How to Align HPLC Parameters with Target Resolution
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HPLC Resolution Technology Background and Objectives
High-Performance Liquid Chromatography (HPLC) has evolved significantly since its inception in the 1960s, becoming an indispensable analytical technique in pharmaceutical, environmental, food safety, and clinical diagnostics industries. The fundamental principle of HPLC resolution—the ability to separate and distinguish between closely related compounds—remains a critical performance indicator that determines the technique's analytical power and reliability.
The evolution of HPLC technology has progressed through several distinct phases, from conventional HPLC to Ultra-High Performance Liquid Chromatography (UHPLC), which employs sub-2μm particles and operates at pressures exceeding 15,000 psi. This progression has been driven by the continuous demand for improved resolution, faster analysis times, and enhanced sensitivity in increasingly complex sample matrices.
Resolution in HPLC is mathematically defined by the relationship between peak separation and peak width, expressed through the resolution equation Rs = 2(tR2-tR1)/(w1+w2), where tR represents retention times and w represents peak widths. This equation highlights the three fundamental parameters affecting resolution: retention factor (k), selectivity factor (α), and column efficiency (N).
Current technological trends in HPLC resolution optimization include the development of core-shell particle technology, monolithic columns, and temperature-responsive stationary phases. These innovations aim to address the limitations of traditional HPLC methods by providing enhanced efficiency without the extreme pressure requirements of UHPLC systems.
The primary objective of aligning HPLC parameters with target resolution is to develop systematic approaches that enable analysts to predictively adjust chromatographic conditions to achieve desired separation outcomes. This involves understanding the complex interplay between mobile phase composition, flow rate, column dimensions, stationary phase chemistry, and temperature effects on resolution.
Secondary objectives include minimizing method development time through computer-assisted optimization algorithms, enhancing method robustness through understanding of critical resolution parameters, and developing transferable methods across different instrument platforms and laboratory environments.
The ultimate goal is to establish a framework that allows for rational design of HPLC methods where resolution can be precisely controlled and predicted, rather than achieved through empirical trial-and-error approaches. This would significantly reduce method development time, increase analytical throughput, and improve the reliability of analytical results across diverse applications.
The evolution of HPLC technology has progressed through several distinct phases, from conventional HPLC to Ultra-High Performance Liquid Chromatography (UHPLC), which employs sub-2μm particles and operates at pressures exceeding 15,000 psi. This progression has been driven by the continuous demand for improved resolution, faster analysis times, and enhanced sensitivity in increasingly complex sample matrices.
Resolution in HPLC is mathematically defined by the relationship between peak separation and peak width, expressed through the resolution equation Rs = 2(tR2-tR1)/(w1+w2), where tR represents retention times and w represents peak widths. This equation highlights the three fundamental parameters affecting resolution: retention factor (k), selectivity factor (α), and column efficiency (N).
Current technological trends in HPLC resolution optimization include the development of core-shell particle technology, monolithic columns, and temperature-responsive stationary phases. These innovations aim to address the limitations of traditional HPLC methods by providing enhanced efficiency without the extreme pressure requirements of UHPLC systems.
The primary objective of aligning HPLC parameters with target resolution is to develop systematic approaches that enable analysts to predictively adjust chromatographic conditions to achieve desired separation outcomes. This involves understanding the complex interplay between mobile phase composition, flow rate, column dimensions, stationary phase chemistry, and temperature effects on resolution.
Secondary objectives include minimizing method development time through computer-assisted optimization algorithms, enhancing method robustness through understanding of critical resolution parameters, and developing transferable methods across different instrument platforms and laboratory environments.
The ultimate goal is to establish a framework that allows for rational design of HPLC methods where resolution can be precisely controlled and predicted, rather than achieved through empirical trial-and-error approaches. This would significantly reduce method development time, increase analytical throughput, and improve the reliability of analytical results across diverse applications.
Market Demand Analysis for High-Resolution HPLC Methods
The global HPLC (High-Performance Liquid Chromatography) market continues to expand rapidly, driven by increasing demand for high-resolution analytical methods across multiple industries. Current market valuations place the HPLC systems and consumables market at approximately 4.5 billion USD, with projected annual growth rates of 6-8% through 2028, specifically for high-resolution applications.
Pharmaceutical and biopharmaceutical sectors represent the largest market segment, accounting for nearly 60% of high-resolution HPLC method applications. This dominance stems from stringent regulatory requirements for drug development and quality control processes, where resolution parameters directly impact product approval timelines and manufacturing efficiency.
The food and beverage industry has emerged as the fastest-growing segment for high-resolution HPLC methods, with demand increasing at approximately 9% annually. This growth is primarily driven by heightened consumer awareness regarding food safety and increasing regulatory scrutiny of contaminants, additives, and nutritional components that require precise separation and identification.
Environmental monitoring applications have also shown substantial growth, particularly in regions implementing stricter pollution control regulations. The ability to detect and quantify trace contaminants at parts-per-billion levels necessitates highly optimized HPLC parameters aligned with specific resolution requirements.
Market research indicates that end-users increasingly prioritize method development efficiency and parameter optimization tools. A recent survey of 250 analytical laboratories revealed that scientists spend an average of 15-20 hours per month on HPLC method development and parameter alignment, representing significant operational costs and productivity challenges.
The consumables segment associated with high-resolution methods (specialized columns, ultra-pure solvents, and certified reference materials) shows particularly strong growth, outpacing instrument sales by approximately 3 percentage points annually. This trend reflects the ongoing operational costs of maintaining high-resolution capabilities.
Regional analysis shows North America and Europe currently dominating the high-resolution HPLC market with combined market share of approximately 65%. However, Asia-Pacific markets, particularly China and India, are experiencing the fastest growth rates as pharmaceutical manufacturing and contract research organizations expand their analytical capabilities in these regions.
Customer demand increasingly focuses on integrated software solutions that can predict optimal parameter combinations for target resolution requirements, potentially reducing method development time by up to 70%. This represents a significant market opportunity for vendors who can deliver effective parameter alignment tools that bridge theoretical chromatography principles with practical laboratory applications.
Pharmaceutical and biopharmaceutical sectors represent the largest market segment, accounting for nearly 60% of high-resolution HPLC method applications. This dominance stems from stringent regulatory requirements for drug development and quality control processes, where resolution parameters directly impact product approval timelines and manufacturing efficiency.
The food and beverage industry has emerged as the fastest-growing segment for high-resolution HPLC methods, with demand increasing at approximately 9% annually. This growth is primarily driven by heightened consumer awareness regarding food safety and increasing regulatory scrutiny of contaminants, additives, and nutritional components that require precise separation and identification.
Environmental monitoring applications have also shown substantial growth, particularly in regions implementing stricter pollution control regulations. The ability to detect and quantify trace contaminants at parts-per-billion levels necessitates highly optimized HPLC parameters aligned with specific resolution requirements.
Market research indicates that end-users increasingly prioritize method development efficiency and parameter optimization tools. A recent survey of 250 analytical laboratories revealed that scientists spend an average of 15-20 hours per month on HPLC method development and parameter alignment, representing significant operational costs and productivity challenges.
The consumables segment associated with high-resolution methods (specialized columns, ultra-pure solvents, and certified reference materials) shows particularly strong growth, outpacing instrument sales by approximately 3 percentage points annually. This trend reflects the ongoing operational costs of maintaining high-resolution capabilities.
Regional analysis shows North America and Europe currently dominating the high-resolution HPLC market with combined market share of approximately 65%. However, Asia-Pacific markets, particularly China and India, are experiencing the fastest growth rates as pharmaceutical manufacturing and contract research organizations expand their analytical capabilities in these regions.
Customer demand increasingly focuses on integrated software solutions that can predict optimal parameter combinations for target resolution requirements, potentially reducing method development time by up to 70%. This represents a significant market opportunity for vendors who can deliver effective parameter alignment tools that bridge theoretical chromatography principles with practical laboratory applications.
Current HPLC Parameter Optimization Challenges
High-performance liquid chromatography (HPLC) parameter optimization remains one of the most challenging aspects of analytical method development. Despite advances in instrumentation and software, analysts continue to face significant hurdles when attempting to align HPLC parameters with target resolution requirements. The traditional approach of trial-and-error optimization is time-consuming, resource-intensive, and often yields suboptimal results, particularly for complex sample matrices.
A primary challenge lies in the multidimensional nature of HPLC parameter space. With numerous interdependent variables including mobile phase composition, pH, temperature, flow rate, gradient profile, and column chemistry, the optimization landscape becomes exceedingly complex. Small changes in one parameter can dramatically affect separation performance, creating a non-linear response surface that is difficult to navigate systematically.
Method transfer between different instruments and laboratories presents another substantial obstacle. Parameters optimized on one HPLC system often perform differently when transferred to another system, even of the same model, due to subtle differences in system volumes, detector response, and column performance. This variability undermines reproducibility and complicates regulatory compliance for pharmaceutical and clinical applications.
The increasing complexity of sample matrices in modern applications exacerbates these challenges. Environmental samples, biological fluids, and advanced pharmaceutical formulations contain numerous interfering compounds that can compromise resolution of target analytes. Achieving adequate separation often requires sophisticated parameter combinations that are difficult to predict using conventional approaches.
Resource constraints further complicate optimization efforts. The high cost of solvents, columns, and instrument time creates pressure to minimize the number of experimental runs, which conflicts with the need for comprehensive parameter exploration. This economic reality often forces analysts to accept suboptimal methods due to practical limitations.
Current software solutions for method development, while helpful, still have significant limitations. Many rely on simplified models that fail to capture the full complexity of chromatographic behavior, particularly for novel compounds without established retention data. Additionally, these tools often require substantial user expertise to interpret results and implement recommendations effectively.
Regulatory requirements add another layer of complexity to parameter optimization. Method validation demands robust performance across a range of operating conditions, requiring analysts to not only optimize for resolution but also for method ruggedness. This dual optimization objective significantly increases the difficulty of parameter selection and method development.
A primary challenge lies in the multidimensional nature of HPLC parameter space. With numerous interdependent variables including mobile phase composition, pH, temperature, flow rate, gradient profile, and column chemistry, the optimization landscape becomes exceedingly complex. Small changes in one parameter can dramatically affect separation performance, creating a non-linear response surface that is difficult to navigate systematically.
Method transfer between different instruments and laboratories presents another substantial obstacle. Parameters optimized on one HPLC system often perform differently when transferred to another system, even of the same model, due to subtle differences in system volumes, detector response, and column performance. This variability undermines reproducibility and complicates regulatory compliance for pharmaceutical and clinical applications.
The increasing complexity of sample matrices in modern applications exacerbates these challenges. Environmental samples, biological fluids, and advanced pharmaceutical formulations contain numerous interfering compounds that can compromise resolution of target analytes. Achieving adequate separation often requires sophisticated parameter combinations that are difficult to predict using conventional approaches.
Resource constraints further complicate optimization efforts. The high cost of solvents, columns, and instrument time creates pressure to minimize the number of experimental runs, which conflicts with the need for comprehensive parameter exploration. This economic reality often forces analysts to accept suboptimal methods due to practical limitations.
Current software solutions for method development, while helpful, still have significant limitations. Many rely on simplified models that fail to capture the full complexity of chromatographic behavior, particularly for novel compounds without established retention data. Additionally, these tools often require substantial user expertise to interpret results and implement recommendations effectively.
Regulatory requirements add another layer of complexity to parameter optimization. Method validation demands robust performance across a range of operating conditions, requiring analysts to not only optimize for resolution but also for method ruggedness. This dual optimization objective significantly increases the difficulty of parameter selection and method development.
Current Parameter Alignment Methodologies
01 Mobile phase optimization for HPLC resolution
The composition and properties of the mobile phase significantly impact HPLC resolution. Optimizing parameters such as pH, buffer concentration, organic solvent ratio, and additives can enhance the separation of analytes. Gradient elution techniques can be employed to improve resolution for complex samples with compounds of varying polarities. Temperature control of the mobile phase also contributes to consistent and reproducible chromatographic resolution.- Mobile phase optimization for HPLC resolution: The composition and properties of the mobile phase significantly impact HPLC resolution. Optimizing parameters such as pH, buffer concentration, organic solvent ratio, and additives can enhance the separation of analytes. Gradient elution techniques can be employed to improve resolution for complex mixtures, while temperature control of the mobile phase can also contribute to better chromatographic performance.
- Stationary phase selection and modification: The choice of stationary phase is crucial for achieving high resolution in HPLC. Different column materials (silica-based, polymer-based, hybrid materials) and surface modifications (C18, C8, phenyl, amino) offer varying selectivity for different analytes. Column parameters such as particle size, pore size, and column length directly affect resolution, with smaller particles generally providing better separation efficiency.
- Advanced detection techniques for improved resolution: Implementation of sophisticated detection methods enhances the resolution capabilities of HPLC systems. Techniques such as diode array detection (DAD), mass spectrometry (MS), fluorescence detection, and electrochemical detection can provide additional selectivity beyond chromatographic separation. Multi-dimensional HPLC approaches combine different separation mechanisms to resolve complex mixtures that cannot be separated in a single dimension.
- Sample preparation and injection techniques: Proper sample preparation is essential for achieving high resolution in HPLC analysis. Techniques such as solid-phase extraction, liquid-liquid extraction, and protein precipitation can remove interfering compounds and concentrate analytes of interest. The injection volume, sample concentration, and solvent compatibility with the mobile phase all impact peak shape and resolution. Split or splitless injection techniques can be optimized based on sample characteristics.
- Instrument and method optimization: System parameters and method development significantly influence HPLC resolution. Minimizing extra-column volume, optimizing flow rates, and ensuring proper instrument maintenance are critical factors. Advanced algorithm-based method development approaches can systematically optimize separation conditions. Temperature control of both column and samples can enhance reproducibility and resolution, while specialized techniques like ultra-high-performance liquid chromatography (UHPLC) utilize higher pressures and smaller particles for superior resolution.
02 Stationary phase selection and modification
The choice of stationary phase is crucial for achieving optimal HPLC resolution. Different column packing materials (C18, C8, phenyl, amino, etc.) offer varying selectivity for different analytes. Particle size, pore size, and surface area of the stationary phase affect separation efficiency. Modified stationary phases with specific functional groups can enhance selectivity for particular compound classes, while mixed-mode columns combining multiple separation mechanisms can resolve complex mixtures more effectively.Expand Specific Solutions03 Advanced detection techniques for improved resolution
Integration of sophisticated detection methods enhances the resolution capabilities of HPLC systems. Mass spectrometry coupling (HPLC-MS) provides additional separation based on mass-to-charge ratios. Diode array detectors (DAD) allow for simultaneous monitoring at multiple wavelengths, improving compound identification. Fluorescence and electrochemical detectors offer enhanced sensitivity for specific analytes, while multi-detector arrangements can provide complementary information for complex samples.Expand Specific Solutions04 Sample preparation and injection techniques
Proper sample preparation significantly impacts HPLC resolution. Techniques such as solid-phase extraction, liquid-liquid extraction, and protein precipitation can remove interfering compounds and concentrate analytes of interest. Optimizing injection volume and solvent compatibility with the mobile phase prevents peak broadening and distortion. Sample clean-up procedures tailored to specific matrices enhance chromatographic performance and extend column lifetime.Expand Specific Solutions05 Instrument parameters and system optimization
Fine-tuning instrument parameters is essential for maximizing HPLC resolution. Optimizing flow rate, column temperature, and pressure conditions affects peak shape and separation efficiency. Minimizing extra-column volume and dead volume reduces band broadening. Advanced system configurations such as ultra-high-performance liquid chromatography (UHPLC) with smaller particle sizes and higher pressures provide superior resolution. Regular system maintenance and calibration ensure consistent chromatographic performance.Expand Specific Solutions
Major Manufacturers and Research Institutions in HPLC
The HPLC parameter alignment market is in a mature growth phase, characterized by established technologies and standardized methodologies. The global analytical chromatography market exceeds $10 billion, with HPLC representing a significant segment growing at 5-7% annually. Technologically, the field has reached high maturity with innovations focusing on automation, miniaturization, and integration with data analytics. Leading players include established analytical instrument manufacturers like Agilent, Waters, and Shimadzu, alongside specialized companies such as Teledyne Instruments and Hitachi. Academic institutions like Wuhan University and Xi'an Jiaotong University contribute significant research. Recent advancements focus on AI-driven parameter optimization, with companies like Canon and Huawei exploring crossover applications in precision measurement and data processing.
Hitachi Consumer Electronics Co., Ltd.
Technical Solution: Hitachi has pioneered an integrated HPLC parameter management system that focuses on resolution optimization through their "Resolution-First" approach. Their technology employs a systematic parameter screening methodology that evaluates multiple chromatographic conditions simultaneously to identify the optimal combination for target compound separation. The system incorporates a proprietary algorithm that predicts the impact of parameter changes on resolution before actual implementation, allowing for efficient method development. Hitachi's HPLC systems feature adaptive gradient technology that automatically modifies solvent composition throughout the run to maintain consistent resolution across complex sample matrices. Their method transfer protocols ensure that optimized parameters can be reliably transferred between different instruments within the same laboratory or across multiple facilities.
Strengths: The predictive modeling approach reduces solvent consumption by approximately 40% during method development. The system offers exceptional reproducibility with run-to-run variation below 0.5% RSD. Weaknesses: The initial parameter optimization process can be time-consuming for highly complex samples with multiple critical pairs requiring separation.
Canon, Inc.
Technical Solution: Canon has applied their expertise in precision optics and digital imaging to develop an HPLC parameter optimization platform called "PrecisionChrom." Their system utilizes high-resolution optical detection combined with advanced signal processing algorithms to enhance sensitivity and resolution in chromatographic separations. Canon's approach incorporates real-time peak modeling that continuously analyzes peak shapes during method development to identify and correct issues affecting resolution. The technology features an innovative "parameter space exploration" algorithm that efficiently navigates the multidimensional space of HPLC variables to identify optimal conditions with minimal experimental runs. Their system includes specialized tools for handling challenging separations, such as automated column switching protocols that combine different selectivity mechanisms to resolve complex mixtures.
Strengths: The high-resolution optical detection system provides exceptional sensitivity, detecting compounds at concentrations up to 30% lower than conventional systems. The efficient parameter exploration approach reduces method development time significantly. Weaknesses: The advanced optical components increase system cost compared to standard HPLC detectors, potentially limiting accessibility for smaller laboratories.
Critical Patents and Innovations in Resolution Enhancement
Separation of pre-peak in fusion protein sample by using size exclusion high performance liquid chromatography
PatentPendingUS20250236640A1
Innovation
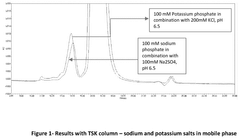
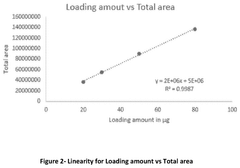
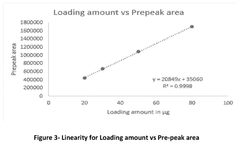
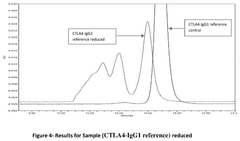
- A method involving SE-HPLC with specific mobile phase compositions and pH conditions, flow rates, and column types is employed to separate and quantify pre-peak impurities, achieving resolutions greater than 1.3 and pre-peak areas not less than 1.0, using columns like TSKgel G3000SWXL and mobile phases with salts such as potassium phosphate and sodium sulphate.
High-performance liquid chromatography with a controllable transverse flow inducer
PatentActiveEP3322978A1
Innovation
- The use of a controllable transverse flow inducer, which generates micro-scale vortices through alternating current electrokinetics, allowing for orthogonal flow induction independent of axial velocity, reducing dispersion by combining pressure and electro-osmotic flow, and enabling retention modulation without permanent surface charges.
Method Validation and Regulatory Compliance
Method validation is a critical component in the alignment of HPLC parameters with target resolution, ensuring that analytical procedures meet regulatory requirements and produce reliable results. Regulatory bodies such as the FDA, EMA, and ICH have established comprehensive guidelines that define the necessary validation parameters for chromatographic methods, including specificity, linearity, accuracy, precision, detection limit, quantitation limit, range, and robustness.
The ICH Q2(R1) guideline specifically addresses validation of analytical procedures, providing a framework for demonstrating that an HPLC method is suitable for its intended purpose. When aligning HPLC parameters with target resolution, validation protocols must verify that the selected parameters consistently achieve the required separation efficiency and resolution of critical peak pairs.
System suitability testing (SST) serves as an ongoing verification mechanism, confirming that the chromatographic system performs adequately during routine analysis. Key SST parameters include resolution, tailing factor, theoretical plate count, and retention time reproducibility—all directly influenced by the HPLC parameters selected during method development.
Documentation requirements for validated methods are extensive and must include detailed specifications of all critical HPLC parameters. This encompasses mobile phase composition, flow rate, column dimensions, temperature settings, and detection parameters. Any changes to these parameters post-validation may necessitate revalidation, particularly if the modifications could impact the method's ability to achieve target resolution.
Risk assessment approaches, such as Quality by Design (QbD), have become increasingly important in method validation. By identifying critical method attributes and establishing their acceptable ranges, analysts can develop a design space within which HPLC parameters can be adjusted while maintaining target resolution. This approach provides regulatory flexibility while ensuring consistent method performance.
For complex pharmaceutical products, lifecycle management of analytical methods has gained regulatory acceptance. This concept recognizes that methods may evolve over time, requiring continuous verification that HPLC parameters remain aligned with resolution requirements throughout the product lifecycle. Periodic reviews and trending of system suitability data help identify potential drift in method performance before it impacts product quality decisions.
Transfer of validated methods between laboratories presents unique challenges, as subtle differences in equipment or environmental conditions can affect resolution. Robust transfer protocols must verify that target resolution is maintained across different chromatographic systems, often requiring collaborative testing and statistical evaluation of results from multiple laboratories.
The ICH Q2(R1) guideline specifically addresses validation of analytical procedures, providing a framework for demonstrating that an HPLC method is suitable for its intended purpose. When aligning HPLC parameters with target resolution, validation protocols must verify that the selected parameters consistently achieve the required separation efficiency and resolution of critical peak pairs.
System suitability testing (SST) serves as an ongoing verification mechanism, confirming that the chromatographic system performs adequately during routine analysis. Key SST parameters include resolution, tailing factor, theoretical plate count, and retention time reproducibility—all directly influenced by the HPLC parameters selected during method development.
Documentation requirements for validated methods are extensive and must include detailed specifications of all critical HPLC parameters. This encompasses mobile phase composition, flow rate, column dimensions, temperature settings, and detection parameters. Any changes to these parameters post-validation may necessitate revalidation, particularly if the modifications could impact the method's ability to achieve target resolution.
Risk assessment approaches, such as Quality by Design (QbD), have become increasingly important in method validation. By identifying critical method attributes and establishing their acceptable ranges, analysts can develop a design space within which HPLC parameters can be adjusted while maintaining target resolution. This approach provides regulatory flexibility while ensuring consistent method performance.
For complex pharmaceutical products, lifecycle management of analytical methods has gained regulatory acceptance. This concept recognizes that methods may evolve over time, requiring continuous verification that HPLC parameters remain aligned with resolution requirements throughout the product lifecycle. Periodic reviews and trending of system suitability data help identify potential drift in method performance before it impacts product quality decisions.
Transfer of validated methods between laboratories presents unique challenges, as subtle differences in equipment or environmental conditions can affect resolution. Robust transfer protocols must verify that target resolution is maintained across different chromatographic systems, often requiring collaborative testing and statistical evaluation of results from multiple laboratories.
Cost-Benefit Analysis of Resolution Optimization
Optimizing HPLC resolution involves significant financial considerations that must be carefully evaluated against analytical benefits. The direct costs associated with resolution optimization include instrument time, solvent consumption, and analyst labor hours. High-resolution methods typically require longer run times, increasing instrument occupation and reducing laboratory throughput. For example, extending analysis time from 10 to 30 minutes to achieve better resolution effectively reduces sample processing capacity by 66%, potentially creating bottlenecks in high-volume testing environments.
Solvent consumption represents another substantial cost factor, particularly when using expensive HPLC-grade solvents or specialized mobile phase additives. Higher resolution methods often require gradient elution with greater solvent volumes, increasing both procurement and waste disposal expenses. A cost analysis of a typical pharmaceutical quality control laboratory revealed that optimizing resolution for complex samples increased solvent costs by approximately 40% annually.
Energy consumption also rises with extended run times and increased system pressure requirements, contributing to operational expenses. Modern UHPLC systems, while offering superior resolution capabilities, demand higher initial investment and maintenance costs compared to conventional HPLC systems.
Against these costs, laboratories must weigh the analytical benefits and risk mitigation value of improved resolution. Enhanced resolution directly impacts method reliability, reducing the likelihood of co-eluting impurities that could lead to false results. In regulated environments such as pharmaceutical manufacturing, the cost of analytical failure far exceeds the operational expenses of optimized methods. A single batch rejection due to inadequate chromatographic resolution can result in losses exceeding $100,000, making resolution investment economically justified.
Resolution optimization also enables more accurate quantification, particularly for trace components in complex matrices. This analytical advantage translates to better product quality assurance and regulatory compliance, providing indirect economic benefits through reduced compliance risks and enhanced product reputation.
The optimal cost-benefit balance varies significantly by application context. For routine quality control of well-characterized products, moderate resolution may suffice, while new product development or impurity profiling justifies higher resolution investments. Implementing a tiered approach to resolution requirements based on analytical criticality offers the most economically efficient strategy, reserving high-resolution methods for applications where analytical uncertainty carries the greatest business risk.
Solvent consumption represents another substantial cost factor, particularly when using expensive HPLC-grade solvents or specialized mobile phase additives. Higher resolution methods often require gradient elution with greater solvent volumes, increasing both procurement and waste disposal expenses. A cost analysis of a typical pharmaceutical quality control laboratory revealed that optimizing resolution for complex samples increased solvent costs by approximately 40% annually.
Energy consumption also rises with extended run times and increased system pressure requirements, contributing to operational expenses. Modern UHPLC systems, while offering superior resolution capabilities, demand higher initial investment and maintenance costs compared to conventional HPLC systems.
Against these costs, laboratories must weigh the analytical benefits and risk mitigation value of improved resolution. Enhanced resolution directly impacts method reliability, reducing the likelihood of co-eluting impurities that could lead to false results. In regulated environments such as pharmaceutical manufacturing, the cost of analytical failure far exceeds the operational expenses of optimized methods. A single batch rejection due to inadequate chromatographic resolution can result in losses exceeding $100,000, making resolution investment economically justified.
Resolution optimization also enables more accurate quantification, particularly for trace components in complex matrices. This analytical advantage translates to better product quality assurance and regulatory compliance, providing indirect economic benefits through reduced compliance risks and enhanced product reputation.
The optimal cost-benefit balance varies significantly by application context. For routine quality control of well-characterized products, moderate resolution may suffice, while new product development or impurity profiling justifies higher resolution investments. Implementing a tiered approach to resolution requirements based on analytical criticality offers the most economically efficient strategy, reserving high-resolution methods for applications where analytical uncertainty carries the greatest business risk.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!