Quantify Non-Labile Compounds Using HPLC—Accuracy Test
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HPLC Non-Labile Compound Analysis Background and Objectives
High-performance liquid chromatography (HPLC) has evolved significantly since its inception in the late 1960s, becoming a cornerstone analytical technique in pharmaceutical, environmental, and food safety applications. The development trajectory of HPLC technology has been characterized by continuous improvements in column technology, detection methods, and data analysis capabilities, particularly for the quantification of non-labile compounds.
Non-labile compounds, characterized by their chemical stability under standard analytical conditions, represent a significant proportion of analytes in various industries. Unlike their labile counterparts, these compounds maintain structural integrity during sample preparation and analysis, making them theoretically more straightforward to quantify. However, achieving high accuracy in their measurement remains challenging due to matrix effects, instrumental variations, and method-specific limitations.
The evolution of HPLC technology for non-labile compound analysis has progressed from conventional systems with limited sensitivity to ultra-high-performance liquid chromatography (UHPLC) with sub-2-micron particle columns, enabling enhanced resolution and faster analysis times. Parallel advancements in detection technologies, from simple UV-Vis to mass spectrometry coupling, have dramatically improved specificity and sensitivity.
Current industry trends indicate a growing demand for more accurate quantification methods, driven by increasingly stringent regulatory requirements in pharmaceutical quality control, environmental monitoring, and food safety. The FDA, EMA, and other regulatory bodies have established guidelines requiring demonstration of method accuracy within specific tolerance limits, typically within ±2% for pharmaceutical applications.
The primary technical objective of this investigation is to evaluate and enhance the accuracy of HPLC methods for quantifying non-labile compounds across diverse matrices. Specifically, we aim to identify key factors affecting measurement accuracy, develop standardized protocols for method validation, and establish best practices for minimizing systematic and random errors in quantitative analysis.
Secondary objectives include assessing the impact of sample preparation techniques on quantification accuracy, comparing the performance of different detector types for specific compound classes, and exploring the relationship between chromatographic parameters and measurement precision. Additionally, we seek to develop mathematical models for predicting method accuracy based on compound properties and analytical conditions.
The long-term technological goal is to establish a comprehensive framework for method development that ensures consistent accuracy in non-labile compound quantification across different HPLC platforms and laboratory environments. This framework would incorporate adaptive calibration strategies, automated system suitability testing, and intelligent data processing algorithms to compensate for instrument-specific variations.
Non-labile compounds, characterized by their chemical stability under standard analytical conditions, represent a significant proportion of analytes in various industries. Unlike their labile counterparts, these compounds maintain structural integrity during sample preparation and analysis, making them theoretically more straightforward to quantify. However, achieving high accuracy in their measurement remains challenging due to matrix effects, instrumental variations, and method-specific limitations.
The evolution of HPLC technology for non-labile compound analysis has progressed from conventional systems with limited sensitivity to ultra-high-performance liquid chromatography (UHPLC) with sub-2-micron particle columns, enabling enhanced resolution and faster analysis times. Parallel advancements in detection technologies, from simple UV-Vis to mass spectrometry coupling, have dramatically improved specificity and sensitivity.
Current industry trends indicate a growing demand for more accurate quantification methods, driven by increasingly stringent regulatory requirements in pharmaceutical quality control, environmental monitoring, and food safety. The FDA, EMA, and other regulatory bodies have established guidelines requiring demonstration of method accuracy within specific tolerance limits, typically within ±2% for pharmaceutical applications.
The primary technical objective of this investigation is to evaluate and enhance the accuracy of HPLC methods for quantifying non-labile compounds across diverse matrices. Specifically, we aim to identify key factors affecting measurement accuracy, develop standardized protocols for method validation, and establish best practices for minimizing systematic and random errors in quantitative analysis.
Secondary objectives include assessing the impact of sample preparation techniques on quantification accuracy, comparing the performance of different detector types for specific compound classes, and exploring the relationship between chromatographic parameters and measurement precision. Additionally, we seek to develop mathematical models for predicting method accuracy based on compound properties and analytical conditions.
The long-term technological goal is to establish a comprehensive framework for method development that ensures consistent accuracy in non-labile compound quantification across different HPLC platforms and laboratory environments. This framework would incorporate adaptive calibration strategies, automated system suitability testing, and intelligent data processing algorithms to compensate for instrument-specific variations.
Market Demand for Accurate HPLC Quantification Methods
The global market for High-Performance Liquid Chromatography (HPLC) quantification methods has experienced substantial growth in recent years, driven by increasing demands for accurate analytical techniques across multiple industries. The worldwide HPLC market was valued at approximately $4.5 billion in 2022 and is projected to reach $6.7 billion by 2027, representing a compound annual growth rate of 8.2%.
Pharmaceutical and biotechnology sectors constitute the largest market segment, accounting for nearly 60% of the total demand. These industries require precise quantification of non-labile compounds for drug development, quality control, and regulatory compliance. The implementation of stringent regulatory frameworks by agencies such as the FDA and EMA has significantly elevated the need for accurate HPLC methods that can reliably quantify active pharmaceutical ingredients and impurities.
Clinical diagnostics represents another rapidly growing segment, with hospitals and diagnostic laboratories increasingly adopting HPLC for therapeutic drug monitoring and biomarker analysis. The market in this sector is expanding at approximately 9.5% annually, reflecting the critical importance of accurate quantification in patient care and treatment optimization.
Food safety testing has emerged as a high-potential market for HPLC quantification methods. Consumer awareness regarding food contaminants and adulterants has prompted regulatory bodies worldwide to implement stricter testing protocols. The food and beverage industry's demand for accurate HPLC methods has grown by 7.8% annually since 2020, with particular emphasis on detecting pesticides, mycotoxins, and unauthorized additives.
Environmental monitoring applications have also contributed significantly to market growth. Government agencies and environmental consulting firms require precise quantification of pollutants in water, soil, and air samples. This segment has seen consistent growth of 6.5% annually, driven by increasingly stringent environmental regulations and public health concerns.
Geographically, North America dominates the market with approximately 38% share, followed by Europe (30%) and Asia-Pacific (25%). However, the Asia-Pacific region is witnessing the fastest growth rate at 10.2% annually, primarily due to expanding pharmaceutical manufacturing, increasing R&D investments, and strengthening regulatory frameworks in countries like China and India.
Market research indicates that end-users are increasingly prioritizing accuracy and reproducibility over cost considerations when selecting HPLC methods. A survey of 250 analytical laboratories revealed that 78% of respondents identified quantification accuracy as the most critical factor in method selection, followed by reproducibility (65%) and ease of validation (52%).
Pharmaceutical and biotechnology sectors constitute the largest market segment, accounting for nearly 60% of the total demand. These industries require precise quantification of non-labile compounds for drug development, quality control, and regulatory compliance. The implementation of stringent regulatory frameworks by agencies such as the FDA and EMA has significantly elevated the need for accurate HPLC methods that can reliably quantify active pharmaceutical ingredients and impurities.
Clinical diagnostics represents another rapidly growing segment, with hospitals and diagnostic laboratories increasingly adopting HPLC for therapeutic drug monitoring and biomarker analysis. The market in this sector is expanding at approximately 9.5% annually, reflecting the critical importance of accurate quantification in patient care and treatment optimization.
Food safety testing has emerged as a high-potential market for HPLC quantification methods. Consumer awareness regarding food contaminants and adulterants has prompted regulatory bodies worldwide to implement stricter testing protocols. The food and beverage industry's demand for accurate HPLC methods has grown by 7.8% annually since 2020, with particular emphasis on detecting pesticides, mycotoxins, and unauthorized additives.
Environmental monitoring applications have also contributed significantly to market growth. Government agencies and environmental consulting firms require precise quantification of pollutants in water, soil, and air samples. This segment has seen consistent growth of 6.5% annually, driven by increasingly stringent environmental regulations and public health concerns.
Geographically, North America dominates the market with approximately 38% share, followed by Europe (30%) and Asia-Pacific (25%). However, the Asia-Pacific region is witnessing the fastest growth rate at 10.2% annually, primarily due to expanding pharmaceutical manufacturing, increasing R&D investments, and strengthening regulatory frameworks in countries like China and India.
Market research indicates that end-users are increasingly prioritizing accuracy and reproducibility over cost considerations when selecting HPLC methods. A survey of 250 analytical laboratories revealed that 78% of respondents identified quantification accuracy as the most critical factor in method selection, followed by reproducibility (65%) and ease of validation (52%).
Current HPLC Technology Limitations and Challenges
Despite significant advancements in High-Performance Liquid Chromatography (HPLC) technology, several limitations and challenges persist when quantifying non-labile compounds. The fundamental challenge lies in achieving consistent accuracy across diverse sample matrices, particularly when dealing with complex biological or environmental samples containing multiple interfering substances. Current HPLC systems often struggle with matrix effects that can suppress or enhance analyte signals, leading to quantification errors that may exceed acceptable thresholds for regulatory compliance.
Resolution limitations represent another significant challenge in current HPLC methodologies. When analyzing structurally similar compounds or isomers, conventional columns may fail to provide adequate separation, resulting in co-elution problems that compromise quantitative accuracy. This becomes particularly problematic in pharmaceutical analysis where impurity profiling demands exceptional resolution capabilities that current systems may not consistently deliver.
Detector sensitivity and linearity constraints also impact quantification accuracy, especially at lower concentration ranges. While modern detectors have improved significantly, they still exhibit variable responses across different compound classes, necessitating careful calibration procedures. The limited linear dynamic range of many detectors requires multiple dilutions and re-runs, introducing additional sources of error in the quantification process.
Method transfer challenges between different HPLC systems represent a persistent industry-wide issue. Variations in instrument design, column manufacturing tolerances, and detector response characteristics often result in significant differences in quantification results when the same method is implemented across different platforms or laboratories. This lack of standardization undermines confidence in analytical results and complicates multi-site studies.
Sample preparation inconsistencies continue to be a major source of error in HPLC quantification. Current automated sample preparation systems still lack the sophistication to handle complex matrices uniformly, leading to variable extraction efficiencies and recovery rates. These pre-analytical variables can significantly impact downstream quantification accuracy regardless of HPLC system performance.
Carryover effects remain problematic in many HPLC applications, particularly when analyzing compounds with strong affinity for stationary phases or system components. Despite advances in autosampler design and washing protocols, residual analyte from previous injections can contaminate subsequent samples, introducing positive bias in quantification results.
The integration of peaks presents another significant challenge, especially for compounds that exhibit tailing or fronting. Current automated integration algorithms often struggle with complex chromatograms, requiring manual adjustments that introduce subjectivity and potential inconsistency in quantification results. This becomes particularly problematic in high-throughput environments where manual review of each chromatogram is impractical.
Resolution limitations represent another significant challenge in current HPLC methodologies. When analyzing structurally similar compounds or isomers, conventional columns may fail to provide adequate separation, resulting in co-elution problems that compromise quantitative accuracy. This becomes particularly problematic in pharmaceutical analysis where impurity profiling demands exceptional resolution capabilities that current systems may not consistently deliver.
Detector sensitivity and linearity constraints also impact quantification accuracy, especially at lower concentration ranges. While modern detectors have improved significantly, they still exhibit variable responses across different compound classes, necessitating careful calibration procedures. The limited linear dynamic range of many detectors requires multiple dilutions and re-runs, introducing additional sources of error in the quantification process.
Method transfer challenges between different HPLC systems represent a persistent industry-wide issue. Variations in instrument design, column manufacturing tolerances, and detector response characteristics often result in significant differences in quantification results when the same method is implemented across different platforms or laboratories. This lack of standardization undermines confidence in analytical results and complicates multi-site studies.
Sample preparation inconsistencies continue to be a major source of error in HPLC quantification. Current automated sample preparation systems still lack the sophistication to handle complex matrices uniformly, leading to variable extraction efficiencies and recovery rates. These pre-analytical variables can significantly impact downstream quantification accuracy regardless of HPLC system performance.
Carryover effects remain problematic in many HPLC applications, particularly when analyzing compounds with strong affinity for stationary phases or system components. Despite advances in autosampler design and washing protocols, residual analyte from previous injections can contaminate subsequent samples, introducing positive bias in quantification results.
The integration of peaks presents another significant challenge, especially for compounds that exhibit tailing or fronting. Current automated integration algorithms often struggle with complex chromatograms, requiring manual adjustments that introduce subjectivity and potential inconsistency in quantification results. This becomes particularly problematic in high-throughput environments where manual review of each chromatogram is impractical.
Established Protocols for Non-Labile Compound Quantification
01 HPLC method optimization for improved accuracy
Optimization of HPLC methods involves adjusting parameters such as mobile phase composition, flow rate, column temperature, and detection wavelength to enhance separation efficiency and accuracy. These optimizations can significantly improve the precision of analyte quantification, reduce interference from matrix components, and ensure reliable results across different sample types. Method validation protocols are essential to confirm that the optimized HPLC method meets the required accuracy standards for specific analytical applications.- HPLC method optimization for improved accuracy: Optimization of HPLC methods involves adjusting parameters such as mobile phase composition, flow rate, column temperature, and detection wavelength to enhance separation efficiency and accuracy. These optimizations can significantly improve the resolution of analytes, reduce interference from matrix components, and enhance the overall accuracy of quantitative analysis. Proper method validation ensures that the optimized conditions consistently deliver accurate and reliable results across different samples.
- Calibration techniques for HPLC accuracy enhancement: Calibration is crucial for ensuring HPLC accuracy. This includes the preparation of standard curves with high-purity reference materials, internal standardization methods, and regular system suitability testing. Proper calibration accounts for instrumental variations and drift, ensuring that quantitative measurements remain accurate over time. Multi-point calibration curves with appropriate concentration ranges relative to sample concentrations provide more reliable quantification and help minimize systematic errors.
- Sample preparation methods affecting HPLC accuracy: Sample preparation techniques significantly impact HPLC accuracy. Methods such as solid-phase extraction, liquid-liquid extraction, and protein precipitation help remove interfering compounds and concentrate analytes of interest. Proper homogenization, filtration, and stabilization of samples prevent degradation and ensure representative sampling. Standardized sample preparation protocols reduce variability between analyses and improve the reproducibility and accuracy of HPLC measurements.
- Advanced detection systems for improving HPLC accuracy: Modern detection systems enhance HPLC accuracy through improved sensitivity, selectivity, and linear response ranges. Mass spectrometry (MS) coupling provides structural information and exceptional selectivity for complex mixtures. Diode array detectors (DAD) offer spectral information across multiple wavelengths simultaneously. Fluorescence and electrochemical detectors provide high sensitivity for specific compound classes. These advanced detection systems minimize interference and improve quantification accuracy, particularly for trace analysis in complex matrices.
- Quality control and validation procedures for HPLC accuracy: Comprehensive validation protocols ensure HPLC accuracy through assessment of parameters including linearity, precision, accuracy, specificity, limit of detection, limit of quantification, and robustness. Regular system performance checks using control samples and reference standards maintain instrument calibration. Statistical analysis of quality control samples helps identify and correct systematic errors. Participation in proficiency testing programs and adherence to regulatory guidelines such as ICH, USP, or FDA standards ensures that HPLC methods consistently deliver accurate results that meet established acceptance criteria.
02 Calibration techniques for HPLC accuracy enhancement
Proper calibration is crucial for ensuring HPLC accuracy. This includes the use of certified reference standards, multi-point calibration curves, and internal standardization techniques. Regular system suitability testing and calibration verification help maintain instrument performance over time. Advanced calibration approaches such as bracketing standards and matrix-matched calibration can compensate for instrumental drift and matrix effects, leading to more accurate quantitative results in complex sample analyses.Expand Specific Solutions03 Sample preparation strategies affecting HPLC accuracy
The accuracy of HPLC analysis is heavily influenced by sample preparation techniques. Methods such as solid-phase extraction, liquid-liquid extraction, and protein precipitation can remove interfering compounds and concentrate analytes of interest. Proper homogenization, filtration, and stabilization of samples prior to injection prevent column contamination and ensure representative sampling. Standardized sample handling protocols minimize variability and improve the reproducibility and accuracy of analytical results.Expand Specific Solutions04 Advanced detection systems for improved HPLC accuracy
Integration of sophisticated detection systems with HPLC enhances analytical accuracy. Mass spectrometry (MS) coupling provides superior selectivity and sensitivity, enabling accurate identification and quantification of compounds in complex mixtures. Diode array detectors (DAD) offer spectral information that helps confirm peak purity and identity. Fluorescence and electrochemical detectors provide enhanced sensitivity for specific compound classes. These advanced detection technologies significantly reduce false positives and improve quantitative accuracy, particularly at trace concentration levels.Expand Specific Solutions05 Quality control and validation procedures for HPLC accuracy
Comprehensive quality control and validation procedures are essential for maintaining HPLC accuracy. This includes system suitability testing, method validation parameters (linearity, precision, accuracy, specificity, robustness), and regular performance verification. Statistical tools for data analysis help identify outliers and systematic errors. Implementation of quality assurance protocols such as proficiency testing, use of control charts, and adherence to regulatory guidelines ensures consistent and reliable analytical performance across different laboratories and over extended periods.Expand Specific Solutions
Leading Manufacturers and Research Institutions in HPLC Technology
The HPLC quantification of non-labile compounds market is currently in a growth phase, with increasing demand driven by pharmaceutical quality control requirements. The global market size for analytical chromatography in pharmaceuticals exceeds $5 billion, growing at 5-7% annually. Technologically, HPLC accuracy testing has reached maturity with established protocols, though innovations continue. Leading players include pharmaceutical manufacturers like Sunshine Lake Pharma, Sanofi-Aventis, and Novo Nordisk who utilize advanced HPLC methods for quality assurance. Research institutions such as Okayama University and Beijing University of Chemical Technology contribute significant methodological advancements. Equipment and reagent suppliers like Daicel Corp and LG Chem provide specialized columns and reference standards, creating a competitive ecosystem balancing innovation with standardization requirements.
Sanofi-Aventis Deutschland GmbH
Technical Solution: Sanofi-Aventis has developed a comprehensive HPLC platform for non-labile compound quantification that emphasizes accuracy through multi-detector approaches. Their method incorporates both UV and mass spectrometric detection in parallel, providing orthogonal confirmation of analyte identity and quantity. The company has implemented automated system suitability testing protocols that ensure consistent chromatographic performance with predefined acceptance criteria for retention time stability (RSD < 0.5%), peak area reproducibility (RSD < 1.0%), and resolution factors (>2.0). Their approach includes internal standardization using stable isotope-labeled analogues, which compensates for matrix effects and improves quantitative accuracy to within ±2% of theoretical values. Sanofi's method validation demonstrates linearity across 0.1-100 μg/mL with correlation coefficients exceeding 0.999 and detection limits in the low nanogram range for pharmaceutical compounds.
Strengths: Comprehensive validation approach with dual detection methods provides exceptional confidence in quantitative results. Their automated system suitability protocols ensure consistent performance over time. Weakness: The complex instrumentation setup requires significant expertise to maintain and troubleshoot, and the method may be overly sophisticated for routine quality control applications.
Alembic Pharmaceuticals Ltd.
Technical Solution: Alembic Pharmaceuticals has developed a robust HPLC methodology for accurate quantification of non-labile compounds in pharmaceutical formulations. Their approach focuses on stability-indicating methods that can separate and quantify active ingredients from potential degradation products and excipients. Alembic's technique employs gradient elution with carefully optimized mobile phase pH control (typically using phosphate buffers at pH 3.0-7.0) to maintain compound stability while achieving excellent peak resolution. Their validated methods demonstrate linearity across 50-150% of target concentrations with correlation coefficients >0.999 and precision metrics showing RSD values below 1.5% for both intra-day and inter-day analyses. Alembic has implemented forced degradation studies as part of their method development process, ensuring that their HPLC procedures can accurately quantify the target compounds even in the presence of degradation products, with demonstrated specificity and peak purity factors exceeding 0.999.
Strengths: Exceptional specificity in complex pharmaceutical matrices with demonstrated stability-indicating capabilities that meet regulatory requirements. Their methods show excellent transferability between different laboratory settings. Weakness: The methods may require longer analysis times compared to some newer techniques, and the approach is primarily optimized for pharmaceutical applications rather than biological samples.
Critical Innovations in HPLC Accuracy Enhancement
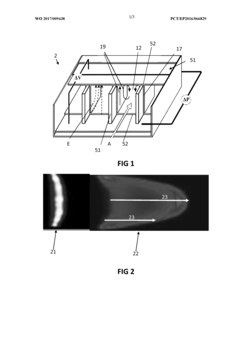
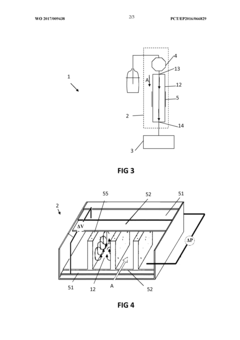
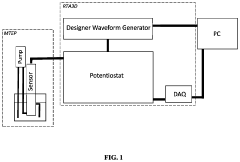
High-performance liquid chromatography with a controllable transverse flow inducer
PatentWO2017009438A1
Innovation
- The use of a controllable transverse flow inducer, such as an array of electrodes generating an alternating current electrokinetic field, to create micro-scale vortices that reduce dispersion and enhance mass transfer between support structures in the chromatography column, allowing for efficient separation without permanent surface charges and minimizing direct contact with electrodes.
System for the Simultaneous Monitoring of Constituents of an Electroplating Bath
PatentPendingUS20240133074A1
Innovation
- The development of novel second-order, consolidated voltammetric waveforms combined with chemometric analysis and data compression techniques allows for the simultaneous measurement and analysis of all electroplating bath constituents without pretreatment, using a multi-frequency, variable amplitude waveform to generate a diagnostic voltammetric output that captures the synergistic interactions and maintains process control within the electroplating process.
Validation Standards and Quality Control Measures
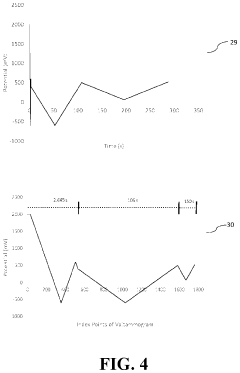
The establishment of robust validation standards and quality control measures is essential for ensuring the reliability and reproducibility of HPLC methods for non-labile compound quantification. International regulatory bodies, including the FDA, EMA, and ICH, have developed comprehensive guidelines that define the parameters for method validation, such as accuracy, precision, specificity, linearity, range, and robustness.
For accuracy testing specifically, the use of certified reference standards with known purity is paramount. These standards should be traceable to recognized authorities like NIST or USP, and their certificates of analysis must be thoroughly documented. The preparation of calibration curves using these standards should follow a minimum of five concentration levels, covering at least 80-120% of the expected analyte concentration range.
Quality control samples (QCs) play a critical role in monitoring method performance during routine analysis. These QCs should be prepared independently from calibration standards, typically at three concentration levels: low (near the lower limit of quantification), medium (middle of the range), and high (near the upper limit). Acceptance criteria for these QCs generally require that measured values fall within ±15% of the nominal concentration (±20% at LLOQ).
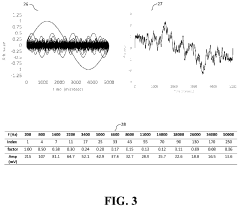
System suitability tests (SSTs) must be performed before each analytical run to verify that the chromatographic system is functioning properly. Key parameters include retention time reproducibility (typically RSD ≤1%), peak area reproducibility (RSD ≤2%), theoretical plate count (>2000), tailing factor (<2.0), and resolution between critical pairs (>1.5).
Statistical evaluation of accuracy data requires calculation of percent recovery, which should fall within 98-102% for pharmaceutical analysis and 80-120% for bioanalytical methods. The relative standard deviation (RSD) should not exceed 2% for pharmaceutical applications or 15% for bioanalytical methods. Additionally, statistical tests such as Student's t-test can be employed to determine if there is significant bias in the analytical method.
Regular instrument qualification and calibration schedules must be established and documented. This includes performance verification tests for critical components such as pumps (flow rate accuracy), detectors (linearity, noise, drift), autosamplers (injection precision), and column ovens (temperature stability). Maintenance logs and calibration certificates should be maintained as part of the laboratory's quality management system.
Proficiency testing and inter-laboratory comparisons provide external validation of method performance and laboratory competence. Participation in such programs should be scheduled regularly, with results documented and any discrepancies investigated through root cause analysis and corrective action plans.
For accuracy testing specifically, the use of certified reference standards with known purity is paramount. These standards should be traceable to recognized authorities like NIST or USP, and their certificates of analysis must be thoroughly documented. The preparation of calibration curves using these standards should follow a minimum of five concentration levels, covering at least 80-120% of the expected analyte concentration range.
Quality control samples (QCs) play a critical role in monitoring method performance during routine analysis. These QCs should be prepared independently from calibration standards, typically at three concentration levels: low (near the lower limit of quantification), medium (middle of the range), and high (near the upper limit). Acceptance criteria for these QCs generally require that measured values fall within ±15% of the nominal concentration (±20% at LLOQ).
System suitability tests (SSTs) must be performed before each analytical run to verify that the chromatographic system is functioning properly. Key parameters include retention time reproducibility (typically RSD ≤1%), peak area reproducibility (RSD ≤2%), theoretical plate count (>2000), tailing factor (<2.0), and resolution between critical pairs (>1.5).
Statistical evaluation of accuracy data requires calculation of percent recovery, which should fall within 98-102% for pharmaceutical analysis and 80-120% for bioanalytical methods. The relative standard deviation (RSD) should not exceed 2% for pharmaceutical applications or 15% for bioanalytical methods. Additionally, statistical tests such as Student's t-test can be employed to determine if there is significant bias in the analytical method.
Regular instrument qualification and calibration schedules must be established and documented. This includes performance verification tests for critical components such as pumps (flow rate accuracy), detectors (linearity, noise, drift), autosamplers (injection precision), and column ovens (temperature stability). Maintenance logs and calibration certificates should be maintained as part of the laboratory's quality management system.
Proficiency testing and inter-laboratory comparisons provide external validation of method performance and laboratory competence. Participation in such programs should be scheduled regularly, with results documented and any discrepancies investigated through root cause analysis and corrective action plans.
Regulatory Compliance for Analytical Method Development
Regulatory compliance represents a critical framework governing analytical method development in pharmaceutical and chemical industries. For HPLC accuracy testing of non-labile compounds, adherence to established regulatory guidelines ensures that analytical results are reliable, reproducible, and accepted by global regulatory bodies.
The primary regulatory bodies influencing HPLC method development include the FDA (Food and Drug Administration), EMA (European Medicines Agency), ICH (International Council for Harmonisation), and USP (United States Pharmacopeia). These organizations have established comprehensive guidelines that dictate the validation parameters for analytical methods, including accuracy testing requirements for non-labile compounds.
ICH Q2(R1) "Validation of Analytical Procedures: Text and Methodology" serves as the cornerstone document for analytical method validation. For accuracy testing of non-labile compounds using HPLC, this guideline specifies that accuracy should be assessed using a minimum of nine determinations across three concentration levels covering the specified range (e.g., three concentrations with three replicates each).
FDA's Guidance for Industry on Analytical Procedures and Methods Validation for Drugs and Biologics emphasizes the importance of demonstrating that the analytical procedure provides accurate results for its intended purpose. For non-labile compounds, this typically requires recovery studies where known amounts of analyte are added to the sample matrix.
Compliance with 21 CFR Part 11 becomes essential when using electronic systems for data acquisition and processing in HPLC accuracy testing. This regulation mandates controls for electronic records and signatures, ensuring data integrity throughout the analytical process.
Method validation protocols must be established prior to conducting accuracy tests, with pre-defined acceptance criteria based on the compound's intended use. For pharmaceutical applications, accuracy recovery typically falls within 98.0-102.0% for active ingredients and 95.0-105.0% for impurities, though these ranges may vary based on concentration levels and specific regulatory requirements.
Documentation practices represent another crucial aspect of regulatory compliance. All experimental data, including chromatograms, calibration curves, and statistical analyses from accuracy tests, must be thoroughly documented and maintained in accordance with GMP (Good Manufacturing Practice) requirements, typically for a minimum of five years after product expiration.
Regular system suitability testing must be performed to ensure the HPLC system meets operational parameters before conducting accuracy tests. This includes verification of resolution, tailing factor, theoretical plates, and retention time reproducibility as specified in USP <621> and similar international standards.
The primary regulatory bodies influencing HPLC method development include the FDA (Food and Drug Administration), EMA (European Medicines Agency), ICH (International Council for Harmonisation), and USP (United States Pharmacopeia). These organizations have established comprehensive guidelines that dictate the validation parameters for analytical methods, including accuracy testing requirements for non-labile compounds.
ICH Q2(R1) "Validation of Analytical Procedures: Text and Methodology" serves as the cornerstone document for analytical method validation. For accuracy testing of non-labile compounds using HPLC, this guideline specifies that accuracy should be assessed using a minimum of nine determinations across three concentration levels covering the specified range (e.g., three concentrations with three replicates each).
FDA's Guidance for Industry on Analytical Procedures and Methods Validation for Drugs and Biologics emphasizes the importance of demonstrating that the analytical procedure provides accurate results for its intended purpose. For non-labile compounds, this typically requires recovery studies where known amounts of analyte are added to the sample matrix.
Compliance with 21 CFR Part 11 becomes essential when using electronic systems for data acquisition and processing in HPLC accuracy testing. This regulation mandates controls for electronic records and signatures, ensuring data integrity throughout the analytical process.
Method validation protocols must be established prior to conducting accuracy tests, with pre-defined acceptance criteria based on the compound's intended use. For pharmaceutical applications, accuracy recovery typically falls within 98.0-102.0% for active ingredients and 95.0-105.0% for impurities, though these ranges may vary based on concentration levels and specific regulatory requirements.
Documentation practices represent another crucial aspect of regulatory compliance. All experimental data, including chromatograms, calibration curves, and statistical analyses from accuracy tests, must be thoroughly documented and maintained in accordance with GMP (Good Manufacturing Practice) requirements, typically for a minimum of five years after product expiration.
Regular system suitability testing must be performed to ensure the HPLC system meets operational parameters before conducting accuracy tests. This includes verification of resolution, tailing factor, theoretical plates, and retention time reproducibility as specified in USP <621> and similar international standards.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!