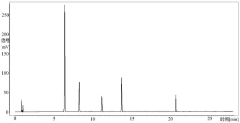
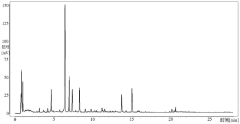
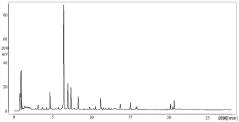
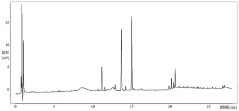
HPLC vs UPLC: Detection Limit and Accuracy Compared
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Chromatography Evolution and Objectives
Chromatography has undergone significant evolution since its inception in the early 20th century. The journey began with simple paper chromatography techniques and gradually progressed to more sophisticated methods including thin-layer chromatography (TLC), gas chromatography (GC), and eventually high-performance liquid chromatography (HPLC) in the 1970s. HPLC represented a major breakthrough, offering improved separation efficiency and reproducibility compared to earlier techniques.
The development of HPLC addressed the growing need for more precise analytical methods in pharmaceuticals, environmental monitoring, and food safety. However, as scientific demands increased, traditional HPLC systems began showing limitations in terms of analysis speed, resolution, and sensitivity. This led to the emergence of Ultra-Performance Liquid Chromatography (UPLC) in the early 2000s, marking another significant milestone in chromatographic technology.
UPLC technology was developed to overcome HPLC limitations by utilizing sub-2μm particle columns and systems capable of handling much higher pressures (up to 15,000 psi compared to HPLC's typical 6,000 psi). This innovation dramatically improved separation efficiency, speed, and sensitivity, allowing for detection of compounds at significantly lower concentrations.
The technological trajectory clearly indicates a continuous push toward greater precision, faster analysis times, and lower detection limits. Modern research increasingly demands the ability to detect trace amounts of compounds in complex matrices, particularly in fields such as biomarker discovery, environmental contaminant monitoring, and pharmaceutical impurity profiling.
The primary objectives of current chromatographic technology development focus on several key areas: enhancing detection limits to identify compounds at increasingly lower concentrations; improving accuracy and reproducibility of quantitative measurements; reducing analysis time without compromising separation quality; minimizing sample and solvent consumption for more sustainable laboratory practices; and developing more robust systems capable of handling diverse sample types.
Specifically regarding HPLC versus UPLC, a central objective is to quantitatively understand the practical differences in detection limits and accuracy between these two technologies across various application domains. This comparison is crucial for laboratories making investment decisions and for researchers selecting the most appropriate methodology for their specific analytical challenges.
The evolution continues with emerging technologies like nano-LC systems, chip-based separations, and the integration of chromatography with advanced detection systems such as high-resolution mass spectrometry. These developments aim to further push the boundaries of what's possible in terms of sensitivity, selectivity, and throughput in analytical chemistry.
The development of HPLC addressed the growing need for more precise analytical methods in pharmaceuticals, environmental monitoring, and food safety. However, as scientific demands increased, traditional HPLC systems began showing limitations in terms of analysis speed, resolution, and sensitivity. This led to the emergence of Ultra-Performance Liquid Chromatography (UPLC) in the early 2000s, marking another significant milestone in chromatographic technology.
UPLC technology was developed to overcome HPLC limitations by utilizing sub-2μm particle columns and systems capable of handling much higher pressures (up to 15,000 psi compared to HPLC's typical 6,000 psi). This innovation dramatically improved separation efficiency, speed, and sensitivity, allowing for detection of compounds at significantly lower concentrations.
The technological trajectory clearly indicates a continuous push toward greater precision, faster analysis times, and lower detection limits. Modern research increasingly demands the ability to detect trace amounts of compounds in complex matrices, particularly in fields such as biomarker discovery, environmental contaminant monitoring, and pharmaceutical impurity profiling.
The primary objectives of current chromatographic technology development focus on several key areas: enhancing detection limits to identify compounds at increasingly lower concentrations; improving accuracy and reproducibility of quantitative measurements; reducing analysis time without compromising separation quality; minimizing sample and solvent consumption for more sustainable laboratory practices; and developing more robust systems capable of handling diverse sample types.
Specifically regarding HPLC versus UPLC, a central objective is to quantitatively understand the practical differences in detection limits and accuracy between these two technologies across various application domains. This comparison is crucial for laboratories making investment decisions and for researchers selecting the most appropriate methodology for their specific analytical challenges.
The evolution continues with emerging technologies like nano-LC systems, chip-based separations, and the integration of chromatography with advanced detection systems such as high-resolution mass spectrometry. These developments aim to further push the boundaries of what's possible in terms of sensitivity, selectivity, and throughput in analytical chemistry.
Market Demand for Advanced Analytical Methods
The analytical chemistry market has witnessed a significant shift towards more advanced and precise analytical methods over the past decade. The global analytical instrumentation market, particularly for liquid chromatography systems, continues to expand at a compound annual growth rate of approximately 6.7%, driven primarily by increasing demands from pharmaceutical, biotechnology, and environmental monitoring sectors.
Pharmaceutical and biopharmaceutical industries represent the largest market segment for high-performance analytical methods, accounting for nearly 40% of the total market share. These industries require increasingly sensitive detection limits and higher accuracy for drug development, quality control, and regulatory compliance. The implementation of stringent regulatory frameworks such as FDA's Process Analytical Technology (PAT) initiative has further accelerated the demand for advanced analytical technologies like UPLC.
Environmental monitoring and food safety sectors have emerged as rapidly growing markets for advanced chromatography methods. With increasing public concern over contaminants in food, water, and the environment, regulatory bodies worldwide have established lower permissible limits for various pollutants, necessitating analytical methods with superior detection capabilities.
Academic and research institutions constitute another significant market segment, where the demand for instruments capable of analyzing complex biological samples with high throughput and sensitivity continues to rise. The growing focus on proteomics, metabolomics, and other "-omics" fields has created substantial demand for analytical systems that can detect trace amounts of biomarkers and metabolites.
Contract research organizations (CROs) and contract manufacturing organizations (CMOs) represent a fast-growing market segment, with many pharmaceutical companies outsourcing analytical testing. These organizations require versatile analytical platforms that can handle diverse sample types while maintaining high accuracy and reproducibility across different analytical methods.
The geographical distribution of market demand shows North America leading with approximately 35% market share, followed closely by Europe at 30% and Asia-Pacific at 25%. However, the Asia-Pacific region, particularly China and India, demonstrates the fastest growth rate due to expanding pharmaceutical manufacturing capabilities and increasing environmental regulations.
Cost considerations remain significant market drivers, with laboratories seeking analytical methods that offer optimal balance between performance and operational expenses. While UPLC systems typically require higher initial investment compared to HPLC, the reduced analysis time, solvent consumption, and enhanced productivity often result in lower cost-per-sample analysis, making them increasingly attractive for high-throughput applications.
Pharmaceutical and biopharmaceutical industries represent the largest market segment for high-performance analytical methods, accounting for nearly 40% of the total market share. These industries require increasingly sensitive detection limits and higher accuracy for drug development, quality control, and regulatory compliance. The implementation of stringent regulatory frameworks such as FDA's Process Analytical Technology (PAT) initiative has further accelerated the demand for advanced analytical technologies like UPLC.
Environmental monitoring and food safety sectors have emerged as rapidly growing markets for advanced chromatography methods. With increasing public concern over contaminants in food, water, and the environment, regulatory bodies worldwide have established lower permissible limits for various pollutants, necessitating analytical methods with superior detection capabilities.
Academic and research institutions constitute another significant market segment, where the demand for instruments capable of analyzing complex biological samples with high throughput and sensitivity continues to rise. The growing focus on proteomics, metabolomics, and other "-omics" fields has created substantial demand for analytical systems that can detect trace amounts of biomarkers and metabolites.
Contract research organizations (CROs) and contract manufacturing organizations (CMOs) represent a fast-growing market segment, with many pharmaceutical companies outsourcing analytical testing. These organizations require versatile analytical platforms that can handle diverse sample types while maintaining high accuracy and reproducibility across different analytical methods.
The geographical distribution of market demand shows North America leading with approximately 35% market share, followed closely by Europe at 30% and Asia-Pacific at 25%. However, the Asia-Pacific region, particularly China and India, demonstrates the fastest growth rate due to expanding pharmaceutical manufacturing capabilities and increasing environmental regulations.
Cost considerations remain significant market drivers, with laboratories seeking analytical methods that offer optimal balance between performance and operational expenses. While UPLC systems typically require higher initial investment compared to HPLC, the reduced analysis time, solvent consumption, and enhanced productivity often result in lower cost-per-sample analysis, making them increasingly attractive for high-throughput applications.
HPLC vs UPLC: Technical Limitations and Challenges
Despite significant advancements in liquid chromatography technology, both HPLC and UPLC systems face distinct technical limitations and challenges that impact their detection limits and accuracy. HPLC systems typically operate at pressures below 6,000 psi, which inherently limits their separation efficiency and speed. This pressure constraint results in broader peaks and reduced sensitivity, particularly when analyzing complex matrices or compounds present at trace levels. The larger particle size columns (typically 3-5 μm) used in HPLC create higher plate heights and reduced theoretical plates, directly impacting resolution capabilities and detection limits.
UPLC systems, while offering superior performance in many aspects, encounter their own set of technical challenges. The ultra-high pressures (up to 15,000 psi) place extreme demands on system components, leading to potential issues with seal integrity, pump reliability, and overall system durability. These mechanical stresses can result in increased maintenance requirements and potential system downtime. Additionally, the narrow-bore columns and small particle sizes (sub-2 μm) used in UPLC are more susceptible to clogging and fouling, particularly when analyzing biological samples or matrices with high particulate content.
Detection limit disparities between the two technologies stem from fundamental differences in peak capacity and signal-to-noise ratios. While UPLC generally provides 3-10 times lower detection limits than HPLC, this advantage can be compromised by sample-specific matrix effects and detector compatibility issues. The faster analysis times in UPLC may sometimes lead to incomplete separation of closely eluting compounds, potentially affecting quantification accuracy for complex samples.
Temperature control represents another critical challenge affecting both systems. UPLC is particularly sensitive to temperature fluctuations due to the frictional heating generated under high-pressure conditions. Even minor temperature variations can significantly impact retention times and peak shapes, necessitating precise thermal management systems that add complexity and cost.
Carryover effects present ongoing challenges for both technologies, though they manifest differently. In HPLC, larger internal volumes and lower flow rates can exacerbate sample-to-sample contamination. UPLC systems, despite smaller internal volumes, may experience more pronounced carryover due to higher pressures driving analyte adsorption to system surfaces.
Method transfer between HPLC and UPLC platforms remains problematic, with scaling factors often failing to account for all variables affecting separation. This creates significant challenges when validating methods across different instrument platforms or when upgrading from legacy HPLC methods to modern UPLC approaches.
Detector response linearity and dynamic range limitations affect both technologies, though UPLC's narrower peaks can sometimes push detector electronics beyond their optimal response range, potentially compromising quantitative accuracy at concentration extremes.
UPLC systems, while offering superior performance in many aspects, encounter their own set of technical challenges. The ultra-high pressures (up to 15,000 psi) place extreme demands on system components, leading to potential issues with seal integrity, pump reliability, and overall system durability. These mechanical stresses can result in increased maintenance requirements and potential system downtime. Additionally, the narrow-bore columns and small particle sizes (sub-2 μm) used in UPLC are more susceptible to clogging and fouling, particularly when analyzing biological samples or matrices with high particulate content.
Detection limit disparities between the two technologies stem from fundamental differences in peak capacity and signal-to-noise ratios. While UPLC generally provides 3-10 times lower detection limits than HPLC, this advantage can be compromised by sample-specific matrix effects and detector compatibility issues. The faster analysis times in UPLC may sometimes lead to incomplete separation of closely eluting compounds, potentially affecting quantification accuracy for complex samples.
Temperature control represents another critical challenge affecting both systems. UPLC is particularly sensitive to temperature fluctuations due to the frictional heating generated under high-pressure conditions. Even minor temperature variations can significantly impact retention times and peak shapes, necessitating precise thermal management systems that add complexity and cost.
Carryover effects present ongoing challenges for both technologies, though they manifest differently. In HPLC, larger internal volumes and lower flow rates can exacerbate sample-to-sample contamination. UPLC systems, despite smaller internal volumes, may experience more pronounced carryover due to higher pressures driving analyte adsorption to system surfaces.
Method transfer between HPLC and UPLC platforms remains problematic, with scaling factors often failing to account for all variables affecting separation. This creates significant challenges when validating methods across different instrument platforms or when upgrading from legacy HPLC methods to modern UPLC approaches.
Detector response linearity and dynamic range limitations affect both technologies, though UPLC's narrower peaks can sometimes push detector electronics beyond their optimal response range, potentially compromising quantitative accuracy at concentration extremes.
Current Detection Limit Enhancement Strategies
01 Detection limit improvement techniques in HPLC/UPLC systems
Various techniques can be employed to improve detection limits in HPLC and UPLC chromatography systems. These include optimizing sample preparation methods, enhancing detector sensitivity, implementing signal amplification technologies, and utilizing advanced data processing algorithms. By reducing background noise and increasing signal-to-noise ratios, these techniques enable the detection of analytes at significantly lower concentrations, thereby improving the overall sensitivity of the chromatographic analysis.- Detection limit improvement techniques in HPLC/UPLC systems: Various techniques can be employed to improve detection limits in HPLC and UPLC chromatography systems. These include optimizing mobile phase composition, using specialized detectors with enhanced sensitivity, implementing sample pre-concentration methods, and utilizing advanced signal processing algorithms. These approaches can significantly lower the minimum detectable concentration of analytes, enabling the analysis of trace compounds in complex matrices.
- Accuracy enhancement methods for chromatographic analysis: Accuracy in HPLC and UPLC systems can be enhanced through several methods, including proper calibration procedures, use of internal standards, temperature control of columns and samples, precise sample preparation techniques, and validation protocols. These methods help minimize systematic errors and ensure that measurement results closely match the true value of the analyte concentration, which is crucial for quantitative analysis in pharmaceutical, environmental, and food safety applications.
- Comparative performance of HPLC versus UPLC systems: UPLC systems generally offer superior detection limits and accuracy compared to traditional HPLC systems due to their ability to operate at higher pressures, use smaller particle size columns, and provide faster analysis times. The improved peak resolution and signal-to-noise ratios in UPLC contribute to better quantification capabilities, particularly for complex samples with multiple analytes or those requiring trace-level detection.
- Novel detector technologies for improved sensitivity: Advanced detector technologies have been developed to enhance the sensitivity and accuracy of HPLC and UPLC systems. These include high-sensitivity mass spectrometry detectors, fluorescence detectors with improved optics, electrochemical detectors with lower noise characteristics, and multi-detector arrangements that combine complementary detection principles. These technologies enable lower detection limits and improved quantification accuracy for challenging analytes.
- Validation and calibration protocols for ensuring accuracy: Robust validation and calibration protocols are essential for ensuring the accuracy and reliability of HPLC and UPLC chromatography systems. These protocols include system suitability testing, linearity assessment across the analytical range, precision studies, accuracy verification using certified reference materials, and regular performance checks. Implementing these protocols helps maintain consistent detection limits and accuracy throughout the operational lifetime of the chromatography system.
02 Accuracy enhancement methods for chromatographic analysis
Accuracy in HPLC and UPLC systems can be enhanced through several methods, including proper calibration procedures, use of internal standards, matrix-matched calibration, and validation protocols. These methods help to compensate for matrix effects, instrument drift, and other factors that can affect measurement accuracy. Implementation of quality control samples and reference materials during analysis further ensures the reliability and reproducibility of quantitative results.Expand Specific Solutions03 Advanced detector technologies for improved sensitivity and accuracy
Modern HPLC and UPLC systems incorporate advanced detector technologies such as mass spectrometry (MS), diode array detection (DAD), fluorescence detection, and electrochemical detection. These detectors offer enhanced sensitivity, selectivity, and accuracy compared to conventional UV-Vis detectors. Multi-detector configurations can provide complementary information about analytes, allowing for more comprehensive characterization and quantification at lower detection limits.Expand Specific Solutions04 System optimization and method development for enhanced performance
Optimization of chromatographic parameters such as mobile phase composition, flow rate, column temperature, and gradient profiles significantly impacts detection limits and accuracy. Method development strategies include design of experiments (DoE) approaches, quality by design principles, and automated method optimization software. These systematic approaches help identify optimal conditions that maximize sensitivity while maintaining chromatographic resolution and peak shape, ultimately leading to improved detection limits and quantitative accuracy.Expand Specific Solutions05 Validation and qualification protocols for ensuring system performance
Comprehensive validation and qualification protocols are essential for ensuring HPLC and UPLC system performance meets required detection limits and accuracy specifications. These protocols include system suitability tests, instrument qualification procedures, method validation parameters (linearity, precision, accuracy, specificity), and ongoing performance verification. Regular performance checks using standard reference materials help maintain system integrity and ensure consistent analytical results over time, particularly for regulated environments requiring high confidence in reported values.Expand Specific Solutions
Leading Manufacturers and Research Institutions
The HPLC vs UPLC detection limit and accuracy comparison market is in a mature growth phase, with an estimated global chromatography market exceeding $10 billion. UPLC technology demonstrates superior detection limits and accuracy compared to traditional HPLC, driving its adoption in pharmaceutical and clinical applications. Leading players include Waters Technology, which pioneered UPLC technology, alongside Phenomenex, IDEX Health & Science, and Daicel Corp offering specialized columns and components. Pharmaceutical giants like Novartis and Janssen Pharmaceutica leverage these advanced systems for drug development, while academic institutions such as University of Michigan and University College Cork contribute significant research advancing chromatographic techniques and applications.
IDEX Health & Science LLC
Technical Solution: IDEX Health & Science has developed comprehensive fluidic and optical component solutions that address the fundamental differences between HPLC and UPLC systems. Their specialized UPLC connection technologies, including PEEK-lined stainless steel tubing and ZircoFit fittings, minimize extra-column band broadening that can significantly impact detection limits, particularly in UPLC where peak volumes are typically 3-5 times smaller than in HPLC. IDEX's advanced flow cell designs for both platforms optimize light path and volume characteristics, with their UPLC-specific cells featuring volumes as low as 100nL compared to traditional 8-10μL HPLC cells, resulting in improved signal-to-noise ratios and lower detection limits. Their proprietary degassing technology addresses the heightened sensitivity of UPLC systems to dissolved gases, which can create baseline instabilities that compromise detection accuracy. IDEX has also pioneered specialized in-line filters and biocompatible flow path components that maintain sample integrity while preventing system contamination, a critical factor in achieving reproducible quantitation at ultra-low detection limits in both chromatographic approaches.
Strengths: Comprehensive system component optimization that addresses critical extra-column effects, specialized solutions for both HPLC and UPLC platforms, and components engineered specifically to enhance detection sensitivity. Weaknesses: Requires system-wide implementation for maximum benefit, component costs can be significant when upgrading entire systems, and some specialized components have more limited pressure ratings or chemical compatibility.
Phenomenex, Inc.
Technical Solution: Phenomenex has developed specialized column technologies optimized for both HPLC and UPLC applications, with particular focus on enhancing detection limits and analytical accuracy. Their Kinetex core-shell particle technology represents a hybrid approach that delivers UPLC-like performance on standard HPLC systems, achieving up to 90% of UPLC efficiency without requiring new instrumentation. For true UPLC applications, their Luna Omega series utilizes sub-2μm fully porous particles with enhanced surface area and specialized bonding chemistry that improves sensitivity by up to 30% compared to conventional columns. Phenomenex's Biozen product line specifically addresses biomolecule analysis challenges, offering improved recovery and reduced carryover that directly impacts detection limits. Their columns demonstrate exceptional batch-to-batch reproducibility (>99.7% similarity), which significantly contributes to analytical accuracy across both platforms. Phenomenex has conducted extensive comparative studies showing their optimized HPLC columns can achieve detection limits within 2-3x of UPLC performance, while their UPLC columns consistently demonstrate 5-10x lower detection limits than standard HPLC approaches.
Strengths: Innovative particle technologies that bridge HPLC and UPLC performance gaps, exceptional column reproducibility contributing to analytical accuracy, and specialized phases for challenging analyte classes. Weaknesses: Premium pricing compared to standard columns, some technologies require specific instrument capabilities to realize full benefits, and method development may require additional optimization steps.
Key Innovations in Column Technology and Particle Size Reduction
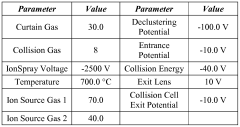
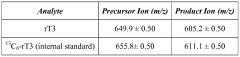
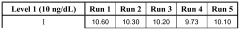
Methods for detecting reverse triiodothyronine by mass spectrometry
PatentWO2013085818A1
Innovation
- The method involves ionizing rT3 from body fluid samples using mass spectrometry, potentially after protein precipitation and liquid chromatography, without prior solid phase extraction, to detect and quantify rT3 using tandem mass spectrometry, with the option of using internal standards and specific ionization modes like electrospray ionization.
Method for determining contents of multiple components in Xintong oral liquid
PatentPendingCN115616095A
Innovation
- Simultaneous determination of puerarin, daidzin, 2,3,5,4'-tetrahydroxystilbene-2-O-β-D-glucoside, naringin and epimedium in Xintong oral liquid using UPLC method The content of glycosides can be measured simultaneously through gradient elution procedures, simplifying operations and improving detection efficiency.
Method Validation and Standardization Protocols
Method validation is a critical component when comparing analytical techniques such as HPLC and UPLC. Both technologies require rigorous standardization protocols to ensure reliable and reproducible results, though their validation approaches differ due to inherent technological characteristics.
For HPLC methods, validation typically follows established guidelines from regulatory bodies such as ICH, FDA, and USP. These protocols generally require assessment of specificity, linearity, accuracy, precision, detection limit, quantitation limit, range, robustness, and system suitability. The validation process for HPLC is well-documented with extensive historical data supporting standardization efforts.
UPLC, being a more recent technology, has necessitated adaptations to traditional validation protocols. The higher pressures, smaller particle sizes, and increased sensitivity of UPLC systems require more stringent system suitability criteria and modified robustness parameters. Particularly, carryover assessment becomes more critical due to the higher sensitivity of UPLC systems.
Detection limit validation represents a significant difference between these technologies. UPLC typically demonstrates lower limits of detection (LOD) and quantification (LOQ), often by a factor of 3-10 times compared to conventional HPLC. This requires validation protocols to incorporate lower concentration standards and more sensitive detection parameters when transitioning methods from HPLC to UPLC.
Accuracy validation also differs between platforms. While both require recovery studies, UPLC's enhanced sensitivity may reveal matrix interferences not previously detected in HPLC methods, necessitating more comprehensive matrix effect evaluations. Standard addition methods and isotopically labeled internal standards are increasingly employed in UPLC validation protocols to address these challenges.
Interlaboratory standardization represents another critical aspect of method validation. UPLC methods often demonstrate improved interlaboratory reproducibility due to reduced system-to-system variability, though this advantage must be formally validated through collaborative studies when establishing new methods.
Transfer protocols between HPLC and UPLC require special consideration. When migrating validated HPLC methods to UPLC platforms, partial revalidation is typically necessary, focusing particularly on detection limits, linearity at lower concentrations, and resolution of closely eluting compounds. Regulatory agencies increasingly accept UPLC as an alternative to HPLC provided that appropriate validation bridges are established.
Automated system suitability testing has become more prevalent with UPLC methods, allowing real-time monitoring of critical method parameters and facilitating compliance with validation requirements. This approach enhances method robustness and provides continuous verification of system performance throughout analytical runs.
For HPLC methods, validation typically follows established guidelines from regulatory bodies such as ICH, FDA, and USP. These protocols generally require assessment of specificity, linearity, accuracy, precision, detection limit, quantitation limit, range, robustness, and system suitability. The validation process for HPLC is well-documented with extensive historical data supporting standardization efforts.
UPLC, being a more recent technology, has necessitated adaptations to traditional validation protocols. The higher pressures, smaller particle sizes, and increased sensitivity of UPLC systems require more stringent system suitability criteria and modified robustness parameters. Particularly, carryover assessment becomes more critical due to the higher sensitivity of UPLC systems.
Detection limit validation represents a significant difference between these technologies. UPLC typically demonstrates lower limits of detection (LOD) and quantification (LOQ), often by a factor of 3-10 times compared to conventional HPLC. This requires validation protocols to incorporate lower concentration standards and more sensitive detection parameters when transitioning methods from HPLC to UPLC.
Accuracy validation also differs between platforms. While both require recovery studies, UPLC's enhanced sensitivity may reveal matrix interferences not previously detected in HPLC methods, necessitating more comprehensive matrix effect evaluations. Standard addition methods and isotopically labeled internal standards are increasingly employed in UPLC validation protocols to address these challenges.
Interlaboratory standardization represents another critical aspect of method validation. UPLC methods often demonstrate improved interlaboratory reproducibility due to reduced system-to-system variability, though this advantage must be formally validated through collaborative studies when establishing new methods.
Transfer protocols between HPLC and UPLC require special consideration. When migrating validated HPLC methods to UPLC platforms, partial revalidation is typically necessary, focusing particularly on detection limits, linearity at lower concentrations, and resolution of closely eluting compounds. Regulatory agencies increasingly accept UPLC as an alternative to HPLC provided that appropriate validation bridges are established.
Automated system suitability testing has become more prevalent with UPLC methods, allowing real-time monitoring of critical method parameters and facilitating compliance with validation requirements. This approach enhances method robustness and provides continuous verification of system performance throughout analytical runs.
Cost-Benefit Analysis of HPLC to UPLC Transition
The transition from High-Performance Liquid Chromatography (HPLC) to Ultra-Performance Liquid Chromatography (UPLC) represents a significant investment decision for laboratories. This cost-benefit analysis examines the financial implications of such a transition while considering the enhanced detection limits and accuracy offered by UPLC technology.
Initial capital expenditure for UPLC systems typically ranges from $75,000 to $150,000, representing a 30-50% premium over comparable HPLC systems. This substantial investment must be weighed against the operational benefits and long-term savings potential. Additionally, laboratories must consider facility modifications to accommodate the higher pressure systems, which may add 5-15% to implementation costs.
Operational cost savings emerge as a primary benefit of UPLC adoption. Analysis time reductions of 50-90% translate directly to increased sample throughput, with many laboratories reporting capacity increases of 200-300%. This efficiency gain allows for more analyses per day using the same staffing resources, effectively reducing the cost per sample by 30-60% in high-volume environments.
Solvent consumption represents another significant area of cost reduction. UPLC methods typically require 70-90% less mobile phase than traditional HPLC methods due to shorter run times and lower flow rates. For laboratories processing thousands of samples monthly, this can translate to annual solvent savings of $5,000-$15,000, with additional reductions in hazardous waste disposal costs of approximately $2,000-$7,000 annually.
The enhanced detection limits of UPLC (typically 2-5 times more sensitive than HPLC) deliver quantifiable economic benefits through reduced sample preparation requirements and fewer repeat analyses. Studies indicate that laboratories can reduce sample preparation costs by 15-25% through smaller required sample volumes and simplified extraction procedures. Furthermore, the improved accuracy (typically showing RSD improvements of 30-50%) reduces costly analytical errors and repeat testing by 10-30%.
Maintenance considerations present a mixed cost profile. While UPLC systems require more specialized maintenance due to their higher pressure tolerances, the reduced run times mean less overall system wear per sample. Annual maintenance costs typically run 10-15% higher for UPLC systems, but when calculated on a per-sample basis, maintenance costs are often 20-40% lower due to higher throughput.
Return on investment calculations suggest that laboratories processing more than 50 samples daily can typically recoup UPLC implementation costs within 18-36 months through combined operational savings. Smaller laboratories with lower throughput may experience longer payback periods of 3-5 years, necessitating careful consideration of workflow demands and future growth projections.
Initial capital expenditure for UPLC systems typically ranges from $75,000 to $150,000, representing a 30-50% premium over comparable HPLC systems. This substantial investment must be weighed against the operational benefits and long-term savings potential. Additionally, laboratories must consider facility modifications to accommodate the higher pressure systems, which may add 5-15% to implementation costs.
Operational cost savings emerge as a primary benefit of UPLC adoption. Analysis time reductions of 50-90% translate directly to increased sample throughput, with many laboratories reporting capacity increases of 200-300%. This efficiency gain allows for more analyses per day using the same staffing resources, effectively reducing the cost per sample by 30-60% in high-volume environments.
Solvent consumption represents another significant area of cost reduction. UPLC methods typically require 70-90% less mobile phase than traditional HPLC methods due to shorter run times and lower flow rates. For laboratories processing thousands of samples monthly, this can translate to annual solvent savings of $5,000-$15,000, with additional reductions in hazardous waste disposal costs of approximately $2,000-$7,000 annually.
The enhanced detection limits of UPLC (typically 2-5 times more sensitive than HPLC) deliver quantifiable economic benefits through reduced sample preparation requirements and fewer repeat analyses. Studies indicate that laboratories can reduce sample preparation costs by 15-25% through smaller required sample volumes and simplified extraction procedures. Furthermore, the improved accuracy (typically showing RSD improvements of 30-50%) reduces costly analytical errors and repeat testing by 10-30%.
Maintenance considerations present a mixed cost profile. While UPLC systems require more specialized maintenance due to their higher pressure tolerances, the reduced run times mean less overall system wear per sample. Annual maintenance costs typically run 10-15% higher for UPLC systems, but when calculated on a per-sample basis, maintenance costs are often 20-40% lower due to higher throughput.
Return on investment calculations suggest that laboratories processing more than 50 samples daily can typically recoup UPLC implementation costs within 18-36 months through combined operational savings. Smaller laboratories with lower throughput may experience longer payback periods of 3-5 years, necessitating careful consideration of workflow demands and future growth projections.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!