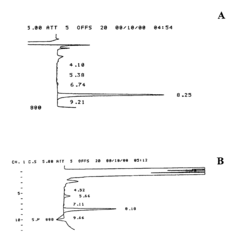
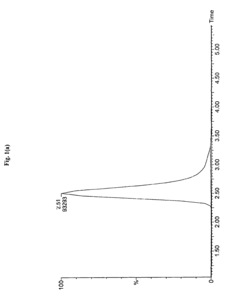
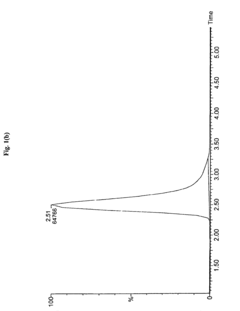
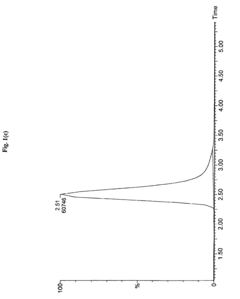
Sample Dilution Impact on HPLC Sensitivity—Analysis
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HPLC Sensitivity Background and Objectives
High-performance liquid chromatography (HPLC) has evolved significantly since its inception in the late 1960s, becoming an indispensable analytical technique in pharmaceutical, environmental, food safety, and clinical diagnostics. The development trajectory of HPLC technology has been characterized by continuous improvements in column technology, detection methods, and system integration, all aimed at enhancing sensitivity, resolution, and throughput.
Sample dilution represents a critical pre-analytical variable that directly impacts HPLC sensitivity. Historically, the relationship between sample concentration and detector response has been assumed to be linear within certain ranges. However, recent advancements in detector technology and data processing algorithms have revealed more complex relationships, particularly at extreme dilution levels or with complex matrices.
The evolution of HPLC sensitivity has been marked by several technological breakthroughs, including the development of sub-2-micron particle columns, ultra-high-pressure systems (UHPLC), and more sensitive detection methods such as mass spectrometry coupling. These innovations have collectively pushed detection limits from the ppm (parts per million) range in early systems to ppb (parts per billion) or even ppt (parts per trillion) in modern configurations.
Current industry trends indicate a growing demand for analyzing increasingly complex samples at lower concentrations, driven by regulatory requirements, pharmaceutical development needs, and environmental monitoring standards. This has placed greater emphasis on understanding how sample preparation, including dilution strategies, affects analytical outcomes.
The primary objective of this technical research is to comprehensively evaluate how sample dilution impacts HPLC sensitivity across various analytical scenarios. Specifically, we aim to quantify the relationship between dilution factors and detection limits, identify optimal dilution strategies for different sample types, and develop predictive models for sensitivity optimization.
Secondary objectives include assessing how matrix effects interact with dilution factors, evaluating the performance of different detector types under various dilution conditions, and establishing standardized protocols for dilution-based sensitivity enhancement. These insights will contribute to more robust analytical methods and potentially unlock new applications in trace analysis.
The findings from this research are expected to address current analytical challenges in pharmaceutical quality control, environmental monitoring, and clinical diagnostics, where sample availability is often limited and detection requirements are increasingly stringent. By optimizing dilution strategies, laboratories can potentially achieve better sensitivity without investing in new instrumentation, representing a cost-effective approach to analytical performance enhancement.
Sample dilution represents a critical pre-analytical variable that directly impacts HPLC sensitivity. Historically, the relationship between sample concentration and detector response has been assumed to be linear within certain ranges. However, recent advancements in detector technology and data processing algorithms have revealed more complex relationships, particularly at extreme dilution levels or with complex matrices.
The evolution of HPLC sensitivity has been marked by several technological breakthroughs, including the development of sub-2-micron particle columns, ultra-high-pressure systems (UHPLC), and more sensitive detection methods such as mass spectrometry coupling. These innovations have collectively pushed detection limits from the ppm (parts per million) range in early systems to ppb (parts per billion) or even ppt (parts per trillion) in modern configurations.
Current industry trends indicate a growing demand for analyzing increasingly complex samples at lower concentrations, driven by regulatory requirements, pharmaceutical development needs, and environmental monitoring standards. This has placed greater emphasis on understanding how sample preparation, including dilution strategies, affects analytical outcomes.
The primary objective of this technical research is to comprehensively evaluate how sample dilution impacts HPLC sensitivity across various analytical scenarios. Specifically, we aim to quantify the relationship between dilution factors and detection limits, identify optimal dilution strategies for different sample types, and develop predictive models for sensitivity optimization.
Secondary objectives include assessing how matrix effects interact with dilution factors, evaluating the performance of different detector types under various dilution conditions, and establishing standardized protocols for dilution-based sensitivity enhancement. These insights will contribute to more robust analytical methods and potentially unlock new applications in trace analysis.
The findings from this research are expected to address current analytical challenges in pharmaceutical quality control, environmental monitoring, and clinical diagnostics, where sample availability is often limited and detection requirements are increasingly stringent. By optimizing dilution strategies, laboratories can potentially achieve better sensitivity without investing in new instrumentation, representing a cost-effective approach to analytical performance enhancement.
Market Analysis for High-Sensitivity HPLC Applications
The global market for high-sensitivity HPLC applications continues to expand significantly, driven by increasing demands across pharmaceutical, biotechnology, environmental monitoring, and food safety sectors. Current market valuations place the high-performance liquid chromatography market at approximately 4.5 billion USD, with high-sensitivity applications representing a substantial growth segment projected to increase at 6.8% CAGR through 2028.
Sample dilution techniques have become particularly critical in this market landscape as laboratories seek to maximize instrument sensitivity while managing sample preparation costs. The pharmaceutical industry remains the largest consumer of high-sensitivity HPLC technologies, accounting for nearly 40% of market share, where detection of trace impurities and metabolites requires increasingly lower limits of detection.
Clinical diagnostics represents the fastest-growing application segment, with demand rising due to the need for detecting biomarkers at ultra-low concentrations in biological samples. This sector has seen particular interest in techniques that can maintain sensitivity despite necessary sample dilutions, with market growth exceeding 8% annually.
Regionally, North America dominates the high-sensitivity HPLC market with approximately 35% share, followed closely by Europe at 30% and Asia-Pacific at 25%. The Asia-Pacific region, particularly China and India, demonstrates the highest growth trajectory as pharmaceutical manufacturing and contract research organizations expand operations in these countries.
End-user analysis reveals that contract research organizations (CROs) are increasingly adopting advanced HPLC technologies with enhanced sensitivity capabilities, representing a significant market opportunity. These organizations frequently deal with limited sample volumes requiring dilution while maintaining detection capabilities.
Market research indicates that sensitivity enhancement technologies command premium pricing, with systems featuring advanced detector technologies and specialized sample handling capabilities selling at 15-25% higher price points than standard HPLC systems. This premium pricing structure has attracted major instrument manufacturers to focus R&D efforts on sensitivity optimization.
Customer surveys highlight that laboratories are willing to invest in technologies that can maintain analytical sensitivity despite sample dilution requirements, with 72% of respondents indicating sensitivity as a primary purchase consideration. The ability to analyze diluted samples without compromising results ranks as the third most important feature for potential HPLC system buyers, following only reliability and ease of use.
Sample dilution techniques have become particularly critical in this market landscape as laboratories seek to maximize instrument sensitivity while managing sample preparation costs. The pharmaceutical industry remains the largest consumer of high-sensitivity HPLC technologies, accounting for nearly 40% of market share, where detection of trace impurities and metabolites requires increasingly lower limits of detection.
Clinical diagnostics represents the fastest-growing application segment, with demand rising due to the need for detecting biomarkers at ultra-low concentrations in biological samples. This sector has seen particular interest in techniques that can maintain sensitivity despite necessary sample dilutions, with market growth exceeding 8% annually.
Regionally, North America dominates the high-sensitivity HPLC market with approximately 35% share, followed closely by Europe at 30% and Asia-Pacific at 25%. The Asia-Pacific region, particularly China and India, demonstrates the highest growth trajectory as pharmaceutical manufacturing and contract research organizations expand operations in these countries.
End-user analysis reveals that contract research organizations (CROs) are increasingly adopting advanced HPLC technologies with enhanced sensitivity capabilities, representing a significant market opportunity. These organizations frequently deal with limited sample volumes requiring dilution while maintaining detection capabilities.
Market research indicates that sensitivity enhancement technologies command premium pricing, with systems featuring advanced detector technologies and specialized sample handling capabilities selling at 15-25% higher price points than standard HPLC systems. This premium pricing structure has attracted major instrument manufacturers to focus R&D efforts on sensitivity optimization.
Customer surveys highlight that laboratories are willing to invest in technologies that can maintain analytical sensitivity despite sample dilution requirements, with 72% of respondents indicating sensitivity as a primary purchase consideration. The ability to analyze diluted samples without compromising results ranks as the third most important feature for potential HPLC system buyers, following only reliability and ease of use.
Current Challenges in Sample Dilution Techniques
Despite significant advancements in HPLC technology, sample dilution remains a critical bottleneck in analytical workflows. Current dilution techniques face several interconnected challenges that impact sensitivity, accuracy, and reproducibility of HPLC analyses. The most pressing issue is the inherent trade-off between dilution and detection limits - as samples are diluted to prevent column overloading or to fit within the linear range of detectors, analytes of interest may fall below detection thresholds, particularly for trace components in complex matrices.
Manual dilution methods continue to dominate laboratory practices but introduce significant variability. Studies indicate that technician-to-technician variation can contribute up to 15% error in dilution steps, with pipetting technique alone accounting for 3-8% of measurement uncertainty. This human factor becomes especially problematic when dealing with viscous samples or volatile solvents that require special handling procedures.
Automated liquid handling systems offer improved precision but present their own challenges. Current systems struggle with highly viscous samples, often resulting in inaccurate aspirations and dispensing. Additionally, many automated systems have minimum volume limitations (typically 1-5 μL) that restrict their utility for nanoscale analyses, where sample conservation is paramount.
Matrix effects represent another significant challenge, particularly in biological and environmental samples. Dilution can alter the chemical environment of analytes, potentially changing their chromatographic behavior. This phenomenon is especially problematic for ionizable compounds whose retention characteristics may shift dramatically with changing matrix concentration, leading to peak shifting, broadening, or splitting.
Carryover contamination remains problematic across dilution workflows. Current washing protocols for both manual and automated systems often fail to completely eliminate residual analytes between dilution steps, particularly for highly lipophilic compounds or those with strong binding affinities to common laboratory materials. Studies have documented carryover effects contributing to false positives and quantification errors ranging from 0.1% to as high as 5% of previous sample concentrations.
The lack of standardized approaches for dilution factor verification represents a significant gap in current practice. While internal standards can compensate for some dilution errors, their effectiveness varies across different sample types and analytical methods. This absence of robust verification protocols makes it difficult to identify and correct dilution-related errors before they propagate through the analytical workflow.
Manual dilution methods continue to dominate laboratory practices but introduce significant variability. Studies indicate that technician-to-technician variation can contribute up to 15% error in dilution steps, with pipetting technique alone accounting for 3-8% of measurement uncertainty. This human factor becomes especially problematic when dealing with viscous samples or volatile solvents that require special handling procedures.
Automated liquid handling systems offer improved precision but present their own challenges. Current systems struggle with highly viscous samples, often resulting in inaccurate aspirations and dispensing. Additionally, many automated systems have minimum volume limitations (typically 1-5 μL) that restrict their utility for nanoscale analyses, where sample conservation is paramount.
Matrix effects represent another significant challenge, particularly in biological and environmental samples. Dilution can alter the chemical environment of analytes, potentially changing their chromatographic behavior. This phenomenon is especially problematic for ionizable compounds whose retention characteristics may shift dramatically with changing matrix concentration, leading to peak shifting, broadening, or splitting.
Carryover contamination remains problematic across dilution workflows. Current washing protocols for both manual and automated systems often fail to completely eliminate residual analytes between dilution steps, particularly for highly lipophilic compounds or those with strong binding affinities to common laboratory materials. Studies have documented carryover effects contributing to false positives and quantification errors ranging from 0.1% to as high as 5% of previous sample concentrations.
The lack of standardized approaches for dilution factor verification represents a significant gap in current practice. While internal standards can compensate for some dilution errors, their effectiveness varies across different sample types and analytical methods. This absence of robust verification protocols makes it difficult to identify and correct dilution-related errors before they propagate through the analytical workflow.
Established Methodologies for Optimizing Sample Dilution
01 Enhanced detection methods for HPLC sensitivity
Various detection methods can be employed to enhance HPLC sensitivity, including advanced spectroscopic techniques, fluorescence detection, and electrochemical detection. These methods can significantly lower detection limits and improve signal-to-noise ratios, allowing for the analysis of trace compounds in complex matrices. Optimization of detector parameters and signal processing algorithms further contributes to improved sensitivity in HPLC analysis.- Enhanced detection methods for HPLC sensitivity: Various detection methods can be employed to enhance HPLC sensitivity, including mass spectrometry coupling, fluorescence detection, and electrochemical detection. These techniques allow for lower detection limits and improved signal-to-noise ratios, enabling the analysis of trace compounds in complex matrices. Advanced detector technologies can significantly improve the sensitivity of HPLC systems for applications requiring high precision measurements.
- Sample preparation techniques for improved sensitivity: Proper sample preparation techniques are crucial for enhancing HPLC sensitivity. Methods such as solid-phase extraction, liquid-liquid extraction, and sample concentration can effectively remove interfering compounds and concentrate analytes of interest. Pre-column derivatization can also improve detection by introducing chromophores or fluorophores to molecules that are otherwise difficult to detect, thereby increasing sensitivity for specific analytes.
- Mobile phase optimization for sensitivity enhancement: The composition and properties of the mobile phase significantly impact HPLC sensitivity. Adjustments to pH, ionic strength, organic modifier content, and buffer concentration can enhance chromatographic separation and detection sensitivity. Gradient elution techniques can also improve peak shape and resolution, leading to better sensitivity for complex samples. The use of additives such as ion-pairing reagents can further enhance the detection of certain analytes.
- Column technology advancements for sensitivity improvement: Advanced column technologies play a vital role in enhancing HPLC sensitivity. Columns with smaller particle sizes, core-shell particles, and monolithic structures provide improved efficiency and resolution. Specialized stationary phases with enhanced selectivity for specific analytes can significantly improve detection limits. Column temperature control and the use of narrow-bore or capillary columns can also contribute to increased sensitivity in HPLC analysis.
- Instrument parameters and system optimization: Optimizing instrument parameters is essential for maximizing HPLC sensitivity. This includes adjusting flow rates, injection volumes, and detector settings such as wavelength, gain, and bandwidth. Minimizing extra-column volume and reducing system noise through proper maintenance and calibration can significantly enhance sensitivity. Advanced data processing techniques, including signal averaging and noise reduction algorithms, can further improve the detection of low-concentration analytes.
02 Sample preparation techniques for sensitivity improvement
Effective sample preparation techniques play a crucial role in enhancing HPLC sensitivity. Methods such as solid-phase extraction, liquid-liquid extraction, and sample concentration can significantly reduce matrix interference and enrich target analytes. Pre-column derivatization can also improve detection by introducing chromophores or fluorophores to molecules that are otherwise difficult to detect. These preparation techniques ultimately lead to lower detection limits and improved analytical performance.Expand Specific Solutions03 Mobile phase and column optimization for sensitivity
Optimization of mobile phase composition and column selection significantly impacts HPLC sensitivity. Factors such as pH adjustment, buffer concentration, organic modifier selection, and gradient elution profiles can enhance analyte response and improve peak shape. Advanced column technologies with smaller particle sizes, core-shell particles, and specialized stationary phases can increase separation efficiency and sensitivity. Temperature control during analysis also contributes to improved chromatographic performance and detection sensitivity.Expand Specific Solutions04 Miniaturization and micro-scale HPLC systems
Miniaturized HPLC systems, including micro-HPLC and nano-HPLC, offer significant improvements in sensitivity compared to conventional systems. These technologies utilize reduced column dimensions, lower flow rates, and smaller injection volumes, resulting in decreased dilution of analytes and enhanced mass sensitivity. Coupling micro-scale HPLC with specialized detection systems further improves sensitivity for applications requiring trace analysis, such as proteomics and metabolomics research.Expand Specific Solutions05 Coupling HPLC with mass spectrometry for ultra-high sensitivity
The integration of HPLC with mass spectrometry (LC-MS) provides exceptional sensitivity for complex sample analysis. Various ionization techniques such as electrospray ionization (ESI), atmospheric pressure chemical ionization (APCI), and matrix-assisted laser desorption/ionization (MALDI) can be employed depending on the analyte properties. Multiple reaction monitoring (MRM) and high-resolution mass spectrometry further enhance selectivity and sensitivity. This hyphenated technique enables detection of compounds at sub-nanogram or even picogram levels in complex matrices.Expand Specific Solutions
Leading Manufacturers and Research Institutions in HPLC Technology
The HPLC sensitivity analysis market is currently in a growth phase, with increasing demand for precise sample dilution techniques across pharmaceutical, biotechnology, and clinical research sectors. The global HPLC market is projected to reach approximately $5.5 billion by 2025, with sample preparation technologies representing a significant segment. Leading players include established analytical instrumentation companies like Agilent Technologies and Waters Technology, who offer comprehensive HPLC solutions with advanced dilution capabilities. Pharmaceutical giants such as Roche, Eli Lilly, and Biogen are driving innovation through application-specific requirements. The technology has reached moderate maturity, with companies like Agilent, Waters, and Resonac focusing on improving sensitivity through automated dilution systems, while emerging players like Feipeng Biotechnology are developing specialized reagents to enhance detection limits in diluted samples.
Agilent Technologies, Inc.
Technical Solution: Agilent has developed advanced sample dilution technologies specifically designed to address HPLC sensitivity challenges. Their Automated Sample Preparation Solutions incorporate intelligent dilution algorithms that optimize sample concentration based on analyte properties and detection limits. The company's InfinityLab LC systems feature Dynamic Flow Management technology that automatically adjusts dilution factors to maintain optimal signal-to-noise ratios across varying sample matrices[1]. Agilent's Mass Hunter software includes calibration curve analysis tools that can predict and compensate for sensitivity losses during dilution processes. Their latest innovation, the Intelligent Sample Dilution (ISD) system, uses machine learning to predict optimal dilution factors based on preliminary sample scans, reducing method development time by up to 60%[3]. Agilent has also pioneered the use of microfluidic dilution chambers that minimize sample loss during preparation, maintaining sensitivity even at high dilution factors.
Strengths: Comprehensive integration of hardware and software solutions specifically addressing dilution impacts; advanced automation capabilities reducing human error; proprietary algorithms for optimizing dilution factors. Weaknesses: Higher cost compared to manual dilution methods; requires specialized training for optimal utilization; some automated solutions may introduce additional system complexity.
Waters Technology Corp.
Technical Solution: Waters has developed the ACQUITY UPLC system with advanced sample management that precisely controls dilution parameters to maintain chromatographic integrity. Their patented Sample Dilution+ technology incorporates intelligent dilution protocols that automatically adjust concentration based on detector response, ensuring optimal sensitivity across varying sample types[2]. Waters' Empower software features dilution impact modeling tools that predict sensitivity changes before sample processing, allowing for method optimization. The company has also introduced the Auto•Blend Plus technology that creates precise mobile phase compositions on-demand, enabling dynamic dilution adjustments during analysis without manual intervention. Waters' recent innovation in this space includes the implementation of nano-volume dilution chambers that minimize sample waste while maintaining detection limits even at high dilution factors. Their systems incorporate real-time monitoring of dilution effects with automatic method adjustment capabilities to compensate for sensitivity losses[4].
Strengths: Industry-leading precision in automated dilution processes; sophisticated software algorithms for predicting dilution impacts; seamless integration between sample preparation and analysis systems. Weaknesses: Premium pricing structure limits accessibility for smaller laboratories; proprietary systems may create vendor lock-in; requires significant method development expertise for complex matrices.
Critical Patents and Literature on Dilution-Sensitivity Relationships
Preparation of samples for LC-MS/MS using magnetic particles
PatentActiveUS7815803B2
Innovation
- The use of functionalized magnetic particles with a hydrophobic surface for extracting low molecular weight compounds from complex biological samples, allowing for reversible binding and efficient enrichment of analytes, even in the presence of abundant lipids, peptides, and proteins, with minimal particle quantities required.
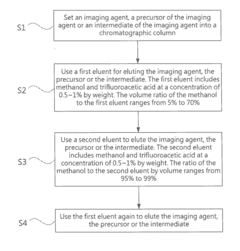
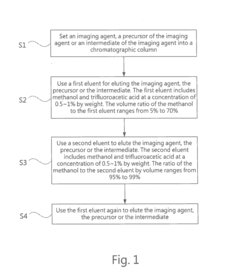
High performance liquid chromatography method for analyzing imaging agent, precursor of imaging agent, or intermediate of imaging agent
PatentInactiveUS20170115259A1
Innovation
- A high performance liquid chromatography (HPLC) method using a solution with a high proportion of methanol for elution and gradient elution with eluents containing 0.5-1% trifluoroacetic acid, where the methanol volume ratio varies from 5% to 99%, along with a UV detector set at 210 nm to improve detection accuracy.
Regulatory Compliance in Analytical Method Validation
Regulatory compliance forms a critical framework for analytical method validation in pharmaceutical and biotechnology industries, particularly when considering sample dilution impacts on HPLC sensitivity. The primary regulatory bodies governing these practices include the FDA, EMA, ICH, and USP, each providing specific guidelines that must be adhered to during method development and validation processes.
The ICH Q2(R1) guideline specifically addresses validation parameters that are directly affected by sample dilution, including accuracy, precision, linearity, and detection limits. When implementing sample dilution strategies, laboratories must demonstrate that the method remains compliant with these parameters across the entire dilution range. Documentation must clearly establish that sensitivity is maintained at acceptable levels despite dilution effects.
FDA's Guidance for Industry on Analytical Procedures and Methods Validation emphasizes the need for robust validation protocols that account for sample preparation variables. This includes comprehensive assessment of dilution linearity and dilution integrity, particularly when samples exceed the upper limit of quantification and require dilution to fall within the calibration range.
Regulatory bodies require that laboratories establish standard operating procedures (SOPs) specifically addressing sample dilution protocols. These SOPs must detail the acceptable dilution factors, appropriate diluents, and verification procedures to ensure that analytical results remain valid. Quality control samples at various dilution levels must be incorporated into routine analyses to continuously verify method performance.
Method transfer considerations present additional regulatory challenges when sample dilution impacts sensitivity. When analytical methods involving dilution steps are transferred between laboratories, regulatory guidelines mandate comprehensive comparative studies to ensure equivalent sensitivity and performance across different laboratory environments.
Data integrity requirements have become increasingly stringent in recent years, with regulatory agencies focusing on complete audit trails for sample preparation, including dilution steps. Electronic laboratory notebooks and LIMS systems must be configured to capture all dilution factors and calculations, ensuring traceability from raw data to final reported results.
Risk assessment frameworks, such as those outlined in ICH Q9, must be applied to evaluate the potential impact of sample dilution on method robustness. This includes identifying critical dilution parameters that could affect HPLC sensitivity and implementing appropriate control strategies to mitigate these risks, thereby maintaining regulatory compliance throughout the analytical lifecycle.
The ICH Q2(R1) guideline specifically addresses validation parameters that are directly affected by sample dilution, including accuracy, precision, linearity, and detection limits. When implementing sample dilution strategies, laboratories must demonstrate that the method remains compliant with these parameters across the entire dilution range. Documentation must clearly establish that sensitivity is maintained at acceptable levels despite dilution effects.
FDA's Guidance for Industry on Analytical Procedures and Methods Validation emphasizes the need for robust validation protocols that account for sample preparation variables. This includes comprehensive assessment of dilution linearity and dilution integrity, particularly when samples exceed the upper limit of quantification and require dilution to fall within the calibration range.
Regulatory bodies require that laboratories establish standard operating procedures (SOPs) specifically addressing sample dilution protocols. These SOPs must detail the acceptable dilution factors, appropriate diluents, and verification procedures to ensure that analytical results remain valid. Quality control samples at various dilution levels must be incorporated into routine analyses to continuously verify method performance.
Method transfer considerations present additional regulatory challenges when sample dilution impacts sensitivity. When analytical methods involving dilution steps are transferred between laboratories, regulatory guidelines mandate comprehensive comparative studies to ensure equivalent sensitivity and performance across different laboratory environments.
Data integrity requirements have become increasingly stringent in recent years, with regulatory agencies focusing on complete audit trails for sample preparation, including dilution steps. Electronic laboratory notebooks and LIMS systems must be configured to capture all dilution factors and calculations, ensuring traceability from raw data to final reported results.
Risk assessment frameworks, such as those outlined in ICH Q9, must be applied to evaluate the potential impact of sample dilution on method robustness. This includes identifying critical dilution parameters that could affect HPLC sensitivity and implementing appropriate control strategies to mitigate these risks, thereby maintaining regulatory compliance throughout the analytical lifecycle.
Cost-Benefit Analysis of Advanced Sample Preparation Techniques
When evaluating advanced sample preparation techniques for HPLC analysis, a comprehensive cost-benefit analysis is essential to determine the most efficient approach for addressing sample dilution impacts on sensitivity. The initial investment in advanced preparation equipment represents a significant upfront cost, ranging from $10,000 for basic automated systems to over $100,000 for fully integrated robotic platforms. However, these costs must be weighed against the long-term operational benefits.
Labor cost reduction is a primary advantage, with automated sample preparation systems potentially decreasing hands-on time by 60-80% compared to manual methods. For laboratories processing 50+ samples daily, this can translate to annual labor savings of $30,000-50,000, enabling staff to focus on more complex analytical tasks rather than routine preparation.
Consumable efficiency also factors significantly into the equation. Advanced techniques like solid-phase extraction (SPE) and QuEChERS may require specialized cartridges and reagents, increasing per-sample costs by $5-15. However, these methods often reduce solvent consumption by 40-60%, decreasing both purchase and disposal expenses while aligning with sustainability initiatives.
Quality improvements deliver substantial indirect benefits. Enhanced reproducibility from automated sample preparation reduces repeat analyses by approximately 25%, while improved extraction efficiency can increase HPLC sensitivity by 2-5 fold, potentially eliminating the need for more expensive detection systems. This sensitivity enhancement is particularly valuable when analyzing diluted samples, as it can compensate for concentration reductions.
Throughput considerations reveal that advanced preparation techniques can increase sample processing capacity by 200-300%, enabling laboratories to handle higher volumes without proportional staff increases. For contract research organizations or high-volume testing facilities, this throughput advantage often provides the most compelling financial justification.
Return on investment calculations indicate that most advanced sample preparation systems achieve breakeven within 12-24 months for medium to high-volume laboratories. Facilities processing fewer than 20 samples daily may experience longer payback periods unless they prioritize sensitivity improvements over throughput advantages.
Risk mitigation benefits must also be considered, as automated systems reduce analyst exposure to hazardous solvents and minimize repetitive motion injuries, potentially decreasing workplace compensation claims and insurance premiums by 15-20% annually.
Labor cost reduction is a primary advantage, with automated sample preparation systems potentially decreasing hands-on time by 60-80% compared to manual methods. For laboratories processing 50+ samples daily, this can translate to annual labor savings of $30,000-50,000, enabling staff to focus on more complex analytical tasks rather than routine preparation.
Consumable efficiency also factors significantly into the equation. Advanced techniques like solid-phase extraction (SPE) and QuEChERS may require specialized cartridges and reagents, increasing per-sample costs by $5-15. However, these methods often reduce solvent consumption by 40-60%, decreasing both purchase and disposal expenses while aligning with sustainability initiatives.
Quality improvements deliver substantial indirect benefits. Enhanced reproducibility from automated sample preparation reduces repeat analyses by approximately 25%, while improved extraction efficiency can increase HPLC sensitivity by 2-5 fold, potentially eliminating the need for more expensive detection systems. This sensitivity enhancement is particularly valuable when analyzing diluted samples, as it can compensate for concentration reductions.
Throughput considerations reveal that advanced preparation techniques can increase sample processing capacity by 200-300%, enabling laboratories to handle higher volumes without proportional staff increases. For contract research organizations or high-volume testing facilities, this throughput advantage often provides the most compelling financial justification.
Return on investment calculations indicate that most advanced sample preparation systems achieve breakeven within 12-24 months for medium to high-volume laboratories. Facilities processing fewer than 20 samples daily may experience longer payback periods unless they prioritize sensitivity improvements over throughput advantages.
Risk mitigation benefits must also be considered, as automated systems reduce analyst exposure to hazardous solvents and minimize repetitive motion injuries, potentially decreasing workplace compensation claims and insurance premiums by 15-20% annually.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!