingly being employed to optimize separation parameters and predict protein behavior.
Looking forward, the objectives for protein separation technology development include achieving single-molecule resolution, developing truly orthogonal separation mechanisms, reducing sample volume requirements to nanoliter levels, and creating fully automated, integrated analytical platforms. There is also significant interest in developing sustainable, environmentally friendly separation methods that reduce solvent consumption and waste generation while maintaining or improving separation performance.
Market Analysis for Protein Separation Methods
The global protein separation market has witnessed substantial growth in recent years, driven by advancements in proteomics research, biopharmaceutical development, and diagnostic applications. As of 2023, the protein separation technologies market is valued at approximately $11.7 billion, with projections indicating a compound annual growth rate (CAGR) of 8.2% through 2028.
High-Performance Liquid Chromatography (HPLC) currently dominates the protein separation market, accounting for roughly 65% of the total market share. This dominance stems from HPLC's established presence in pharmaceutical quality control, academic research, and clinical diagnostics. The HPLC segment has benefited from continuous technological improvements in column chemistry, detector sensitivity, and automation capabilities.
Capillary Electrophoresis (CE), while representing a smaller market segment at approximately 15% market share, is experiencing faster growth with a CAGR of 9.7%. This accelerated growth is attributed to CE's advantages in handling smaller sample volumes, reduced analysis time, and lower operational costs compared to traditional HPLC methods.
Regional analysis reveals North America as the largest market for protein separation technologies, holding 42% of the global market share, followed by Europe (28%) and Asia-Pacific (22%). The Asia-Pacific region, particularly China and India, is expected to witness the highest growth rate due to increasing investments in biotechnology research and expanding pharmaceutical manufacturing capabilities.
End-user segmentation shows that pharmaceutical and biotechnology companies constitute the largest consumer base (58%), followed by academic and research institutions (24%), and clinical diagnostic laboratories (12%). The remaining market share is distributed among food testing laboratories and environmental monitoring agencies.
Key market drivers include the growing demand for biopharmaceuticals, increasing research in proteomics, technological advancements in separation techniques, and rising prevalence of chronic diseases necessitating protein-based diagnostics and therapeutics. The shift toward personalized medicine has further accelerated the need for efficient protein separation methods capable of handling complex biological samples.
Market challenges include the high cost of advanced separation systems, technical complexity requiring specialized training, and regulatory hurdles for clinical applications. Additionally, the market faces pressure from alternative technologies such as mass spectrometry-based approaches that can sometimes bypass traditional separation steps.
Emerging trends indicate growing interest in hybrid systems that combine the strengths of both HPLC and CE technologies, miniaturization for point-of-care applications, and integration with artificial intelligence for automated method development and optimization. The demand for single-use, disposable separation technologies is also rising, particularly in clinical settings where cross-contamination concerns are paramount.
High-Performance Liquid Chromatography (HPLC) currently dominates the protein separation market, accounting for roughly 65% of the total market share. This dominance stems from HPLC's established presence in pharmaceutical quality control, academic research, and clinical diagnostics. The HPLC segment has benefited from continuous technological improvements in column chemistry, detector sensitivity, and automation capabilities.
Capillary Electrophoresis (CE), while representing a smaller market segment at approximately 15% market share, is experiencing faster growth with a CAGR of 9.7%. This accelerated growth is attributed to CE's advantages in handling smaller sample volumes, reduced analysis time, and lower operational costs compared to traditional HPLC methods.
Regional analysis reveals North America as the largest market for protein separation technologies, holding 42% of the global market share, followed by Europe (28%) and Asia-Pacific (22%). The Asia-Pacific region, particularly China and India, is expected to witness the highest growth rate due to increasing investments in biotechnology research and expanding pharmaceutical manufacturing capabilities.
End-user segmentation shows that pharmaceutical and biotechnology companies constitute the largest consumer base (58%), followed by academic and research institutions (24%), and clinical diagnostic laboratories (12%). The remaining market share is distributed among food testing laboratories and environmental monitoring agencies.
Key market drivers include the growing demand for biopharmaceuticals, increasing research in proteomics, technological advancements in separation techniques, and rising prevalence of chronic diseases necessitating protein-based diagnostics and therapeutics. The shift toward personalized medicine has further accelerated the need for efficient protein separation methods capable of handling complex biological samples.
Market challenges include the high cost of advanced separation systems, technical complexity requiring specialized training, and regulatory hurdles for clinical applications. Additionally, the market faces pressure from alternative technologies such as mass spectrometry-based approaches that can sometimes bypass traditional separation steps.
Emerging trends indicate growing interest in hybrid systems that combine the strengths of both HPLC and CE technologies, miniaturization for point-of-care applications, and integration with artificial intelligence for automated method development and optimization. The demand for single-use, disposable separation technologies is also rising, particularly in clinical settings where cross-contamination concerns are paramount.
HPLC and CE Current Status and Technical Barriers
High-Performance Liquid Chromatography (HPLC) and Capillary Electrophoresis (CE) represent two dominant analytical techniques in protein separation. Currently, HPLC dominates the market with approximately 85% share in protein analysis applications, primarily due to its robust methodology and extensive historical development since the 1960s. The technique has evolved significantly with the introduction of Ultra-High Performance Liquid Chromatography (UHPLC) systems capable of operating at pressures exceeding 15,000 psi, dramatically improving resolution and analysis speed.
CE technology, though less widely adopted (approximately 15% market share), has experienced substantial growth in recent years, particularly in biopharmaceutical applications. The technique offers exceptional resolution for charged biomolecules and has gained traction with the development of capillary gel electrophoresis (CGE) and capillary isoelectric focusing (CIEF) variants specifically optimized for protein characterization.
Despite their widespread use, both technologies face significant technical barriers. HPLC systems encounter challenges with protein adsorption to stationary phases, leading to peak broadening and reduced separation efficiency. This issue becomes particularly problematic with hydrophobic proteins and those prone to aggregation. Additionally, the high backpressure in UHPLC systems can cause protein denaturation, potentially altering the biological activity of sensitive biomolecules.
CE faces limitations in sensitivity compared to HPLC, with typical UV detection limits approximately 10-fold higher. Sample loading capacity constraints further restrict its application for low-abundance proteins. The technique also suffers from reproducibility issues, with migration time variations of 2-5% between runs being common, compared to retention time variations of less than 1% in modern HPLC systems.
Geographically, HPLC technology development remains concentrated in North America (40%), Europe (35%), and Japan (15%), with emerging contributions from China (10%). CE innovation shows a different distribution pattern, with significant developments in Europe (45%), followed by North America (30%), Japan (15%), and China (10%).
Recent technological advancements are addressing these barriers. For HPLC, the development of biocompatible stationary phases with reduced non-specific interactions has improved protein recovery rates by up to 95%. Meanwhile, CE has seen innovations in detection sensitivity through the integration of laser-induced fluorescence and mass spectrometry interfaces, reducing detection limits by two orders of magnitude.
The miniaturization trend continues to influence both technologies, with microchip-based CE systems and nano-HPLC platforms emerging as promising directions for reduced sample consumption and increased throughput. However, challenges in manufacturing consistency and system integration currently limit widespread adoption of these miniaturized platforms.
CE technology, though less widely adopted (approximately 15% market share), has experienced substantial growth in recent years, particularly in biopharmaceutical applications. The technique offers exceptional resolution for charged biomolecules and has gained traction with the development of capillary gel electrophoresis (CGE) and capillary isoelectric focusing (CIEF) variants specifically optimized for protein characterization.
Despite their widespread use, both technologies face significant technical barriers. HPLC systems encounter challenges with protein adsorption to stationary phases, leading to peak broadening and reduced separation efficiency. This issue becomes particularly problematic with hydrophobic proteins and those prone to aggregation. Additionally, the high backpressure in UHPLC systems can cause protein denaturation, potentially altering the biological activity of sensitive biomolecules.
CE faces limitations in sensitivity compared to HPLC, with typical UV detection limits approximately 10-fold higher. Sample loading capacity constraints further restrict its application for low-abundance proteins. The technique also suffers from reproducibility issues, with migration time variations of 2-5% between runs being common, compared to retention time variations of less than 1% in modern HPLC systems.
Geographically, HPLC technology development remains concentrated in North America (40%), Europe (35%), and Japan (15%), with emerging contributions from China (10%). CE innovation shows a different distribution pattern, with significant developments in Europe (45%), followed by North America (30%), Japan (15%), and China (10%).
Recent technological advancements are addressing these barriers. For HPLC, the development of biocompatible stationary phases with reduced non-specific interactions has improved protein recovery rates by up to 95%. Meanwhile, CE has seen innovations in detection sensitivity through the integration of laser-induced fluorescence and mass spectrometry interfaces, reducing detection limits by two orders of magnitude.
The miniaturization trend continues to influence both technologies, with microchip-based CE systems and nano-HPLC platforms emerging as promising directions for reduced sample consumption and increased throughput. However, challenges in manufacturing consistency and system integration currently limit widespread adoption of these miniaturized platforms.
Comparative Analysis of HPLC and CE Methodologies
01 Improved column technology for HPLC efficiency
Advanced column technologies have been developed to enhance HPLC efficiency, including specialized packing materials, monolithic columns, and core-shell particles. These innovations reduce band broadening, improve peak resolution, and allow for faster analysis times. Optimized column dimensions and particle size distribution contribute to higher theoretical plate counts and better separation performance in high-performance liquid chromatography systems.- Column and capillary design improvements for enhanced efficiency: Innovations in column and capillary design significantly improve separation efficiency in both HPLC and CE systems. These include specialized column packing materials, monolithic columns, and capillaries with modified inner surfaces that reduce band broadening and improve resolution. Advanced geometries and materials enable faster analysis times while maintaining or improving separation quality, particularly for complex sample matrices.
- Mobile phase and buffer optimization techniques: Optimization of mobile phases in HPLC and buffer systems in CE significantly impacts separation efficiency. This includes the development of specialized buffer compositions, pH adjustments, and additives that enhance selectivity and resolution. Gradient elution techniques and dynamic buffer modifications during analysis allow for improved separation of complex mixtures while reducing analysis time and increasing sensitivity.
- Detection and quantification method enhancements: Advanced detection systems improve the overall efficiency of HPLC and CE analyses. These include high-sensitivity detectors, multi-wavelength detection capabilities, and specialized detection methods for specific analytes. Integration of mass spectrometry, fluorescence, and electrochemical detection techniques enhances both qualitative and quantitative analysis capabilities, allowing for lower detection limits and improved accuracy in complex samples.
- Miniaturization and microfluidic integration: Miniaturization of HPLC and CE systems through microfluidic technologies significantly improves efficiency by reducing sample and reagent consumption while increasing analysis speed. These systems feature integrated components on chip-based platforms, allowing for parallel processing and automation. Miniaturized systems also offer advantages in portability, reduced waste generation, and the ability to analyze extremely small sample volumes with high precision.
- Automation and data processing advancements: Automation technologies and advanced data processing methods enhance the operational efficiency of HPLC and CE systems. These include automated sample preparation, injection systems, and method development tools that reduce manual intervention and human error. Sophisticated software algorithms for peak detection, integration, and data analysis improve result interpretation and reproducibility, while machine learning approaches optimize separation conditions and predict chromatographic behavior.
02 Buffer composition optimization for CE performance
The composition of buffer solutions significantly impacts capillary electrophoresis efficiency. Optimizing parameters such as pH, ionic strength, and additives can enhance separation selectivity and resolution. Specialized buffer systems can reduce electroosmotic flow variations, minimize analyte-wall interactions, and improve migration time reproducibility. These optimizations lead to sharper peaks, improved sensitivity, and more reliable quantitative analysis in CE applications.Expand Specific Solutions03 Integration of detection systems for enhanced sensitivity
Advanced detection systems integrated with HPLC and CE platforms significantly improve analytical sensitivity and selectivity. These include mass spectrometry coupling, laser-induced fluorescence, electrochemical detection, and multi-wavelength UV-Vis arrays. Enhanced detection capabilities allow for lower limits of detection, better quantification of trace components, and improved characterization of complex samples across various applications in pharmaceutical, environmental, and biological analysis.Expand Specific Solutions04 Miniaturization and microfluidic approaches
Miniaturization of HPLC and CE systems through microfluidic technologies has led to significant efficiency improvements. These approaches reduce sample and solvent consumption, decrease analysis time, and enable parallel processing. Chip-based platforms integrate multiple analytical functions including sample preparation, separation, and detection in compact formats. Miniaturized systems offer advantages in portability, throughput, and automation while maintaining or improving separation efficiency.Expand Specific Solutions05 Automation and method development tools
Advanced automation and method development tools have revolutionized HPLC and CE efficiency. These include software for method optimization, automated sample preparation systems, and intelligent data processing algorithms. Machine learning approaches help predict separation parameters and optimize conditions with minimal experimental runs. Automated method development reduces analysis time, improves reproducibility, and enables more efficient method transfer across different instruments and laboratories.Expand Specific Solutions
Leading Companies and Research Institutions in Separation Science
The protein separation technology landscape is currently in a mature phase with HPLC (High-Performance Liquid Chromatography) dominating the market, while CE (Capillary Electrophoresis) represents a growing alternative with distinct advantages. The global protein separation market exceeds $10 billion, with steady annual growth of 6-8%. Leading companies like Agilent Technologies, Beckman Coulter, and Revvity Health Sciences have established robust HPLC platforms, while Beckman Coulter and Leidos have made significant advances in CE technology. Academic institutions including University of Michigan and California Institute of Technology continue driving innovation through fundamental research. The technology maturity varies, with HPLC being highly mature and standardized, while CE offers emerging advantages in efficiency, resolution, and sample conservation for specific protein separation applications.
Beckman Coulter, Inc.
Technical Solution: Beckman Coulter has pioneered capillary electrophoresis (CE) technology for protein separation with their PA 800 Plus Pharmaceutical Analysis System. This platform utilizes fused silica capillaries with proprietary coatings that minimize protein adsorption and enhance separation efficiency. Their system employs high voltage (up to 30kV) separation with advanced temperature control (±0.1°C) to maintain protein stability during analysis. Beckman's CE technology incorporates patented dynamic coating processes that prevent protein-wall interactions, significantly reducing band broadening effects common in traditional CE. The system features laser-induced fluorescence detection with sensitivity down to picogram levels, enabling analysis of low-abundance proteins. Their 32 Karat software provides specialized tools for CE method development, including automated optimization of separation parameters based on protein characteristics.
Strengths: Minimal sample consumption (nanoliter volumes); higher theoretical plate counts than HPLC; faster analysis times; reduced solvent usage; excellent for charged protein variants. Weaknesses: Lower loading capacity compared to HPLC; more sensitive to sample matrix effects; may require specialized expertise for method development; potentially lower reproducibility for complex samples.
The Regents of the University of California
Technical Solution: The University of California system has made significant contributions to protein separation technology through research across multiple campuses. At UC San Diego, researchers have developed novel microchip electrophoresis platforms incorporating 3D-printed components that dramatically reduce manufacturing costs while maintaining separation efficiency comparable to commercial CE systems. UC Berkeley scientists have pioneered multidimensional separation approaches combining HPLC with CE for comprehensive protein characterization, achieving peak capacities exceeding 5,000 for complex proteome samples. At UCLA, researchers have developed specialized capillary coatings using zwitterionic polymers that significantly reduce protein adsorption in CE separations, improving resolution for hydrophobic proteins that traditionally perform poorly in CE. UC Davis has focused on green chemistry approaches to HPLC protein separations, developing water-based mobile phases that eliminate organic solvents while maintaining separation efficiency through temperature modulation and specialized stationary phases with enhanced selectivity for protein functional groups.
Strengths: Cutting-edge innovation in hybrid separation approaches; development of environmentally sustainable methods; cost-effective technology adaptations; specialized solutions for challenging protein classes. Weaknesses: Technologies often at research/prototype stage rather than commercial readiness; variable performance across different implementations; potential intellectual property fragmentation across multiple campuses; limited standardization.
Breakthrough Innovations in Protein Separation Science
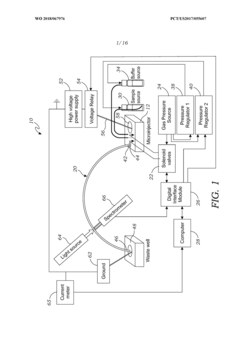
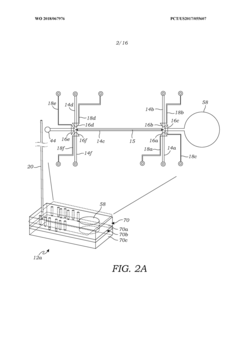
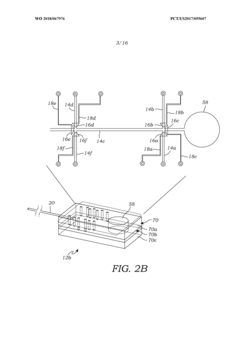
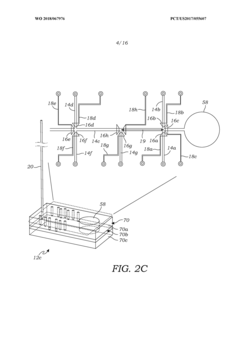
Volumetric micro-injector for capillary electrophoresis
PatentWO2018067976A1
Innovation
- A volumetric micro-injector for CE is developed, featuring a microfluidic injector chip with a defined volume channel and on-chip micro-valves, allowing for precise control of sample injection, eliminating biases and achieving high repeatability by isolating a plug of sample between valves and applying electrophoretic potential for separation.
Scalability and Cost-Efficiency Considerations
When evaluating HPLC and CE technologies for protein separation at scale, significant differences emerge in their operational economics and scalability profiles. HPLC systems typically require substantial initial capital investment, with high-end analytical instruments costing between $30,000 and $150,000, while preparative-scale systems can exceed $500,000. This considerable upfront cost must be factored against the technology's robust throughput capabilities, particularly in industrial settings.
CE systems, by comparison, offer a more modest entry point, with standard instruments ranging from $25,000 to $80,000. This lower initial investment makes CE particularly attractive for research institutions and smaller biotechnology firms with limited capital resources. The reduced footprint of CE equipment—typically 30-50% smaller than comparable HPLC systems—further enhances its appeal in laboratory environments where space optimization is critical.
Operational expenditures reveal additional distinctions between these technologies. HPLC consumes significantly more mobile phase solvents, with typical flow rates of 0.5-5 mL/min for analytical separations and up to 100 mL/min for preparative applications. These solvent requirements translate to recurring costs of $5,000-$15,000 annually for a moderately active laboratory. CE dramatically reduces this expense with nanoliter-scale sample volumes and minimal buffer requirements, potentially decreasing solvent costs by 80-90% compared to HPLC.
Column lifetime and replacement costs represent another critical economic consideration. HPLC columns typically endure 200-500 injections before performance degradation necessitates replacement, with specialized protein separation columns costing $500-$2,000 each. CE capillaries, while more fragile, cost substantially less ($50-$200) and can be prepared in-house by trained technicians, further reducing operational expenses.
Scaling considerations favor different technologies depending on application requirements. HPLC demonstrates superior scalability for production environments, with established pathways from analytical to preparative and process-scale separations. This scalability makes HPLC the predominant choice for pharmaceutical manufacturing and large-scale protein purification, despite its higher resource consumption. CE excels in high-throughput analytical applications but faces challenges in scaling to preparative volumes due to limited loading capacity.
Energy efficiency metrics also favor CE, which typically consumes 40-60% less electricity than comparable HPLC systems. This reduced energy footprint, combined with lower solvent usage, positions CE as the more environmentally sustainable option—an increasingly important consideration as organizations prioritize green chemistry initiatives and sustainable laboratory practices.
CE systems, by comparison, offer a more modest entry point, with standard instruments ranging from $25,000 to $80,000. This lower initial investment makes CE particularly attractive for research institutions and smaller biotechnology firms with limited capital resources. The reduced footprint of CE equipment—typically 30-50% smaller than comparable HPLC systems—further enhances its appeal in laboratory environments where space optimization is critical.
Operational expenditures reveal additional distinctions between these technologies. HPLC consumes significantly more mobile phase solvents, with typical flow rates of 0.5-5 mL/min for analytical separations and up to 100 mL/min for preparative applications. These solvent requirements translate to recurring costs of $5,000-$15,000 annually for a moderately active laboratory. CE dramatically reduces this expense with nanoliter-scale sample volumes and minimal buffer requirements, potentially decreasing solvent costs by 80-90% compared to HPLC.
Column lifetime and replacement costs represent another critical economic consideration. HPLC columns typically endure 200-500 injections before performance degradation necessitates replacement, with specialized protein separation columns costing $500-$2,000 each. CE capillaries, while more fragile, cost substantially less ($50-$200) and can be prepared in-house by trained technicians, further reducing operational expenses.
Scaling considerations favor different technologies depending on application requirements. HPLC demonstrates superior scalability for production environments, with established pathways from analytical to preparative and process-scale separations. This scalability makes HPLC the predominant choice for pharmaceutical manufacturing and large-scale protein purification, despite its higher resource consumption. CE excels in high-throughput analytical applications but faces challenges in scaling to preparative volumes due to limited loading capacity.
Energy efficiency metrics also favor CE, which typically consumes 40-60% less electricity than comparable HPLC systems. This reduced energy footprint, combined with lower solvent usage, positions CE as the more environmentally sustainable option—an increasingly important consideration as organizations prioritize green chemistry initiatives and sustainable laboratory practices.
Regulatory Compliance in Biopharmaceutical Analysis
Regulatory compliance represents a critical dimension in the evaluation of HPLC versus CE technologies for protein separation in biopharmaceutical analysis. Both techniques must adhere to stringent regulatory frameworks established by agencies such as the FDA, EMA, and ICH, which govern analytical method validation, documentation, and quality control in pharmaceutical development and manufacturing.
HPLC has historically maintained a dominant position in regulatory compliance due to its established presence in pharmacopeial methods. The United States Pharmacopeia (USP), European Pharmacopoeia (Ph. Eur.), and Japanese Pharmacopoeia (JP) contain numerous monographs that specify HPLC as the reference method for protein analysis. This regulatory entrenchment provides HPLC with significant advantages in method approval processes.
Capillary electrophoresis, while less represented in official compendia, has gained increasing regulatory acceptance over the past decade. The FDA's Process Analytical Technology (PAT) initiative and Quality by Design (QbD) approach have created opportunities for CE adoption, particularly where its superior resolution capabilities offer enhanced characterization of protein heterogeneity. CE-SDS methods have received regulatory approval for release testing of monoclonal antibodies, demonstrating the technique's growing compliance status.
Method validation requirements differ subtly between these technologies. HPLC validation protocols are extensively documented and standardized, with established parameters for specificity, linearity, accuracy, precision, detection limits, and robustness. CE validation may require additional considerations regarding system suitability, including capillary conditioning procedures and migration time reproducibility, which can present additional regulatory hurdles.
From a GMP perspective, both technologies must demonstrate appropriate system qualification, calibration, and maintenance. HPLC systems typically have more comprehensive vendor-supplied qualification protocols, while CE may require more customized approaches to satisfy regulatory expectations for instrument qualification.
Data integrity considerations also influence compliance strategies. Modern HPLC systems generally offer robust data management features aligned with 21 CFR Part 11 requirements for electronic records. CE systems have improved significantly in this regard, though legacy systems may present greater compliance challenges for electronic data handling and audit trail functionality.
Recent regulatory trends indicate increasing flexibility toward alternative analytical approaches when justified by improved performance. The ICH Q2(R2) revision and complementary Q14 guideline on analytical procedure development acknowledge the value of enhanced separation techniques like CE when they provide demonstrable benefits for protein characterization. This regulatory evolution suggests a gradual shift toward performance-based rather than technology-specific compliance requirements.
HPLC has historically maintained a dominant position in regulatory compliance due to its established presence in pharmacopeial methods. The United States Pharmacopeia (USP), European Pharmacopoeia (Ph. Eur.), and Japanese Pharmacopoeia (JP) contain numerous monographs that specify HPLC as the reference method for protein analysis. This regulatory entrenchment provides HPLC with significant advantages in method approval processes.
Capillary electrophoresis, while less represented in official compendia, has gained increasing regulatory acceptance over the past decade. The FDA's Process Analytical Technology (PAT) initiative and Quality by Design (QbD) approach have created opportunities for CE adoption, particularly where its superior resolution capabilities offer enhanced characterization of protein heterogeneity. CE-SDS methods have received regulatory approval for release testing of monoclonal antibodies, demonstrating the technique's growing compliance status.
Method validation requirements differ subtly between these technologies. HPLC validation protocols are extensively documented and standardized, with established parameters for specificity, linearity, accuracy, precision, detection limits, and robustness. CE validation may require additional considerations regarding system suitability, including capillary conditioning procedures and migration time reproducibility, which can present additional regulatory hurdles.
From a GMP perspective, both technologies must demonstrate appropriate system qualification, calibration, and maintenance. HPLC systems typically have more comprehensive vendor-supplied qualification protocols, while CE may require more customized approaches to satisfy regulatory expectations for instrument qualification.
Data integrity considerations also influence compliance strategies. Modern HPLC systems generally offer robust data management features aligned with 21 CFR Part 11 requirements for electronic records. CE systems have improved significantly in this regard, though legacy systems may present greater compliance challenges for electronic data handling and audit trail functionality.
Recent regulatory trends indicate increasing flexibility toward alternative analytical approaches when justified by improved performance. The ICH Q2(R2) revision and complementary Q14 guideline on analytical procedure development acknowledge the value of enhanced separation techniques like CE when they provide demonstrable benefits for protein characterization. This regulatory evolution suggests a gradual shift toward performance-based rather than technology-specific compliance requirements.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!