Quantify Mobile Phase Stability in HPLC—Consistency Tests
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HPLC Mobile Phase Stability Background and Objectives
High-performance liquid chromatography (HPLC) has evolved significantly since its inception in the 1960s, becoming an indispensable analytical technique in pharmaceutical, environmental, food safety, and clinical laboratories. The mobile phase, a critical component of HPLC systems, directly influences separation efficiency, resolution, and overall analytical performance. Despite its importance, mobile phase stability has historically received less attention than stationary phase developments, creating a significant gap in analytical methodology.
Mobile phase stability refers to the consistency of the solvent mixture's physical and chemical properties over time. Variations in stability can lead to retention time shifts, baseline drift, changes in peak shape, and ultimately compromised data integrity. These inconsistencies pose significant challenges for method validation, quality control processes, and regulatory compliance, particularly in pharmaceutical and clinical applications where precision is paramount.
The evolution of HPLC technology has seen a transition from isocratic to gradient elution methods, from conventional to ultra-high-pressure systems, and from simple to complex mobile phase compositions. Each advancement has introduced new variables affecting mobile phase stability, necessitating more sophisticated approaches to stability assessment and control.
Current industry practices for evaluating mobile phase stability often rely on subjective assessments or indirect measurements, lacking standardized quantitative methodologies. This inconsistency in approach has led to variability in analytical results across laboratories and hindered the establishment of universal best practices for mobile phase preparation and storage.
The primary objective of this technical research is to develop and validate comprehensive, quantitative methods for assessing mobile phase stability in HPLC systems. Specifically, we aim to establish reproducible consistency tests that can objectively measure key stability parameters across different mobile phase compositions, storage conditions, and time intervals.
Secondary objectives include identifying critical factors affecting mobile phase stability, determining optimal preparation and storage conditions for various mobile phase types, and establishing threshold values for acceptable stability parameters. Additionally, we seek to correlate mobile phase stability metrics with chromatographic performance indicators to provide practical guidance for analytical scientists.
The anticipated outcomes of this research include standardized protocols for mobile phase stability testing, predictive models for stability under various conditions, and practical recommendations for extending mobile phase shelf-life without compromising analytical performance. These developments would significantly enhance method robustness, reduce analytical variability, and improve laboratory efficiency by minimizing the frequency of mobile phase preparation and system equilibration.
Mobile phase stability refers to the consistency of the solvent mixture's physical and chemical properties over time. Variations in stability can lead to retention time shifts, baseline drift, changes in peak shape, and ultimately compromised data integrity. These inconsistencies pose significant challenges for method validation, quality control processes, and regulatory compliance, particularly in pharmaceutical and clinical applications where precision is paramount.
The evolution of HPLC technology has seen a transition from isocratic to gradient elution methods, from conventional to ultra-high-pressure systems, and from simple to complex mobile phase compositions. Each advancement has introduced new variables affecting mobile phase stability, necessitating more sophisticated approaches to stability assessment and control.
Current industry practices for evaluating mobile phase stability often rely on subjective assessments or indirect measurements, lacking standardized quantitative methodologies. This inconsistency in approach has led to variability in analytical results across laboratories and hindered the establishment of universal best practices for mobile phase preparation and storage.
The primary objective of this technical research is to develop and validate comprehensive, quantitative methods for assessing mobile phase stability in HPLC systems. Specifically, we aim to establish reproducible consistency tests that can objectively measure key stability parameters across different mobile phase compositions, storage conditions, and time intervals.
Secondary objectives include identifying critical factors affecting mobile phase stability, determining optimal preparation and storage conditions for various mobile phase types, and establishing threshold values for acceptable stability parameters. Additionally, we seek to correlate mobile phase stability metrics with chromatographic performance indicators to provide practical guidance for analytical scientists.
The anticipated outcomes of this research include standardized protocols for mobile phase stability testing, predictive models for stability under various conditions, and practical recommendations for extending mobile phase shelf-life without compromising analytical performance. These developments would significantly enhance method robustness, reduce analytical variability, and improve laboratory efficiency by minimizing the frequency of mobile phase preparation and system equilibration.
Market Demand Analysis for HPLC Consistency Solutions
The global HPLC market has been experiencing robust growth, with a market value reaching $4.5 billion in 2022 and projected to grow at a CAGR of 5.8% through 2028. Within this expanding market, the demand for mobile phase stability solutions and consistency testing technologies has emerged as a critical segment, driven by increasing regulatory requirements and quality control standards in pharmaceutical, biotechnology, and food safety industries.
Pharmaceutical companies represent the largest market segment for HPLC consistency solutions, accounting for approximately 45% of the total demand. These organizations face stringent regulatory requirements from bodies such as the FDA, EMA, and ICH, which mandate consistent and reproducible analytical results. The cost of non-compliance can be substantial, with product recalls potentially costing pharmaceutical companies between $10 million to $30 million per incident, highlighting the economic incentive for investing in mobile phase stability solutions.
Contract Research Organizations (CROs) constitute the fastest-growing segment for HPLC consistency technologies, with a growth rate of 7.2% annually. This surge is attributed to the increasing outsourcing of analytical testing by pharmaceutical companies and the expansion of drug development pipelines globally. CROs require reliable and consistent HPLC methods to maintain their competitive edge and meet client expectations.
Regional analysis reveals North America dominates the market with a 38% share, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is witnessing the highest growth rate at 8.3% annually, driven by expanding pharmaceutical manufacturing capabilities in China and India, and increasing adoption of international quality standards.
End-user surveys indicate that 78% of laboratory managers consider mobile phase stability a significant challenge in their daily operations, with 65% reporting method inconsistency as a major source of result variability. This translates to an average of 12-15 hours of productivity loss per analyst per month due to troubleshooting inconsistent HPLC results.
The economic impact of improved HPLC consistency solutions is substantial. Laboratories implementing advanced mobile phase stability technologies report a 30% reduction in method development time and a 25% decrease in failed analyses. For a typical pharmaceutical quality control laboratory, this represents annual savings of approximately $120,000 to $180,000.
Market forecasts suggest that demand for automated mobile phase preparation systems will grow by 9.2% annually, while software solutions for monitoring and predicting mobile phase stability will see a 10.5% growth rate. This trend reflects the industry's shift toward integrated solutions that combine hardware, consumables, and software to address the multifaceted challenges of HPLC consistency.
Pharmaceutical companies represent the largest market segment for HPLC consistency solutions, accounting for approximately 45% of the total demand. These organizations face stringent regulatory requirements from bodies such as the FDA, EMA, and ICH, which mandate consistent and reproducible analytical results. The cost of non-compliance can be substantial, with product recalls potentially costing pharmaceutical companies between $10 million to $30 million per incident, highlighting the economic incentive for investing in mobile phase stability solutions.
Contract Research Organizations (CROs) constitute the fastest-growing segment for HPLC consistency technologies, with a growth rate of 7.2% annually. This surge is attributed to the increasing outsourcing of analytical testing by pharmaceutical companies and the expansion of drug development pipelines globally. CROs require reliable and consistent HPLC methods to maintain their competitive edge and meet client expectations.
Regional analysis reveals North America dominates the market with a 38% share, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is witnessing the highest growth rate at 8.3% annually, driven by expanding pharmaceutical manufacturing capabilities in China and India, and increasing adoption of international quality standards.
End-user surveys indicate that 78% of laboratory managers consider mobile phase stability a significant challenge in their daily operations, with 65% reporting method inconsistency as a major source of result variability. This translates to an average of 12-15 hours of productivity loss per analyst per month due to troubleshooting inconsistent HPLC results.
The economic impact of improved HPLC consistency solutions is substantial. Laboratories implementing advanced mobile phase stability technologies report a 30% reduction in method development time and a 25% decrease in failed analyses. For a typical pharmaceutical quality control laboratory, this represents annual savings of approximately $120,000 to $180,000.
Market forecasts suggest that demand for automated mobile phase preparation systems will grow by 9.2% annually, while software solutions for monitoring and predicting mobile phase stability will see a 10.5% growth rate. This trend reflects the industry's shift toward integrated solutions that combine hardware, consumables, and software to address the multifaceted challenges of HPLC consistency.
Current Challenges in Mobile Phase Stability Quantification
Despite significant advancements in HPLC technology, quantifying mobile phase stability remains a persistent challenge in analytical laboratories. Current methodologies for assessing mobile phase consistency suffer from several critical limitations that impact the reliability and reproducibility of chromatographic analyses. The absence of standardized protocols for evaluating mobile phase stability across different instrument configurations and laboratory environments creates significant variability in test results.
One major challenge is the detection and measurement of subtle chemical changes in mobile phases over time. Organic solvents commonly used in HPLC, such as acetonitrile and methanol, can undergo gradual degradation through oxidation, hydrolysis, or photochemical reactions. These changes often occur below the threshold of visual detection yet significantly impact chromatographic performance. Current analytical methods lack sufficient sensitivity to monitor these incremental changes in real-time.
Temperature fluctuations present another substantial obstacle in maintaining mobile phase stability. Even minor variations in ambient temperature can alter solvent miscibility, viscosity, and elution characteristics. Most laboratories lack precise temperature control systems for mobile phase storage and delivery, resulting in inconsistent chromatographic behavior across analytical runs. The relationship between temperature variations and mobile phase stability remains inadequately characterized in current literature.
Microbial contamination represents an underestimated factor affecting mobile phase stability, particularly in aqueous components. Conventional preservation methods such as filtration and addition of antimicrobial agents may themselves introduce variables that affect chromatographic separation. Current detection methods for microbial growth in mobile phases lack the necessary sensitivity and specificity for early intervention.
Buffer degradation kinetics pose significant challenges, especially for phosphate and acetate buffers commonly used in HPLC. The gradual shift in pH over time affects retention times and peak shapes, yet continuous pH monitoring systems integrated with HPLC instrumentation remain underdeveloped. The correlation between buffer aging and separation efficiency requires more sophisticated monitoring tools than currently available.
Instrument-specific variables further complicate mobile phase stability assessment. Different pump designs, tubing materials, and degassing systems interact uniquely with mobile phase components. The lack of standardized testing protocols that account for these instrument-specific factors limits the transferability of stability data between laboratory settings.
Finally, there exists a significant gap in predictive modeling capabilities for mobile phase stability. Current approaches rely heavily on empirical observations rather than fundamental understanding of the physicochemical interactions between mobile phase components under various conditions. This reactive rather than proactive approach to mobile phase management increases method development time and reduces analytical efficiency.
One major challenge is the detection and measurement of subtle chemical changes in mobile phases over time. Organic solvents commonly used in HPLC, such as acetonitrile and methanol, can undergo gradual degradation through oxidation, hydrolysis, or photochemical reactions. These changes often occur below the threshold of visual detection yet significantly impact chromatographic performance. Current analytical methods lack sufficient sensitivity to monitor these incremental changes in real-time.
Temperature fluctuations present another substantial obstacle in maintaining mobile phase stability. Even minor variations in ambient temperature can alter solvent miscibility, viscosity, and elution characteristics. Most laboratories lack precise temperature control systems for mobile phase storage and delivery, resulting in inconsistent chromatographic behavior across analytical runs. The relationship between temperature variations and mobile phase stability remains inadequately characterized in current literature.
Microbial contamination represents an underestimated factor affecting mobile phase stability, particularly in aqueous components. Conventional preservation methods such as filtration and addition of antimicrobial agents may themselves introduce variables that affect chromatographic separation. Current detection methods for microbial growth in mobile phases lack the necessary sensitivity and specificity for early intervention.
Buffer degradation kinetics pose significant challenges, especially for phosphate and acetate buffers commonly used in HPLC. The gradual shift in pH over time affects retention times and peak shapes, yet continuous pH monitoring systems integrated with HPLC instrumentation remain underdeveloped. The correlation between buffer aging and separation efficiency requires more sophisticated monitoring tools than currently available.
Instrument-specific variables further complicate mobile phase stability assessment. Different pump designs, tubing materials, and degassing systems interact uniquely with mobile phase components. The lack of standardized testing protocols that account for these instrument-specific factors limits the transferability of stability data between laboratory settings.
Finally, there exists a significant gap in predictive modeling capabilities for mobile phase stability. Current approaches rely heavily on empirical observations rather than fundamental understanding of the physicochemical interactions between mobile phase components under various conditions. This reactive rather than proactive approach to mobile phase management increases method development time and reduces analytical efficiency.
Established Protocols for Mobile Phase Consistency Testing
01 Buffer composition for mobile phase stability
The composition of buffer solutions in HPLC mobile phases significantly impacts stability. Proper selection of buffer type, concentration, and pH is crucial for maintaining consistent chromatographic conditions. Phosphate, acetate, and citrate buffers are commonly used, with optimal concentrations typically between 10-50 mM. The buffer capacity should be maintained within ±1 unit of the buffer's pKa value to ensure maximum stability and reproducibility of retention times.- Buffer composition and pH control in mobile phase: The stability of HPLC mobile phases is significantly influenced by buffer composition and pH control. Proper selection of buffer systems helps maintain consistent pH throughout analysis, preventing peak shifting and improving reproducibility. Optimized buffer concentrations and pH values can enhance the stability of analytes during separation, particularly for compounds sensitive to pH changes. Phosphate, acetate, and citrate buffers are commonly used, with their selection depending on the target analyte properties and detection method.
- Organic modifier selection and concentration: The choice and concentration of organic modifiers in HPLC mobile phases significantly impact stability. Methanol, acetonitrile, and tetrahydrofuran are commonly used organic modifiers that affect selectivity, resolution, and retention time. The ratio of organic modifier to aqueous phase must be optimized to ensure mobile phase homogeneity and prevent phase separation during analysis. Proper selection helps minimize baseline drift, improve peak shape, and enhance overall chromatographic performance while maintaining stability during extended analytical runs.
- Additives for mobile phase stabilization: Various additives can be incorporated into HPLC mobile phases to enhance stability. Ion-pairing reagents, chelating agents, and antioxidants help prevent degradation of sensitive analytes and improve chromatographic performance. Triethylamine and formic acid can reduce peak tailing and improve peak symmetry. EDTA may be added to sequester metal ions that could catalyze degradation reactions. These additives help maintain consistent retention times, improve peak shapes, and extend the shelf life of prepared mobile phases while ensuring reproducible analytical results.
- Temperature control and storage conditions: Temperature control and proper storage conditions are critical for maintaining HPLC mobile phase stability. Mobile phases should be stored in appropriate containers (amber glass or certain plastics) to prevent light-induced degradation and contamination. Temperature fluctuations can lead to changes in solubility, viscosity, and separation characteristics. Refrigeration may be necessary for certain mobile phases, but they should be equilibrated to operating temperature before use. Proper degassing techniques help prevent air bubble formation and ensure consistent chromatographic performance.
- Innovative approaches for extended mobile phase stability: Recent innovations have focused on extending HPLC mobile phase stability through novel formulation approaches. These include the development of self-regenerating mobile phases, stabilized gradient systems, and mobile phase recycling technologies. Advanced preservation techniques incorporate antioxidants and stabilizers that prevent microbial growth and chemical degradation. Automated systems for real-time monitoring of mobile phase composition ensure consistency throughout analysis. These approaches extend the usable lifetime of mobile phases, reduce analytical variability, and minimize waste in high-throughput environments.
02 Organic modifier selection and ratio optimization
The selection and ratio of organic modifiers in HPLC mobile phases affect stability and separation efficiency. Acetonitrile, methanol, and tetrahydrofuran are commonly used organic modifiers, each offering different selectivity and stability profiles. The ratio of organic modifier to aqueous phase must be optimized to achieve desired separation while maintaining stability. Lower percentages of organic modifiers generally provide better stability but may compromise separation efficiency, requiring a balanced approach.Expand Specific Solutions03 Temperature control and stabilization techniques
Temperature control is critical for HPLC mobile phase stability. Fluctuations in temperature can alter viscosity, solubility, and retention characteristics. Maintaining consistent temperature through column ovens and mobile phase pre-heaters helps ensure reproducible results. Some methods employ gradient temperature programming alongside solvent gradients to enhance separation while maintaining stability. Advanced systems incorporate real-time temperature compensation algorithms to adjust for environmental variations.Expand Specific Solutions04 Additives for enhancing mobile phase stability
Various additives can be incorporated into HPLC mobile phases to enhance stability. Ion-pairing reagents, chelating agents, and antioxidants prevent degradation and improve chromatographic performance. Triethylamine and formic acid are commonly used to reduce peak tailing and improve peak shape. EDTA can be added to sequester metal ions that might catalyze degradation reactions. The concentration of these additives must be carefully controlled to avoid introducing new stability issues.Expand Specific Solutions05 Storage conditions and preparation protocols
Proper storage conditions and preparation protocols are essential for maintaining HPLC mobile phase stability. Degassing techniques, such as helium sparging or vacuum degassing, remove dissolved gases that can form bubbles and affect detector performance. Mobile phases should be stored in appropriate containers (amber glass for light-sensitive solutions) and at controlled temperatures. Regular preparation of fresh mobile phases according to standardized protocols helps ensure consistent chromatographic performance and reliable analytical results.Expand Specific Solutions
Leading Manufacturers and Research Institutions in HPLC Technology
The mobile phase stability in HPLC consistency testing market is in a mature growth stage, with an estimated global market size of $1.5-2 billion. Leading analytical instrument manufacturers like Agilent Technologies, Waters Technology, and Shimadzu dominate with comprehensive solutions that ensure chromatographic reproducibility. These companies have developed advanced technologies for quantifying mobile phase parameters including pH stability, solvent composition consistency, and degassing efficiency. Pharmaceutical companies such as Daiichi Sankyo and Ranbaxy leverage these technologies for quality control in drug development. The field continues to evolve with innovations in real-time monitoring systems and automated stability verification protocols that enhance analytical precision and regulatory compliance.
Agilent Technologies, Inc.
Technical Solution: Agilent Technologies has developed a comprehensive approach to quantify mobile phase stability in HPLC through their Advanced Consistency Testing (ACT) framework. Their solution incorporates real-time monitoring systems that track critical parameters including pH fluctuations, buffer concentration changes, and organic modifier evaporation rates during chromatographic runs. The technology employs proprietary algorithms that can detect subtle changes in mobile phase composition before they impact separation quality. Agilent's system includes automated calibration protocols that establish baseline performance metrics and continuously compare current conditions against these standards[1]. Their InfinityLab LC systems feature built-in sensors that monitor mobile phase conditions and provide early warning indicators when stability parameters drift beyond acceptable thresholds. The technology also incorporates predictive maintenance capabilities that can forecast potential stability issues based on historical performance data and usage patterns[3].
Strengths: Superior integration with existing Agilent HPLC systems providing seamless workflow implementation; exceptional data reproducibility through automated monitoring systems; comprehensive documentation capabilities for regulatory compliance. Weaknesses: Higher initial investment compared to basic testing methods; requires specific Agilent consumables for optimal performance; some advanced features may require specialized training for full utilization.
Shimadzu Corp.
Technical Solution: Shimadzu has engineered a sophisticated Mobile Phase Stability Monitoring System (MPSMS) specifically designed for their Nexera and Prominence HPLC platforms. This technology utilizes advanced optical sensors integrated directly into the solvent delivery system to continuously monitor mobile phase composition in real-time. Their approach incorporates proprietary algorithms that can detect minute changes in refractive index, absorbance characteristics, and viscosity that might indicate mobile phase degradation or contamination. The system features automated stability testing protocols that can be programmed to run at predetermined intervals, establishing baseline performance metrics and tracking deviations over time[2]. Shimadzu's technology also includes temperature-controlled solvent compartments with precision regulation to ±0.1°C, minimizing thermal expansion effects that can impact mobile phase stability. Their Mobile Phase Monitor software provides graphical representations of stability trends and automatically flags anomalies that exceed user-defined thresholds, enabling proactive intervention before separation quality is compromised[4].
Strengths: Exceptional sensitivity for detecting subtle mobile phase changes before they affect chromatographic performance; seamless integration with Shimadzu's LabSolutions software platform; robust temperature control systems that minimize environmental variables. Weaknesses: Limited compatibility with non-Shimadzu HPLC systems; requires regular calibration to maintain optimal performance; higher complexity may present a steeper learning curve for new users.
Critical Technologies for Quantifying Mobile Phase Stability
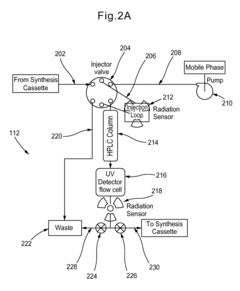
System for radiopharmaceutical preparation involving solid and liquid phase interactions
PatentInactiveUS20130023657A1
Innovation
- A modular system incorporating a high-performance liquid chromatography (HPLC) module with Cadmium Zinc Telluride (CZT) detectors for radiation detection, allowing for flexible operation, reduced radiation exposure, and automated purification of radiopharmaceuticals, enabling efficient production and quality control.
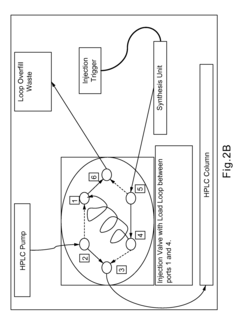
Improvements in or relating to high performance liquid chromatography systems
PatentInactiveGB2174016A
Innovation
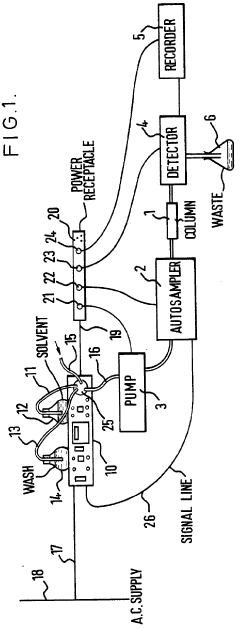
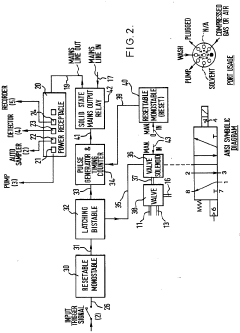
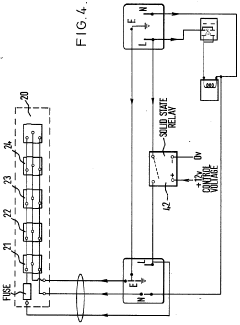
- An ancillary apparatus that includes a valve system for selecting between mobile phase and wash liquid sources, a timing mechanism initiated by a trigger pulse from the HPLC system, and an electric switch to disconnect power to the HPLC system units after a predetermined wash liquid supply, ensuring a controlled shutdown and washout process.
Regulatory Standards for Analytical Method Validation
Regulatory standards for analytical method validation in HPLC mobile phase stability testing are governed by several international frameworks that ensure consistency and reliability across pharmaceutical and analytical laboratories. The International Conference on Harmonisation (ICH) guidelines, particularly ICH Q2(R1), provide comprehensive requirements for validation of analytical procedures, including specificity, accuracy, precision, and robustness parameters that directly impact mobile phase stability assessment.
The United States Pharmacopeia (USP) chapters <621>, <1225>, and <1226> establish specific criteria for chromatographic methods, validation parameters, and system suitability tests that must be considered when evaluating mobile phase stability. These standards require demonstration that the analytical method remains unaffected by deliberate variations in mobile phase composition, pH, and other critical parameters.
European Pharmacopoeia (Ph. Eur.) and Japanese Pharmacopoeia (JP) similarly provide regulatory frameworks that address method validation requirements applicable to HPLC mobile phase consistency testing. These pharmacopoeial standards emphasize the importance of establishing acceptance criteria for system suitability tests that can effectively detect changes in mobile phase performance.
FDA Guidance for Industry on Analytical Procedures and Methods Validation specifically addresses stability-indicating methods, requiring that procedures demonstrate the ability to accurately measure the active ingredients without interference from degradation products, impurities, or excipients. This is particularly relevant when quantifying mobile phase stability, as degraded mobile phases can significantly impact separation efficiency and detection sensitivity.
ISO/IEC 17025 standards for testing and calibration laboratories establish general requirements for competence, impartiality, and consistent operation of laboratories, including specific provisions for method validation that apply to chromatographic techniques and mobile phase stability assessment.
Regulatory bodies increasingly require risk-based approaches to method validation, as outlined in ICH Q9 Quality Risk Management. This approach necessitates identification of critical mobile phase parameters that could impact method performance, followed by appropriate validation studies to demonstrate control of these parameters.
Current regulatory trends show increasing emphasis on lifecycle management of analytical methods, as described in USP <1220> and the proposed ICH Q14 guideline. These frameworks require ongoing verification that methods remain fit for purpose throughout their lifecycle, including periodic evaluation of mobile phase stability and its impact on method performance.
The United States Pharmacopeia (USP) chapters <621>, <1225>, and <1226> establish specific criteria for chromatographic methods, validation parameters, and system suitability tests that must be considered when evaluating mobile phase stability. These standards require demonstration that the analytical method remains unaffected by deliberate variations in mobile phase composition, pH, and other critical parameters.
European Pharmacopoeia (Ph. Eur.) and Japanese Pharmacopoeia (JP) similarly provide regulatory frameworks that address method validation requirements applicable to HPLC mobile phase consistency testing. These pharmacopoeial standards emphasize the importance of establishing acceptance criteria for system suitability tests that can effectively detect changes in mobile phase performance.
FDA Guidance for Industry on Analytical Procedures and Methods Validation specifically addresses stability-indicating methods, requiring that procedures demonstrate the ability to accurately measure the active ingredients without interference from degradation products, impurities, or excipients. This is particularly relevant when quantifying mobile phase stability, as degraded mobile phases can significantly impact separation efficiency and detection sensitivity.
ISO/IEC 17025 standards for testing and calibration laboratories establish general requirements for competence, impartiality, and consistent operation of laboratories, including specific provisions for method validation that apply to chromatographic techniques and mobile phase stability assessment.
Regulatory bodies increasingly require risk-based approaches to method validation, as outlined in ICH Q9 Quality Risk Management. This approach necessitates identification of critical mobile phase parameters that could impact method performance, followed by appropriate validation studies to demonstrate control of these parameters.
Current regulatory trends show increasing emphasis on lifecycle management of analytical methods, as described in USP <1220> and the proposed ICH Q14 guideline. These frameworks require ongoing verification that methods remain fit for purpose throughout their lifecycle, including periodic evaluation of mobile phase stability and its impact on method performance.
Cost-Benefit Analysis of Improved Mobile Phase Stability Methods
Implementing improved mobile phase stability methods in HPLC systems requires careful evaluation of associated costs against potential benefits. Initial investment costs for enhanced mobile phase stability include specialized equipment such as advanced solvent delivery systems with improved precision pumps, temperature-controlled solvent compartments, and automated degassing units. These hardware upgrades typically range from $15,000 to $50,000 depending on laboratory scale and precision requirements. Additionally, implementation of standardized preparation protocols and staff training programs represents a significant upfront investment, typically $5,000-$10,000 for comprehensive training programs.
Operational costs must also be considered, including higher-grade solvents, specialized storage containers, and increased maintenance requirements. Premium-grade solvents can cost 20-30% more than standard alternatives, while specialized containers with inert materials may increase consumable expenses by 15-25%. Regular system calibration and maintenance adds approximately $3,000-$8,000 annually to operational budgets.
Against these costs, laboratories must weigh substantial benefits. Improved mobile phase stability directly enhances analytical precision, with studies demonstrating reduction in retention time variability by 30-50% and peak area reproducibility improvements of 15-25%. This translates to fewer failed runs and repeated analyses, potentially reducing solvent consumption by 10-20% and analyst time by 15-30% annually.
Quality improvements represent perhaps the most significant benefit, though harder to quantify directly. Enhanced data reliability reduces the risk of erroneous results, particularly critical in regulated environments like pharmaceutical quality control. One pharmaceutical industry report estimated that improved mobile phase stability reduced out-of-specification investigations by approximately 25%, representing substantial cost savings considering each investigation typically costs $5,000-$15,000 in resources and delayed production.
Long-term ROI analysis indicates that most laboratories recover initial investments within 12-24 months through reduced operational costs and improved productivity. Facilities processing high sample volumes achieve faster returns, sometimes within 8-12 months. Small research laboratories with lower throughput may experience longer payback periods of 24-36 months, though still benefiting from improved data quality.
Risk mitigation value must also be considered, particularly in regulated environments where analytical failures can trigger costly compliance issues. The prevention of even one major compliance finding can justify the entire investment in improved mobile phase stability methods, as regulatory actions can cost organizations hundreds of thousands in remediation efforts and lost productivity.
Operational costs must also be considered, including higher-grade solvents, specialized storage containers, and increased maintenance requirements. Premium-grade solvents can cost 20-30% more than standard alternatives, while specialized containers with inert materials may increase consumable expenses by 15-25%. Regular system calibration and maintenance adds approximately $3,000-$8,000 annually to operational budgets.
Against these costs, laboratories must weigh substantial benefits. Improved mobile phase stability directly enhances analytical precision, with studies demonstrating reduction in retention time variability by 30-50% and peak area reproducibility improvements of 15-25%. This translates to fewer failed runs and repeated analyses, potentially reducing solvent consumption by 10-20% and analyst time by 15-30% annually.
Quality improvements represent perhaps the most significant benefit, though harder to quantify directly. Enhanced data reliability reduces the risk of erroneous results, particularly critical in regulated environments like pharmaceutical quality control. One pharmaceutical industry report estimated that improved mobile phase stability reduced out-of-specification investigations by approximately 25%, representing substantial cost savings considering each investigation typically costs $5,000-$15,000 in resources and delayed production.
Long-term ROI analysis indicates that most laboratories recover initial investments within 12-24 months through reduced operational costs and improved productivity. Facilities processing high sample volumes achieve faster returns, sometimes within 8-12 months. Small research laboratories with lower throughput may experience longer payback periods of 24-36 months, though still benefiting from improved data quality.
Risk mitigation value must also be considered, particularly in regulated environments where analytical failures can trigger costly compliance issues. The prevention of even one major compliance finding can justify the entire investment in improved mobile phase stability methods, as regulatory actions can cost organizations hundreds of thousands in remediation efforts and lost productivity.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!