HPLC Method Development: Steps for New Analytes
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HPLC Technology Background and Objectives
High-Performance Liquid Chromatography (HPLC) has evolved significantly since its inception in the late 1960s, becoming one of the most powerful and versatile analytical techniques in modern chemistry. The development trajectory of HPLC technology has been characterized by continuous improvements in separation efficiency, detection sensitivity, and automation capabilities. From early systems with limited pressure capabilities to today's ultra-high-performance instruments, HPLC has transformed analytical chemistry across pharmaceutical, environmental, food safety, and clinical diagnostics sectors.
The evolution of HPLC column technology represents a critical advancement path, progressing from large particle sizes (10-20 μm) to sub-2 μm particles, enabling dramatically improved resolution and faster analyses. Parallel developments in stationary phase chemistry have expanded from simple silica-based materials to highly specialized phases including C18, C8, phenyl, amino, and various chiral selectors, each optimized for specific analytical challenges.
Detection technology has similarly advanced from simple UV-Vis detectors to sophisticated mass spectrometry interfaces, diode array detectors, fluorescence, and electrochemical detection systems. This expansion has enabled both targeted and untargeted analysis of increasingly complex sample matrices with remarkable sensitivity and specificity.
The primary objective of HPLC method development for new analytes is to establish robust, reproducible, and efficient analytical procedures that can accurately identify and quantify target compounds. This process requires systematic optimization of multiple parameters including mobile phase composition, pH, temperature, flow rate, and detection conditions to achieve optimal separation and sensitivity.
Current technological trends in HPLC method development include increasing automation through software algorithms that can predict chromatographic behavior, reducing method development time from weeks to days or even hours. Machine learning approaches are beginning to transform method development by analyzing historical data to suggest optimal starting conditions for new analytes.
Green chemistry principles are also influencing HPLC technology development, with emphasis on reducing solvent consumption through miniaturization (micro and nano-HPLC), using more environmentally friendly mobile phases, and implementing more efficient separation mechanisms that require less sample preparation.
The integration of HPLC with other analytical techniques, particularly mass spectrometry, has created powerful hyphenated methods that provide structural information alongside chromatographic separation. This integration represents a significant frontier in analytical chemistry, enabling comprehensive characterization of complex biological and environmental samples.
Future objectives for HPLC method development include further simplification of workflows, increased throughput capabilities, enhanced robustness for diverse sample types, and improved transferability of methods between different laboratory settings and instrument platforms.
The evolution of HPLC column technology represents a critical advancement path, progressing from large particle sizes (10-20 μm) to sub-2 μm particles, enabling dramatically improved resolution and faster analyses. Parallel developments in stationary phase chemistry have expanded from simple silica-based materials to highly specialized phases including C18, C8, phenyl, amino, and various chiral selectors, each optimized for specific analytical challenges.
Detection technology has similarly advanced from simple UV-Vis detectors to sophisticated mass spectrometry interfaces, diode array detectors, fluorescence, and electrochemical detection systems. This expansion has enabled both targeted and untargeted analysis of increasingly complex sample matrices with remarkable sensitivity and specificity.
The primary objective of HPLC method development for new analytes is to establish robust, reproducible, and efficient analytical procedures that can accurately identify and quantify target compounds. This process requires systematic optimization of multiple parameters including mobile phase composition, pH, temperature, flow rate, and detection conditions to achieve optimal separation and sensitivity.
Current technological trends in HPLC method development include increasing automation through software algorithms that can predict chromatographic behavior, reducing method development time from weeks to days or even hours. Machine learning approaches are beginning to transform method development by analyzing historical data to suggest optimal starting conditions for new analytes.
Green chemistry principles are also influencing HPLC technology development, with emphasis on reducing solvent consumption through miniaturization (micro and nano-HPLC), using more environmentally friendly mobile phases, and implementing more efficient separation mechanisms that require less sample preparation.
The integration of HPLC with other analytical techniques, particularly mass spectrometry, has created powerful hyphenated methods that provide structural information alongside chromatographic separation. This integration represents a significant frontier in analytical chemistry, enabling comprehensive characterization of complex biological and environmental samples.
Future objectives for HPLC method development include further simplification of workflows, increased throughput capabilities, enhanced robustness for diverse sample types, and improved transferability of methods between different laboratory settings and instrument platforms.
Market Demand for Advanced Analytical Methods
The analytical chemistry market has witnessed substantial growth in recent years, driven primarily by increasing demands for precise analytical methods across various industries. The global HPLC market specifically was valued at approximately 4.5 billion USD in 2022 and is projected to reach 6.7 billion USD by 2028, representing a compound annual growth rate of 6.8%. This growth trajectory underscores the expanding market demand for advanced analytical methods, particularly HPLC techniques for new analytes.
Pharmaceutical and biopharmaceutical sectors remain the largest consumers of HPLC technology, accounting for nearly 45% of the total market share. The stringent regulatory requirements for drug development and quality control have necessitated more sophisticated analytical methods capable of detecting increasingly complex drug molecules and their metabolites at lower concentrations. The FDA's continuous updates to analytical method validation guidelines further emphasize the critical need for robust HPLC method development protocols.
Environmental monitoring represents another rapidly growing segment, with a 9.2% annual growth rate in demand for advanced analytical methods. Water quality testing, soil contamination analysis, and air pollution monitoring all require highly sensitive detection of diverse chemical compounds, often at trace levels. Regulatory bodies worldwide have established stricter limits for environmental contaminants, driving the need for more sensitive and selective analytical techniques.
The food and beverage industry has also emerged as a significant market driver, particularly with increasing consumer awareness regarding food safety and authenticity. The detection of pesticides, mycotoxins, allergens, and food additives requires specialized analytical methods that can handle complex matrices while maintaining high sensitivity and selectivity. This sector's demand for HPLC methods has grown by approximately 7.5% annually over the past five years.
Clinical diagnostics represents another expanding application area, with the growing importance of therapeutic drug monitoring, metabolomics, and biomarker discovery. The shift toward personalized medicine has created demand for analytical methods capable of detecting and quantifying biomarkers in complex biological samples with high precision and reproducibility.
Academic and research institutions continue to drive innovation in analytical methodologies, with increasing research funding directed toward developing novel separation techniques and detection methods. The publication rate of research papers focusing on HPLC method development has increased by 12% annually since 2018, indicating robust academic interest in advancing this field.
Pharmaceutical and biopharmaceutical sectors remain the largest consumers of HPLC technology, accounting for nearly 45% of the total market share. The stringent regulatory requirements for drug development and quality control have necessitated more sophisticated analytical methods capable of detecting increasingly complex drug molecules and their metabolites at lower concentrations. The FDA's continuous updates to analytical method validation guidelines further emphasize the critical need for robust HPLC method development protocols.
Environmental monitoring represents another rapidly growing segment, with a 9.2% annual growth rate in demand for advanced analytical methods. Water quality testing, soil contamination analysis, and air pollution monitoring all require highly sensitive detection of diverse chemical compounds, often at trace levels. Regulatory bodies worldwide have established stricter limits for environmental contaminants, driving the need for more sensitive and selective analytical techniques.
The food and beverage industry has also emerged as a significant market driver, particularly with increasing consumer awareness regarding food safety and authenticity. The detection of pesticides, mycotoxins, allergens, and food additives requires specialized analytical methods that can handle complex matrices while maintaining high sensitivity and selectivity. This sector's demand for HPLC methods has grown by approximately 7.5% annually over the past five years.
Clinical diagnostics represents another expanding application area, with the growing importance of therapeutic drug monitoring, metabolomics, and biomarker discovery. The shift toward personalized medicine has created demand for analytical methods capable of detecting and quantifying biomarkers in complex biological samples with high precision and reproducibility.
Academic and research institutions continue to drive innovation in analytical methodologies, with increasing research funding directed toward developing novel separation techniques and detection methods. The publication rate of research papers focusing on HPLC method development has increased by 12% annually since 2018, indicating robust academic interest in advancing this field.
Current HPLC Methodologies and Challenges
High-performance liquid chromatography (HPLC) has evolved significantly over the past decades, becoming an indispensable analytical technique in pharmaceutical, environmental, food, and clinical laboratories. Current HPLC methodologies encompass a diverse range of approaches, each with specific advantages and limitations when developing methods for new analytes.
Reversed-phase chromatography remains the dominant HPLC methodology, accounting for approximately 70-80% of all applications due to its versatility and robustness. This approach utilizes non-polar stationary phases (typically C18, C8, or phenyl columns) with polar mobile phases, making it suitable for a wide range of compounds. However, challenges arise when analyzing highly polar compounds that exhibit poor retention or when dealing with complex biological matrices that cause interference.
Ultra-high-performance liquid chromatography (UHPLC) represents a significant advancement, utilizing sub-2μm particles and higher pressure systems (up to 15,000 psi) to achieve superior resolution, sensitivity, and throughput. While UHPLC offers faster analysis times and reduced solvent consumption, it presents challenges related to system backpressure, instrument compatibility, and method transfer from conventional HPLC systems.
Hydrophilic interaction liquid chromatography (HILIC) has gained prominence for analyzing polar and hydrophilic compounds that are poorly retained in reversed-phase systems. HILIC employs polar stationary phases with high organic content mobile phases, but method development can be complex due to multimodal retention mechanisms and longer equilibration times.
Ion-exchange chromatography and size-exclusion chromatography serve specialized applications for charged molecules and large biomolecules, respectively, though they often require specific instrumentation and expertise.
A significant challenge in current HPLC method development is the time-consuming nature of traditional trial-and-error approaches. Method development for new analytes typically requires 2-4 weeks of laboratory work, with multiple iterations of column selection, mobile phase optimization, and validation experiments.
Quality-by-Design (QbD) approaches have emerged to address this inefficiency, employing statistical design of experiments (DoE) to systematically explore the method parameter space. However, implementation of QbD requires specialized software and expertise that may not be available in all laboratories.
Automation in HPLC method development has advanced with column-switching systems and automated method scouting platforms, but these technologies remain expensive and are primarily adopted by larger pharmaceutical companies and research institutions.
Method transfer between laboratories and instrument platforms presents another significant challenge, particularly when developing methods for global deployment. Differences in instrument configurations, column lots, and laboratory environments can impact method robustness and reproducibility.
Reversed-phase chromatography remains the dominant HPLC methodology, accounting for approximately 70-80% of all applications due to its versatility and robustness. This approach utilizes non-polar stationary phases (typically C18, C8, or phenyl columns) with polar mobile phases, making it suitable for a wide range of compounds. However, challenges arise when analyzing highly polar compounds that exhibit poor retention or when dealing with complex biological matrices that cause interference.
Ultra-high-performance liquid chromatography (UHPLC) represents a significant advancement, utilizing sub-2μm particles and higher pressure systems (up to 15,000 psi) to achieve superior resolution, sensitivity, and throughput. While UHPLC offers faster analysis times and reduced solvent consumption, it presents challenges related to system backpressure, instrument compatibility, and method transfer from conventional HPLC systems.
Hydrophilic interaction liquid chromatography (HILIC) has gained prominence for analyzing polar and hydrophilic compounds that are poorly retained in reversed-phase systems. HILIC employs polar stationary phases with high organic content mobile phases, but method development can be complex due to multimodal retention mechanisms and longer equilibration times.
Ion-exchange chromatography and size-exclusion chromatography serve specialized applications for charged molecules and large biomolecules, respectively, though they often require specific instrumentation and expertise.
A significant challenge in current HPLC method development is the time-consuming nature of traditional trial-and-error approaches. Method development for new analytes typically requires 2-4 weeks of laboratory work, with multiple iterations of column selection, mobile phase optimization, and validation experiments.
Quality-by-Design (QbD) approaches have emerged to address this inefficiency, employing statistical design of experiments (DoE) to systematically explore the method parameter space. However, implementation of QbD requires specialized software and expertise that may not be available in all laboratories.
Automation in HPLC method development has advanced with column-switching systems and automated method scouting platforms, but these technologies remain expensive and are primarily adopted by larger pharmaceutical companies and research institutions.
Method transfer between laboratories and instrument platforms presents another significant challenge, particularly when developing methods for global deployment. Differences in instrument configurations, column lots, and laboratory environments can impact method robustness and reproducibility.
Current Method Development Approaches
01 Mobile phase optimization for HPLC method development
Optimization of mobile phase composition is crucial for HPLC method development. This involves selecting appropriate solvents, adjusting pH, and modifying buffer concentrations to achieve optimal separation of analytes. The selection of organic modifiers (like acetonitrile or methanol) and their ratios with aqueous components significantly impacts retention time, peak shape, and resolution. Gradient elution techniques can be employed for complex samples to improve separation efficiency.- Mobile phase optimization for HPLC method development: Optimization of mobile phase composition is crucial for HPLC method development. This involves selecting appropriate solvents, adjusting pH, and modifying buffer concentrations to achieve optimal separation of analytes. The selection of organic modifiers like acetonitrile or methanol, along with proper buffer systems, significantly impacts resolution, retention time, and peak shape in chromatographic separations.
- Column selection and stationary phase considerations: Selection of appropriate column and stationary phase is fundamental to HPLC method development. This includes considerations of column dimensions, particle size, pore size, and chemical properties of the stationary phase. Different stationary phases (C18, C8, phenyl, amino, etc.) offer varying selectivity for different analytes, affecting separation efficiency and resolution of complex mixtures.
- Detection techniques and parameter optimization: Various detection techniques are employed in HPLC method development, including UV-Vis, fluorescence, mass spectrometry, and refractive index detection. Optimization of detection parameters such as wavelength selection for UV detection, ionization conditions for mass spectrometry, or excitation/emission wavelengths for fluorescence detection is essential for achieving high sensitivity and selectivity in analytical methods.
- Sample preparation and extraction methods: Effective sample preparation techniques are critical for successful HPLC analysis. This includes various extraction methods such as liquid-liquid extraction, solid-phase extraction, protein precipitation, and filtration. Proper sample preparation enhances method sensitivity, reduces matrix effects, extends column life, and improves overall chromatographic performance by removing interfering compounds.
- Method validation and robustness testing: Validation of HPLC methods involves systematic evaluation of parameters such as accuracy, precision, linearity, range, specificity, detection limit, and quantitation limit. Robustness testing assesses the reliability of the method when small variations in method parameters occur. This includes evaluating the effects of changes in mobile phase composition, pH, flow rate, column temperature, and other critical factors on method performance.
02 Column selection and stationary phase considerations
Selection of appropriate HPLC columns and stationary phases is fundamental to method development. Factors such as column dimensions, particle size, pore size, and chemical composition of the stationary phase must be considered based on the analytes' properties. Reversed-phase, normal-phase, ion-exchange, and size-exclusion columns offer different separation mechanisms suitable for various applications. Column temperature control and conditioning procedures also play important roles in achieving reproducible results.Expand Specific Solutions03 Sample preparation techniques for HPLC analysis
Effective sample preparation is essential for successful HPLC method development. Techniques include filtration, centrifugation, liquid-liquid extraction, solid-phase extraction, and protein precipitation to remove interfering substances and concentrate analytes of interest. Proper sample dissolution in compatible solvents ensures good chromatographic performance and prevents column damage. Sample stability during preparation and analysis must be considered to maintain the integrity of results.Expand Specific Solutions04 Method validation parameters and procedures
Validation of HPLC methods involves evaluating parameters such as specificity, linearity, accuracy, precision, detection limit, quantification limit, robustness, and system suitability. These parameters ensure the reliability and reproducibility of analytical results. Validation protocols typically follow regulatory guidelines such as ICH, USP, or FDA requirements. Stress testing and forced degradation studies may be included to evaluate the stability-indicating nature of the method.Expand Specific Solutions05 Advanced detection techniques and method optimization
Various detection techniques enhance the sensitivity and selectivity of HPLC methods. These include UV-Visible, fluorescence, refractive index, electrochemical, and mass spectrometric detection. Method optimization strategies involve design of experiments (DoE), quality by design (QbD) approaches, and the use of software tools to systematically evaluate critical method parameters. Transfer of methods between different HPLC systems requires consideration of instrument characteristics to maintain method performance.Expand Specific Solutions
Key Players in Analytical Instrumentation
The HPLC method development landscape for new analytes is currently in a mature growth phase, with an estimated global market size exceeding $4 billion. Leading players include Agilent Technologies and Waters Technology, who dominate with comprehensive instrument portfolios and method development software. Pharmaceutical giants like F. Hoffmann-La Roche, Bristol Myers Squibb, and Merck contribute significantly through application-specific developments. The technology shows high maturity with standardized workflows, yet continues evolving through innovations from Shimadzu and Bio-Rad in specialized detection methods. Academic-industry partnerships with institutions like California Institute of Technology and University of Chicago are driving next-generation approaches for complex analytes, particularly in biologics and biomarkers.
Agilent Technologies, Inc.
Technical Solution: Agilent's HPLC method development approach employs a systematic workflow starting with defining separation goals based on analyte properties. Their technology utilizes automated method scouting with intelligent software that evaluates multiple columns and mobile phase combinations simultaneously. The Agilent Method Development Solution incorporates their InfinityLab LC systems with column selection valves and solvent selection valves to test up to 8 columns and 15 different mobile phases in a single automated sequence. Their proprietary OpenLAB CDS software includes method optimization tools that analyze chromatographic results and suggest optimal conditions based on resolution, peak capacity, and analysis time. Agilent's approach also includes Quality by Design (QbD) principles, using Design of Experiments (DoE) to establish a design space where critical method parameters are identified and optimized to ensure method robustness. For challenging separations, they offer specialized columns with unique selectivities and advanced detection technologies including diode array, mass spectrometry, and charged aerosol detection.
Strengths: Comprehensive automation capabilities reduce manual intervention and accelerate development time; integrated software provides sophisticated data analysis and visualization tools; extensive column chemistry portfolio offers solutions for diverse analyte types. Weaknesses: Higher initial investment cost compared to manual approaches; requires specialized training for optimal utilization of advanced features; system complexity may present challenges for laboratories with limited resources.
Waters Technology Corp.
Technical Solution: Waters' HPLC method development platform centers around their Arc HPLC System and Empower software, creating an integrated ecosystem for new analyte characterization. Their methodology begins with a thorough understanding of analyte physicochemical properties, followed by implementation of column screening protocols using their Method Screening Tool. Waters employs a unique "column-centric" approach where multiple column chemistries (including their patented BEH, CSH, and HSS technologies) are systematically evaluated against standardized mobile phase conditions. Their Auto•Blend Plus technology enables precise quaternary solvent blending for pH and organic modifier screening without manual preparation. For complex samples, Waters utilizes their 2D-LC technology which combines orthogonal separation mechanisms to resolve co-eluting compounds. Their method development incorporates predictive modeling through DryLab integration, allowing in silico optimization before experimental verification. Waters' approach also emphasizes method transfer considerations, ensuring developed methods can be implemented across different instrument platforms with their Method Transfer Kit that accounts for system variance in dwell volume, extra-column dispersion, and detector cell design.
Strengths: Exceptional column technology portfolio with complementary selectivities; sophisticated software tools for method optimization and transfer; comprehensive support for regulatory compliance including audit trails and electronic signatures. Weaknesses: Proprietary ecosystem may limit flexibility with third-party components; higher cost structure compared to some competitors; steeper learning curve for utilizing advanced features of their software platform.
Critical Parameters in HPLC Method Optimization
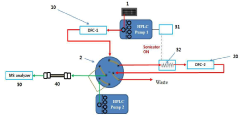
High performance liquid chromatography apparatus and method for screening substance using thereof
PatentActiveKR1020180009057A
Innovation
- A high-performance liquid chromatography (HPLC) apparatus and method that includes a first column for separating target materials, a dissociation device, a second column for further separation, and an analyzer, allowing for rapid screening of candidate substances without separate sample pretreatment.
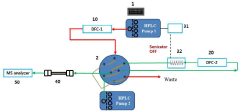
High-performance liquid chromatography with a controllable transverse flow inducer
PatentActiveEP3322978A1
Innovation
- The use of a controllable transverse flow inducer, which generates micro-scale vortices through alternating current electrokinetics, allowing for orthogonal flow induction independent of axial velocity, reducing dispersion by combining pressure and electro-osmotic flow, and enabling retention modulation without permanent surface charges.
Regulatory Compliance in Analytical Method Validation
Regulatory compliance represents a critical dimension in analytical method validation, particularly for HPLC methods developed for new analytes. The pharmaceutical and biopharmaceutical industries operate under stringent regulatory frameworks established by authorities such as the FDA, EMA, ICH, and WHO. These regulatory bodies have established comprehensive guidelines that govern the validation process of analytical methods to ensure data integrity, reproducibility, and reliability.
The ICH Q2(R1) guideline serves as the cornerstone document for analytical method validation, outlining essential parameters including specificity, linearity, accuracy, precision, detection limit, quantitation limit, range, and robustness. For HPLC method development specifically, compliance with these parameters requires systematic documentation of validation experiments and results that demonstrate the method's fitness for its intended purpose.
FDA's guidance on Analytical Procedures and Methods Validation emphasizes a lifecycle approach to method validation, requiring continuous verification throughout the method's operational life. This approach necessitates ongoing monitoring and periodic revalidation to ensure the method maintains its performance characteristics over time, especially when analyzing new analytes or when modifications are made to existing methods.
The validation master plan (VMP) represents a crucial regulatory document that outlines the overall validation strategy, responsibilities, and documentation requirements. For HPLC methods targeting new analytes, the VMP must specifically address how method development steps align with regulatory expectations, including risk assessment procedures and acceptance criteria that reflect the critical quality attributes of the analyte.
Data integrity compliance has gained increased regulatory focus, with requirements for electronic records management under 21 CFR Part 11 (FDA) and Annex 11 (EMA). HPLC method development must incorporate appropriate data handling procedures, audit trails, and electronic signatures to ensure compliant data management throughout the validation process.
Method transfer protocols constitute another essential regulatory consideration, particularly when HPLC methods developed in R&D laboratories are transferred to quality control or manufacturing environments. These protocols must demonstrate that the method performs consistently across different laboratories, instruments, and analysts, with clearly defined acceptance criteria for successful transfer.
Regulatory agencies increasingly expect risk-based approaches to method validation, focusing validation efforts on critical method parameters that significantly impact method performance. For new analytes, this requires thorough risk assessment during development to identify potential failure modes and establish appropriate control strategies that ensure consistent method performance under varying conditions.
The ICH Q2(R1) guideline serves as the cornerstone document for analytical method validation, outlining essential parameters including specificity, linearity, accuracy, precision, detection limit, quantitation limit, range, and robustness. For HPLC method development specifically, compliance with these parameters requires systematic documentation of validation experiments and results that demonstrate the method's fitness for its intended purpose.
FDA's guidance on Analytical Procedures and Methods Validation emphasizes a lifecycle approach to method validation, requiring continuous verification throughout the method's operational life. This approach necessitates ongoing monitoring and periodic revalidation to ensure the method maintains its performance characteristics over time, especially when analyzing new analytes or when modifications are made to existing methods.
The validation master plan (VMP) represents a crucial regulatory document that outlines the overall validation strategy, responsibilities, and documentation requirements. For HPLC methods targeting new analytes, the VMP must specifically address how method development steps align with regulatory expectations, including risk assessment procedures and acceptance criteria that reflect the critical quality attributes of the analyte.
Data integrity compliance has gained increased regulatory focus, with requirements for electronic records management under 21 CFR Part 11 (FDA) and Annex 11 (EMA). HPLC method development must incorporate appropriate data handling procedures, audit trails, and electronic signatures to ensure compliant data management throughout the validation process.
Method transfer protocols constitute another essential regulatory consideration, particularly when HPLC methods developed in R&D laboratories are transferred to quality control or manufacturing environments. These protocols must demonstrate that the method performs consistently across different laboratories, instruments, and analysts, with clearly defined acceptance criteria for successful transfer.
Regulatory agencies increasingly expect risk-based approaches to method validation, focusing validation efforts on critical method parameters that significantly impact method performance. For new analytes, this requires thorough risk assessment during development to identify potential failure modes and establish appropriate control strategies that ensure consistent method performance under varying conditions.
Cost-Benefit Analysis of Method Development
The cost-benefit analysis of HPLC method development requires careful consideration of both direct and indirect expenses against potential returns. Initial investment in method development typically ranges from $10,000 to $50,000, depending on complexity, with labor costs representing 60-70% of this expenditure. A skilled analyst may require 2-4 weeks for straightforward methods and up to 3 months for complex multi-analyte procedures, translating to significant salary allocation.
Equipment and consumables constitute another substantial cost factor. Modern HPLC systems cost between $30,000 and $150,000, while specialty columns range from $500 to $2,500 each. Method development often requires testing multiple column chemistries, mobile phase compositions, and detection parameters, with each iteration consuming valuable resources and time.
The benefits, however, can far outweigh these investments. A well-developed HPLC method delivers consistent, reliable results that directly impact product quality and regulatory compliance. For pharmaceutical companies, optimized methods can accelerate drug development timelines by 15-20%, potentially worth millions in earlier market entry. In quality control environments, efficient methods reduce analysis time by 30-50%, increasing laboratory throughput and reducing per-sample costs.
Risk mitigation represents another significant benefit. Robust methods minimize false positives/negatives, preventing costly product recalls or regulatory penalties. The average cost of a pharmaceutical product recall exceeds $10 million, making method reliability a critical economic consideration. Additionally, transferable methods reduce technology transfer costs between R&D and manufacturing sites by approximately 40%.
Long-term sustainability must also factor into the analysis. Methods requiring exotic solvents or specialized conditions incur higher operational costs over their lifecycle. Conversely, "green chemistry" approaches using reduced solvent volumes or environmentally friendly alternatives may increase initial development costs but reduce long-term expenses and environmental impact by 25-35%.
Return on investment typically occurs within 6-18 months for routine quality control methods and 2-3 years for complex research applications. Organizations should implement systematic review processes to evaluate method performance against predetermined financial and operational metrics, ensuring continued alignment with business objectives throughout the method lifecycle.
Equipment and consumables constitute another substantial cost factor. Modern HPLC systems cost between $30,000 and $150,000, while specialty columns range from $500 to $2,500 each. Method development often requires testing multiple column chemistries, mobile phase compositions, and detection parameters, with each iteration consuming valuable resources and time.
The benefits, however, can far outweigh these investments. A well-developed HPLC method delivers consistent, reliable results that directly impact product quality and regulatory compliance. For pharmaceutical companies, optimized methods can accelerate drug development timelines by 15-20%, potentially worth millions in earlier market entry. In quality control environments, efficient methods reduce analysis time by 30-50%, increasing laboratory throughput and reducing per-sample costs.
Risk mitigation represents another significant benefit. Robust methods minimize false positives/negatives, preventing costly product recalls or regulatory penalties. The average cost of a pharmaceutical product recall exceeds $10 million, making method reliability a critical economic consideration. Additionally, transferable methods reduce technology transfer costs between R&D and manufacturing sites by approximately 40%.
Long-term sustainability must also factor into the analysis. Methods requiring exotic solvents or specialized conditions incur higher operational costs over their lifecycle. Conversely, "green chemistry" approaches using reduced solvent volumes or environmentally friendly alternatives may increase initial development costs but reduce long-term expenses and environmental impact by 25-35%.
Return on investment typically occurs within 6-18 months for routine quality control methods and 2-3 years for complex research applications. Organizations should implement systematic review processes to evaluate method performance against predetermined financial and operational metrics, ensuring continued alignment with business objectives throughout the method lifecycle.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!